Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients Selection and Data Collection
2.3. Muscle Mass Index Values Calculated by Computed Tomography Scans
2.4. Definition of Sarcopenia
2.5. Measurement of Inflammation-and Nutritional Status-Based Makers
2.6. Follow-Up and Time-Course of Changes in Variables after Infusion of Pembrolizumab
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics and the Relationship with Sarcopenia
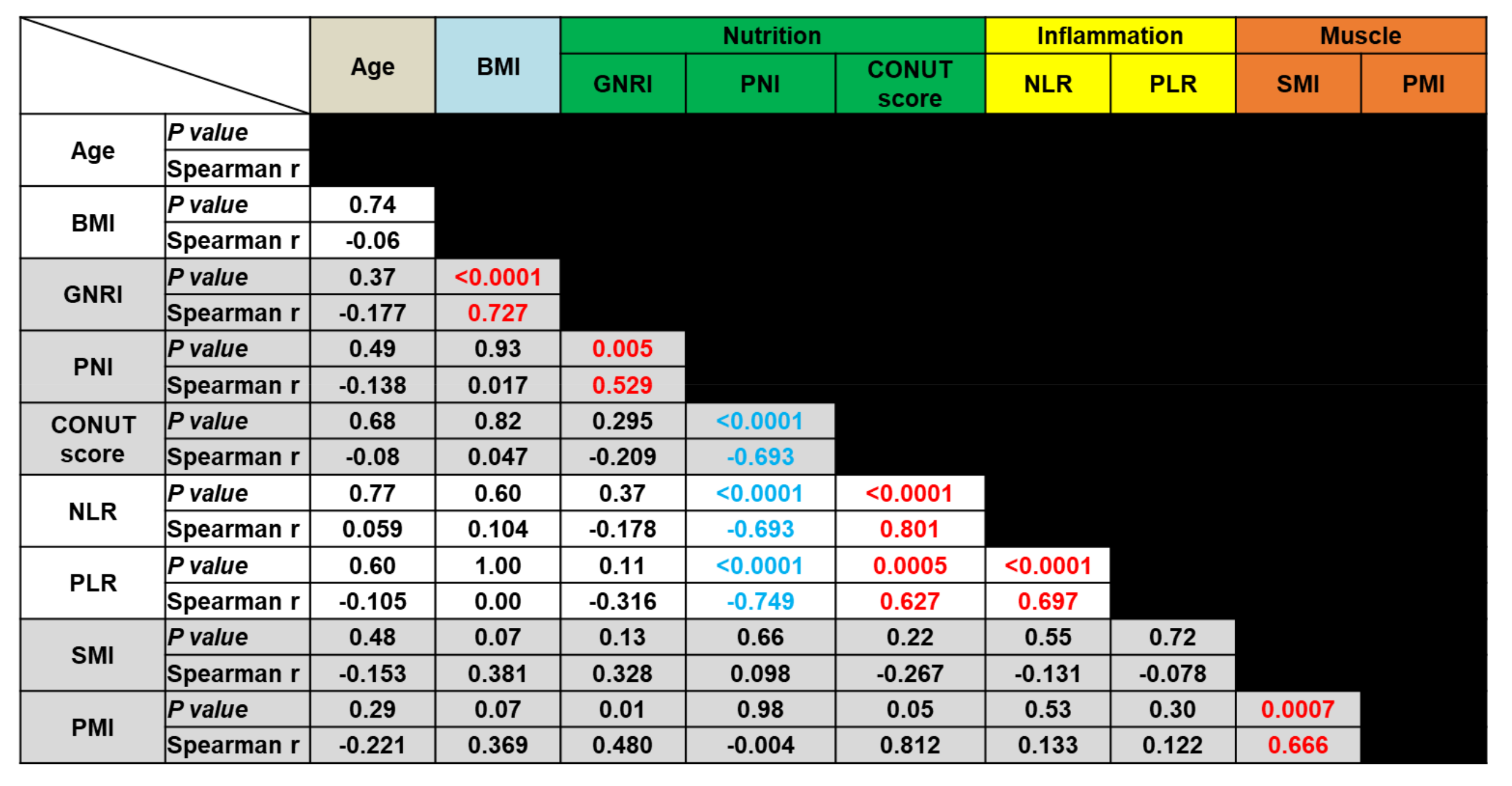
3.2. Correlation Analyses of Biomarkers
3.3. Prognostic Values of Nutritional-, Inflammation- and Muscle Mass-Based Markers
3.4. Time-Course of Change on Nutritional-, Inflammation- and Muscle Mass-Based Markers
3.5. Adverse Event Rates and Effects of Sarcopenia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for pd-l1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: Results from a multicenter, phase Ib study. J. Clin. Oncol. 2017, 35, 2117–2124. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Miyake, M.; Morizawa, Y.; Hori, S.; Marugami, N.; Iida, K.; Ohnishi, K.; Gotoh, D.; Tatsumi, Y.; Nakai, Y.; Inoue, T.; et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology 2017, 93, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Morizawa, Y.; Hori, S.; Marugami, N.; Shimada, K.; Gotoh, D.; Tatsumi, Y.; Nakai, Y.; Inoue, T.; Anai, S.; et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer 2017, 17, 237. [Google Scholar] [CrossRef]
- Itami, Y.; Miyake, M.; Tatsumi, Y.; Gotoh, D.; Hori, S.; Morizawa, Y.; Iida, K.; Ohnishi, K.; Nakai, Y.; Inoue, T.; et al. Preoperative predictive factors focused on inflammation-, nutrition-, and muscle-status in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy. Int. J. Clin. Oncol. 2019, 24, 533–545. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Moller, M.; Turzer, S.; Schutte, W.; Seliger, B.; Riemann, D. Blood immune cell biomarkers in patient with lung cancer undergoing treatment with checkpoint blockade. J. Immunother. 2020, 43, 57–66. [Google Scholar] [CrossRef]
