Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical Data
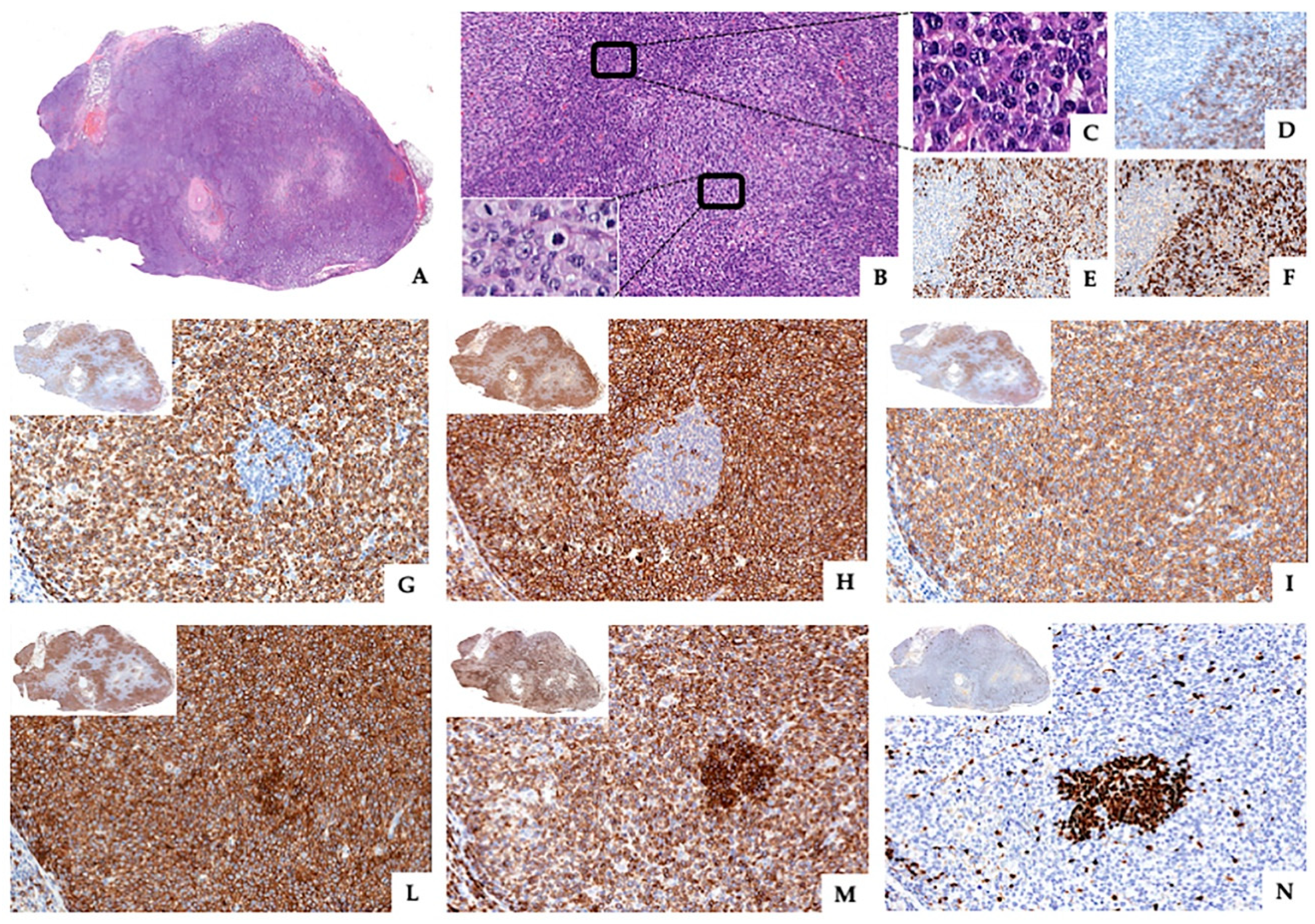
2.2. Pathological and Molecular Findings
2.3. Therapeutic Strategies
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CHOEP-14 | cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone |
| EBV | Epstein–Barr virus |
| IGH | immunoglobulin heavy-chain gene |
| IGK | immunoglobulin light-chain gene |
| IPI | international prognostic index |
| PCR | polymerase chain reaction |
| PTLC-NOS | peripheral T-cell lymphoma, not otherwise specified |
| R | rituximab |
| R-DHAP | rituximab plus dexamethasone, cisplatin and cytosine arabinoside |
| R-(p)GEMOX | rituximab plus (L-asparaginase), gemcitabine and oxaliplatin |
| TCR | T-cell receptor |
| TRG | T-cell receptor gamma gene |
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- International T-Cell Lymphoma Project; Vose, J.; Armitage, J.; Weisenburger, D. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Ferreri, A.J.M.; Zinzani, P.L.; Pileri, S.A. Peripheral T-cell lymphoma–Not otherwise specified. Crit. Rev. Oncol. 2011, 79, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; World Health Organization; International Agency for Research on Cancer. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; p. 585. [Google Scholar]
- Quintanilla-Martinez, L.; Preffer, F.; Rubin, D.; Ferry, J.A.; Harris, N.L. CD20+ T-Cell Lymphoma:Neoplastic Transformation of a Normal T-Cell Subset. Am. J. Clin. Pathol. 1994, 102, 483–489. [Google Scholar] [CrossRef]
- Pilozzi, E.; Pulford, K.; Jones, M.; Müller-Hermelink, H.-K.; Falini, B.; Ralfkiaer, E.; Pileri, S.; Pezzella, F.; De Wolf-Peeters, C.; Arber, D.; et al. Co-expression of CD79a (JCB117) and CD3 by lymphoblastic lymphoma. J. Pathol. 1998, 186, 140–143. [Google Scholar] [CrossRef]
- Takami, A.; Saito, M.; Nakao, S.; Asakura, H.; Nozue, T.; Onoe, Y.; Yachie, A.; Shiobara, S.; Matsuda, T. CD20-positive T-cell chronic lymphocytic leukaemia. Br. J. Haematol. 1998, 102, 1327–1329. [Google Scholar]
- Hashimoto, M.; Yamashita, Y.; Mori, N. Immunohistochemical detection of CD79a expression in precursor T cell lymphoblastic lymphoma/leukaemias. J. Pathol. 2002, 197, 341–347. [Google Scholar] [CrossRef]
- Rahemtullah, A.; Longtine, J.A.; Harris, N.L.; Dorn, M.; Zembowicz, A.; Quintanilla-Fend, L.; Preffer, F.I.; Ferry, J.A. CD20+ T-cell lymphoma: Clinicopathologic analysis of 9 cases and a review of the literature. Am. J. Surg. Pathol. 2008, 32, 1593–1607. [Google Scholar] [CrossRef]
- Balmer, N.N.; Hughey, L.; Busam, K.J.; Reddy, V.; Andea, A.A. Primary Cutaneous Peripheral T-Cell Lymphoma With Aberrant Coexpression of CD20: Case Report and Review of the Literature. Am. J. Dermatopathol. 2009, 31, 187–192. [Google Scholar] [CrossRef]
- Martín, B.; Stefanato, C.; Whittaker, S.; Robson, A. Primary cutaneous CD20-positive T-cell lymphoma. J. Cutan. Pathol. 2011, 38, 663–669. [Google Scholar] [CrossRef]