- Ohba, T.; Takamori, S.; Toyozawa, R.; Nosaki, K.; Umeyama, Y.; Haratake, N.; Miura, N.; Yamaguchi, M.; Taguchi, K.; Seto, T.; et al. Prognostic impact of the Controlling Nutritional Status score in patients with non-small cell lung cancer treated with pembrolizumab. J. Thorac. Dis. 2019, 11, 3757–3768. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef]
- Hori, S.; Morizawa, Y.; Gotoh, D.; Itami, Y.; Nakai, Y.; Miyake, M.; Anai, S.; Torimoto, K.; Aoki, K.; Yoneda, T.; et al. Evaluation of preoperative abdominal adipose tissue-, Inflammation-, muscle mass-, and nutritional status-based prognostic markers to assess renal dysfunction in living kidney donors. Transpl. Proc. 2019, 51, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef]
- Shiroyama, T.; Suzuki, H.; Tamiya, M.; Tamiya, A.; Tanaka, A.; Okamoto, N.; Nakahama, K.; Taniguchi, Y.; Isa, S.I.; Inoue, T.; et al. Clinical characteristics of liver metastasis in nivolumab-treated patients with non-small cell lung cancer. Anticancer Res. 2018, 38, 4723–4729. [Google Scholar] [CrossRef]
- Heidelberger, V.; Goldwasser, F.; Kramkimel, N.; Jouinot, A.; Huillard, O.; Boudou-Rouquette, P.; Chanal, J.; Arrondeau, J.; Franck, N.; Alexandre, J.; et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Investig. New Drugs 2017, 35, 436–441. [Google Scholar] [CrossRef]