- Banz, Y.; Krasniqi, F.; Dirnhofer, S.; Tzankov, A. Relapsed angioimmunoblastic T-cell lymphoma with acquired expression of CD20: A case report and review of the literature. BMC Clin. Pathol. 2013, 13, 18. [Google Scholar] [CrossRef]
- Frings, V.; Roth, S.; Riedmiller, A.; Schäfer, K.; Goebeler, M.; Rosenwald, A.; Geissinger, E.; Wobser, M. Primary Cutaneous T-cell Lymphoma with Aberrant Expression of CD20. Acta Derm.-Venereol. 2017, 97, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Blakolmer, K.; Vesely, M.; Kummer, J.A.; Jurecka, W.; Mannhalter, C.; Chott, A. Immunoreactivity of B-Cell Markers (CD79a, L26) in Rare Cases of Extranodal Cytotoxic Peripheral T- (NK/T-) Cell Lymphomas. Mod. Pathol. 2000, 13, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Teruya-Feldstein, J.; Raffeld, M.; Sorbara, L.; Jaffe, E.S. Peripheral T-Cell Lymphoma with Aberrant Expression of CD79a and CD20: A Diagnostic Pitfall. Mod. Pathol. 2001, 14, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Matnani, R.G.; Stewart, R.; Pulliam, J.; Jennings, C.D.; Kesler, M. Peripheral T-Cell Lymphoma with Aberrant Expression of CD19, CD20, and CD79a: Case Report and Literature Review. Case Rep. Hematol. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Seilstad, K.H.; Porcu, P.; Morrison, C.D. Primary CD20+CD10+CD8+ T-Cell Lymphoma of the Skin With IgH and TCRβ Gene Rearrangement. Am. J. Clin. Pathol. 2006, 126, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lazzi, S.; Bellan, C.; Onnis, A.; De Falco, G.; Sayed, S.; Kostopoulos, I.; Onorati, M.; D’Amuri, A.; Santopietro, R.; Vindigni, C.; et al. Rare lymphoid neoplasms coexpressing B- and T-cell antigens. The role of PAX-5 gene methylation in their pathogenesis. Hum. Pathol. 2009, 40, 1252–1261. [Google Scholar] [CrossRef]
- Kakinoki, Y.; Hashiguchi, J.; Ishio, T.; Chiba, K.; Niino, D.; Ohshima, K. CD20-positive primary gastric T-cell lymphoma poorly responding to initial treatment with rituximab plus CHOP, and a literature review. Int. J. Hematol. 2015, 102, 702–708. [Google Scholar] [CrossRef]
- Shao, Y.; Bai, C.; Sun, J.; Gao, X. T-cell lymphoma with abundant CD20 expression showing a good response to rituximab with gemcitabine, oxiplatin, and L-asparaginase (R-pGEMOX): A case report. Medicine 2018, 97, e0199. [Google Scholar] [CrossRef]
- Teshima, K.; Ohyagi, H.; Kume, M.; Takahashi, S.; Saito, M.; Takahashi, N. Refractory CD20-positive peripheral T-cell lymphoma showing loss of CD20 expression after rituximab therapy and gain of CD20 expression after administration of vorinostat and gemcitabine. Jpn. J. Clin. Hematol. 2017, 58, 2227–2231. [Google Scholar] [CrossRef]
- Hirata, Y.; Yokote, T.; Kobayashi, K.; Nakayama, S.; Miyoshi, T.; Akioka, T.; Hara, S.; Tsuji, M.; Takubo, T.; Hanafusa, T. Rituximab for the treatment of CD20-positive peripheral T-cell lymphoma, unspecified. Leuk. Res. 2009, 33, e13–e16. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, J.; Tomita, A.; Sugimoto, T.; Shimada, K.; Ito, M.; Nakamura, S.; Kiyoi, H.; Kinoshita, T.; Naoe, T. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: Its prevalence and clinical significance. Blood 2009, 113, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Buckner, C.L.; Christiansen, L.R.; Bourgeois, D.; Lazarchick, J.J. CD20 positive T-cell lymphoma/leukemia: A rare entity with potential diagnostic pitfalls. Ann. Clin. Lab. Sci. 2007, 37, 263–267. [Google Scholar] [PubMed]
- Cumiskey, J.; Noonan, S.; Cummins, R.; Quinn, F.; Fennelly, D.; O’Briain, D.S.; Kay, E.W. T cell lymphoma co-expressing CD20. Diagn. Histopathol. 2010, 16, 111–113. [Google Scholar] [CrossRef]
- Makita, M.; Murakami, I.; Yoshioka, T.; Tanaka, H.; Yamamoto, K.; Imajo, K.; Takata, K.; Yoshino, T. Extranodal CD20-positive peripheral T-cell lymphoma presenting with adrenal and testicular masses. Jpn. J. Clin. Hematol. 2009, 50, 413–418. [Google Scholar]
- Kamata, M.; Sugaya, M.; Miyagaki, T.; Sonoda, K.; Ichimura, Y.; Mitsui, H.; Sato, S.; Kamikubo, Y.; Kurokawa, M. A case of CD20-positive peripheral T-cell lymphoma treated with rituximab and multiagent chemotherapy. Int. J. Dermatol. 2013, 53, e24–e26. [Google Scholar] [CrossRef]
- Di Napoli, A.; Al-Jadiri, M.F.; Ms, C.T.; Duranti, E.; Pilozzi, E.; Trivedi, P.; Anastasiadou, E.; Alsaadawi, A.R.; Al-Darraji, A.F.; Al-Hadad, S.A.; et al. Epstein-Barr virus (EBV) positive classical Hodgkin lymphoma of Iraqi children: An immunophenotypic and molecular characterization of Hodgkin/Reed-Sternberg cells. Pediatr. Blood Cancer 2013, 60, 2068–2072. [Google Scholar] [CrossRef]
- Macardle, P.J.; Nicholson, I.C. CD20. J. Biol. Regul. Homeost. Agents 2002, 16, 136–138. [Google Scholar]
- Hultin, L.E.; Hausner, M.A.; Hultin, P.M.; Giorgi, J.V. Cd20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. J. Int. Soc. Anal. Cytol. 1993, 14, 196–204. [Google Scholar] [CrossRef]
- Algino, K.M.; Thomason, R.W.; King, D.E.; Montiel, M.M.; Craig, F.E. CD20 (Pan-B Cell Antigen) Expression on Bone Marrow-Derived T Cells. Am. J. Clin. Pathol. 1996, 106, 78–81. [Google Scholar] [CrossRef]
- Murayama, Y.; Mukai, R.; Sata, T.; Matsunaga, S.; Noguchi, A.; Yoshikawa, Y. Transient Expression of CD20 Antigen (Pan B Cell Marker) in Activated Lymph Node T Cells. Microbiol. Immunol. 1996, 40, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Joly, E.; Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003, 4, 815. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Arber, D.A. CD79: A Review. Appl. Immunohistochem. Mol. Morphol. 2001, 9, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, H.C.; Stetler-Stevenson, M.; Gagneten, D.; Kingma, D.W.; Raffeld, M.; Jaffe, E.S. Immunodeficiency-associated malignant lymphoma. Three cases showing genotypic evidence of both T- and B-cell lineages. Am. J. Surg. Pathol. 1994, 18, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Souabni, A.; Cobaleda, C.; Schebesta, M.; Busslinger, M. Pax5 Promotes B Lymphopoiesis and Blocks T Cell Development by Repressing Notch1. Immunity 2002, 17, 781–793. [Google Scholar] [CrossRef]