- Daly, L.E.; Power, D.G.; O’Reilly, A.; Donnellan, P.; Cushen, S.J.; O’Sullivan, K.; Twomey, M.; Woodlock, D.P.; Redmond, H.P.; Ryan, A.M. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer 2017, 116, 310–317. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Shoji, F.; Takeoka, H.; Kozuma, Y.; Toyokawa, G.; Yamazaki, K.; Ichiki, M.; Takeo, S. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer 2019, 136, 45–51. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total, n (%) | Non-Sarcopenia, n (%) | Sarcopenia, n (%) | p Value | |
|---|---|---|---|---|---|
| Total | 27 (100%) | 12 (44%) | 15 (56%) | ||
| Age | Median (range) | 73 (52–82) | 69 (52–82) | 74 (68–82) | 0.1 † |
| Sex | Male Female | 23 (85%) 4 (15%) | 11 (92%) 1 (8%) | 12 (80%) 3 (20%) | 0.61 |
| ECOG-PS | 0–1 ≥2 | 15 (56%) 12 (44%) | 8 (67%) 4 (33%) | 7 (47%) 8 (53%) | 0.44 |
| BMI (kg/m2) | Median (range) | 21.9 (17.3–29.4) | 23.1 (18.5–29.4) | 20.6 (17.3–28.1) | 0.07 † |
| CCI | <8 ≥8 | 20 (74%) 7 (26%) | 10 (83%) 2 (17%) | 10 (67%) 5 (33%) | 0.4 |
| Autoimmune disease history | No Yes | 26 (96%) 1 (4%) | 12 (100%) 0 (0%) | 14 (93%) 1 (7%) | 1.0 |
| Primary site | BT UTUC | 15 (56%) 12 (44%) | 7 (58%) 5 (42%) | 8 (53%) 7 (47%) | 1.0 |
| Variant histology | No Yes | 22 (81%) 5 (19%) | 9 (75%) 3 (25%) | 13 (87%) 2 (13%) | 0.63 |
| No. of prior chemotherapeutic regimens | 1 ≥2 | 17 (63%) 10 (37%) | 6 (50%) 6 (50%) | 11 (73%) 4 (27%) | 0.26 |
| Evaluable lesion | |||||
| Primary site | No Recurrence Progression | 19 (70%) 5 (19%) 3 (11%) | 11 (92%) 0 (0%) 1 (8%) | 8 (53%) 5 (33%) 2 (13%) | 0.06 |
| Regional lymph nodes | No Yes | 11 (41%) 16 (59%) | 4 (33%) 8 (67%) | 7 (47%) 8 (53%) | 0.7 |
| Non-regional lymph node | No Yes | 20 (74%) 7 (26%) | 8 (67%) 4 (33%) | 12 (80%) 3 (20%) | 0.66 |
| Lung | No Yes | 12 (44%) 15 (56%) | 7 (58%) 5 (42%) | 5 (33%) 10 (67%) | 0.26 |
| Liver | No Yes | 21 (78%) 6 (22%) | 10 (83%) 2 (17%) | 11 (73%) 4 (275) | 0.66 |
| Bone | No Yes | 25 (93%) 2 (7%) | 12 (100%) 0 (0%) | 13 (87%) 2 (13%) | 0.49 |
| Nutrition markers | |||||
| GNRI | Median (range) | 101.1 (72.9–122.8) | 106.1 (98.9–121.1) | 95.0 (72.9–122.8) | 0.002 † |
| PNI | Median (range) | 48.5 (28.5–56.0) | 48.5 (44–56) | 46.0 (28.5–54.5) | 0.09 † |
| CONUT score | Median (range) | 1 (0–8) | 1 (0–6) | 1 (0–8) | 0.8 † |
| Inflammation markers | |||||
| CRP (mg/dL) | Median (range) | 0.54 (0.03–18.3) | 0.25 (0.03–4.17) | 0.66 (0.06–18.3) | 0.15 † |
| NLR | Median (range) | 3.1 (0.9–37) | 3.0 (0.9–7.0) | 3.1 (1.2–37) | 0.64 † |
| PLR | Median (range) | 185 (78–957) | 178 (78–356) | 214 (105–957) | 0.48 † |
| Muscle markers | |||||
| PMI at L3 (cm2/m2) | Median (range) | 5.83 (2.21–8.94) | 6.81 (5.99–8.94) | 3.94 (2.21–6.27) | <0.0001 † |
| Men | Median (range) | 5.80 (2.21–8.94) | 7.01 (6.37–8.94) | 4.25 (2.21–6.27) | 0.0002 † |
| Women * | Median (range) | 3.61 (2.95–5.99) | 5.99 | 2.95, 3.61 | NA |
| Follow-Up (month) | Median (range) | 7 (1–20) | 12 (5–19) | 5 (1–20) | |
| Variables | PFS | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Sex | Female Male | 1 1.84 | 0.38–8.78 | 0.44 | |||
| Age | ≥75 <75 | 1 0.59 | 0.23–1.56 | 0.29 | |||
| ECOG-PS | 0 ≥2 | 1 2.55 | 0.95–6.86 | 0.06 | |||
| BMI | ≥22 <22 | 1 1.43 | 0.56–3.61 | 0.45 | |||
| CCI | <8 ≥8 | 1 1.54 | 0.50–4.73 | 0.45 | |||
| Primary site | BT UTUC | 1 1.75 | 0.68–4.49 | 0.24 | |||
| Variant histology | No Yes | 1 0.78 | 0.26–2.36 | 0.66 | |||
| Number of platinumchemotherapy courses | ≥4 ≤3 | 1 2.25 | 0.88–5.76 | 0.091 | |||
| Lung metastasis | No Yes | 1 1.90 | 0.76–4.78 | 0.17 | |||
| Liver metastasis | No Yes | 1 1.89 | 0.53–6.73 | 0.32 | |||
| GNRI | ≥100 <100 | 1 1.95 | 0.76–4.95 | 0.16 | |||
| PNI | ≥45 <45 | 1 2.10 | 0.75–5.93 | 0.16 | |||
| CONUT score | ≤1 ≥2 | 1 1.60 | 0.59–3.92 | 0.38 | |||
| CRP | <0.5 ≥0.5 | 1 1.15 | 0.46–2.86 | 0.77 | |||