- Höflinger, S.; Kesavan, K.; Fuxa, M.; Hutter, C.; Heavey, B.; Radtke, F.; Busslinger, M. Analysis of Notch1 function by in vitro T cell differentiation of Pax5 mutant lymphoid progenitors. J. Immunol. 2004, 173, 3935–3944. [Google Scholar] [CrossRef]
- Rothenberg, E.V. Regulatory factors for initial T lymphocyte lineage specification. Curr. Opin. Hematol. 2007, 14, 322–329. [Google Scholar] [CrossRef]
- Medvedovic, J.; Ebert, A.; Tagoh, H.; Busslinger, M. Pax5: A master regulator of B cell development and leukemogenesis. Adv. Immunol. 2011, 111, 179–206. [Google Scholar] [CrossRef]
- Cobaleda, C.; Schebesta, A.; Delogu, A.; Busslinger, M. Pax5: The guardian of B cell identity and function. Nat. Immunol. 2007, 8, 463–470. [Google Scholar] [CrossRef]
- Mikkola, I.; Freeman, B.C.; Yamamoto, K.R. Reversion of B Cell Commitment upon Loss of Pax5 Expression. Science 2002, 297, 110–113. [Google Scholar] [CrossRef]
- Hu, S.; He, X.; Shi, Y.; Yang, S.; Qin, Y.; Yang, J.; Dong, M.; Zhou, S.; Liu, P.; Gui, L.; et al. Clinical Features and Prognosis of CD20-Positive Peripheral T-Cell Lymphoma, Not Otherwise Specified (PTCL-NOS) in Chinese Patients: A Retrospective Single Institution Analysis. Blood 2015, 126, 5045. [Google Scholar] [CrossRef]
- Henry, C.; Deschamps, M.; Rohrlich, P.-S.; Pallandre, J.-R.; Rémy-Martin, J.-P.; Callanan, M.; Traverse-Glehen, A.; Grandclement, C.; Garnache-Ottou, F.; Gressin, R.; et al. Identification of an alternative CD20 transcript variant in B-cell malignancies coding for a novel protein associated to rituximab resistance. Blood 2010, 115, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Czuczman, M.S.; Olejniczak, S.H.; Gowda, A.; Kotowski, A.; Binder, A.; Kaur, H.; Knight, J.; Starostik, P.; Deans, J.; Hernandez-Ilizaliturri, F.J. Acquirement of Rituximab Resistance in Lymphoma Cell Lines Is Associated with Both Global CD20 Gene and Protein Down-Regulation Regulated at the Pretranscriptional and Posttranscriptional Levels. Clin. Cancer Res. 2008, 14, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Terui, Y.; Sugimura, N.; Kojima, K.; Sakurai, T.; Mishima, Y.; Kuniyoshi, R.; Taniyama, A.; Yokoyama, M.; Sakajiri, S.; Takeuchi, K.; et al. Identification of CD20 C-Terminal Deletion Mutations Associated with Loss of CD20 Expression in Non-Hodgkin’s Lymphoma. Clin. Cancer Res. 2009, 15, 2523–2530. [Google Scholar] [CrossRef]
- Pedersen, A.E.; Jungersen, M.B.; Pedersen, C.D. Monocytes mediate shaving of B-cell-bound anti-CD20 antibodies. Immunology 2011, 133, 239–245. [Google Scholar] [CrossRef]
- Beum, P.V.; Lindorfer, M.A.; Taylor, R.P. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J. Immunol. 2008, 181, 2916–2924. [Google Scholar] [CrossRef]
- Jazirehi, A.R.; Vega, M.I.; Bonavida, B.; O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; et al. Development of Rituximab-Resistant Lymphoma Clones with Altered Cell Signaling and Cross-Resistance to Chemotherapy. Cancer Res. 2007, 67, 1270–1281. [Google Scholar] [CrossRef]