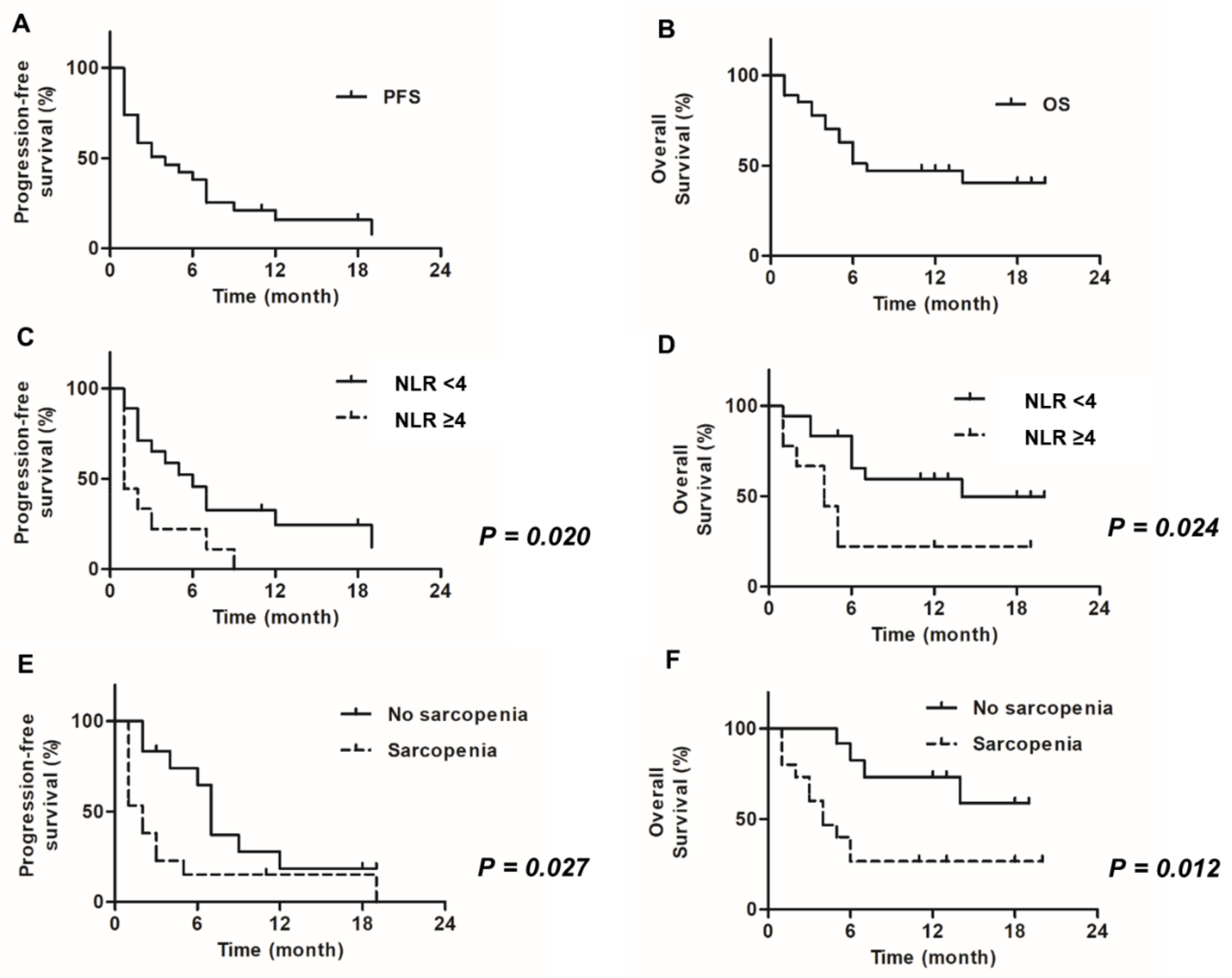
| NLR | <4.0 ≥4.0 | 1 3.81 | 1.23–11.7 | 0.020 | 1 2.89 | 1.10–7.03 | 0.025 |
| PLR | <200 ≥200 | 1 1.94 | 0.74–5.08 | 0.18 | |||
| Sarcopenia | No Yes | 1 2.99 | 1.14–7.85 | 0.027 | 1 2.79 | 1.14–7.32 | 0.030 |
| Variables | OS | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Sex | Female Male | 1 1.84 | 0.38–8.78 | 0.59 | |||
| Age | ≥75 <75 | 1 2.54 | 0.83–7.76 | 0.10 | |||
| ECOG-PS | 0 ≥2 | 1 3.17 | 1.06–9.51 | 0.040 | 1 1.99 | 0.50–7.89 | 0.33 |
| BMI | ≥22 <22 | 1 1.73 | 0.60–4.97 | 0.031 | |||
| CCI | <8 ≥8 | 1 3.24 | 0.87–11.2 | 0.071 | |||
| Primary site | BT UTUC | 1 1.61 | 0.54–4.80 | 0.39 | |||
| Variant histology | No Yes | 1 0.80 | 0.23–2.86 | 0.81 | |||
| Number of platinumchemotherapy courses | ≥4 ≤3 | 1 1.69 | 0.58–4.91 | 0.33 | |||
| Lung metastasis | No Yes | 1 2.18 | 0.76–6.26 | 0.15 | |||
| Liver metastasis | No Yes | 1 4.59 | 1.06–19.9 | 0.040 | 1 1.64 | 0.41–6.48 | 0.48 |
| GNRI | ≥100 <100 | 1 2.55 | 0.88–7.34 | 0.083 | |||
| PNI | ≥45 <45 | 1 3.44 | 1.03–11.6 | 0.046 | 1 2.15 | 0.57–8.11 | 0.26 |
| CONUT score | ≤1 ≥2 | 1 1.58 | 0.54–4.58 | 0.4 | |||
| CRP | <0.5 ≥0.5 | 1 1.77 | 0.62–5.04 | 0.29 | |||
| NLR | <4.0 ≥4.0 | 1 4.26 | 1.21–15.0 | 0.024 | 1 1.22 | 0.21–7.20 | 0.82 |
| PLR | <200 ≥200 | 1 2.37 | 0.83–6.78 | 0.11 | |||
| Sarcopenia | No Yes | 1 3.92 | 1.34–11.5 | 0.012 | 1 4.00 | 1.18–13.6 | 0.026 |
| Variable | PFS | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| irAE | Yes No | 1 0.98 | 0.26–3.75 | 0.98 | |||
| PNI decrease | <10% ≥10% | 1 1.16 | 0.29–4.63 | 0.83 | |||
| CONUT score increase | No Yes | 1 3.01 | 0.83–11.0 | 0.09 | |||
| CRP change | Low→Low High→Low the others | 1 1.71 | 0.66–4.42 | 0.27 | |||
| NLR increase | <15% ≥15% | 1 2.58 | 0.98–6.79 | 0.055 | |||
| PLR increase | <15% ≥ 15% | 1 4.08 | 1.34–12.4 | 0.013 | 1 3.10 | 0.51–18.7 | 0.22 |
| SMI decrease | <5% ≥ 5% | 1 1.24 | 0.33–4.64 | 0.75 | |||
| PMI decrease | <5% ≥5% | 1 17.1 | 3.18–91.7 | 0.001 | 1 12.8 | 1.91–85.4 | 0.008 |
| Variable | OS | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| irAE | Yes No | 1 1.05 | 0.22–5.08 | 0.95 | |||
| PNI decrease | <10% ≥10% | 1 2.18 | 0.52–9.04 | 0.28 | |||
| CONUT score increase | No Yes | 1 8.77 | 2.16–35.6 | 0.024 | 1 2.88 | 0.39–21.5 | 0.30 |
| CRP change | Low→Low High→Low the others | 1 2.21 | 0.72–6.77 | 0.17 | |||
| NLR increase | <15% ≥15% | 1 3.76 | 1.22–11.6 | 0.02 | 1 2.78 | 0.21–36.3 | 0.44 |
| PLR increase | <15% ≥15% | 1 3.74 | 1.17–11.9 | 0.026 | 1 1.96 | 0.20–19.4 | 0.56 |
| SMI decrease | <5% ≥5% | 1 3.15 | 0.75–13.3 | 0.12 | |||
| PMI decrease | <5% ≥5% | 1 7.84 | 1.52–40.4 | 0.014 | 1 6.21 | 1.12–34.3 | 0.036 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. https://doi.org/10.3390/diagnostics10050310
Shimizu T, Miyake M, Hori S, Ichikawa K, Omori C, Iemura Y, Owari T, Itami Y, Nakai Y, Anai S, et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics. 2020; 10(5):310. https://doi.org/10.3390/diagnostics10050310
Chicago/Turabian StyleShimizu, Takuto, Makito Miyake, Shunta Hori, Kazuki Ichikawa, Chihiro Omori, Yusuke Iemura, Takuya Owari, Yoshitaka Itami, Yasushi Nakai, Satoshi Anai, and et al. 2020. "Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab" Diagnostics 10, no. 5: 310. https://doi.org/10.3390/diagnostics10050310
APA StyleShimizu, T., Miyake, M., Hori, S., Ichikawa, K., Omori, C., Iemura, Y., Owari, T., Itami, Y., Nakai, Y., Anai, S., Tomioka, A., Tanaka, N., & Fujimoto, K. (2020). Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics, 10(5), 310. https://doi.org/10.3390/diagnostics10050310