- Delfau-Larue, M.-H.; De Leval, L.; Joly, B.; Plonquet, A.; Challine, D.; Parrens, M.; Delmer, A.; Salles, G.; Morschhauser, F.; Delarue, R.; et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica 2012, 97, 1594–1602. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Ito, K.; Bantilan, K.; Moskowitz, A.J.; Sauter, C.; Horwitz, S.; Schöder, H. Baseline and interim functional imaging with PET effectively risk stratifies patients with peripheral T-cell lymphoma. Blood Adv. 2019, 3, 187–197. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Pro, B.; Pinter-Brown, L.; Bartlett, N.L.; Popplewell, L.; Coiffier, B.; Lechowicz, M.J.; Savage, K.J.; Shustov, A.R.; Gisselbrecht, C.; et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. J. Clin. Oncol. 2011, 29, 1182–1189. [Google Scholar] [CrossRef]
- Zhentang, L.; Phipps, C.; Hwang, W.Y.K.; Tan, S.-Y.; Yeap, C.H.; Chan, Y.H.; Tay, K.; Lim, S.T.; Lee, Y.S.; Kumar, S.G.; et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: An open-label, multicentre phase 2 trial. Lancet Haematol. 2015, 2, e326–e333. [Google Scholar] [CrossRef]
- Coiffier, B.; Pro, B.; Prince, H.M.; Foss, F.; Sokol, L.; Greenwood, M.; Caballero, D.; Borchmann, P.; Morschhauser, F.; Wilhelm, M.; et al. Results from a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma After Prior Systemic Therapy. J. Clin. Oncol. 2012, 30, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-delta, gamma inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 2018, 131, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Chen, R.W.; Flinn, I.; Maris, M.B.; O’Connor, O.A.; Wieland, E.; Sievers, E.L. A phase 1 study of TTI-621, a novel immune checkpoint inhibitor targeting CD47, in subjects with relapsed or refractory hematologic malignancies. J. Clin. Oncol. 2016, 34, TPS7585. [Google Scholar] [CrossRef]
- Marchi, E.; O’Connor, O.A. The rapidly changing landscape in mature T-cell lymphoma (MTCL) biology and management. CA A Cancer J. Clin. 2019, 70, 47–70. [Google Scholar] [CrossRef]
- Kharfan-Dabaja, M.A.; Kumar, A.; Ayala, E.; Hamadani, M.; Reimer, P.; Gisselbrecht, C.; D’Amore, F.; Jantunen, E.; Ishida, T.; Bazarbachi, A.; et al. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Boil. Blood Marrow Transplant. 2017, 23, 1826–1838. [Google Scholar] [CrossRef]
- Cox, M.C.; Musuraca, G.; Battistini, R.; Casaroli, I.; Zoli, V.; Anticoli-Borza, P.; Arcari, A.; Naso, V.; Di Landro, F.; Fabbri, F.; et al. Aggressive lymphomas of the elderly: The DEVEC metronomic chemotherapy schedule fits the unfit. Br. J. Haematol. 2017, 183, 819–822. [Google Scholar] [CrossRef]



| Reference | Age (yr)/Sex | Clinical Stage | T-cell Antigens | B-cell Antigens | CD30 Antigen Expression | Molecular Findings | Therapy | Response |
|---|---|---|---|---|---|---|---|---|
| Magro et al. [17] | 65/F | I | CD3+, CD5+, CD2+, CD4+/-, CD8+ | CD20+ variable, CD79a-, CD22- | - | TRB clonal IGH clonal | R | Progressive disease |
| Buckner et al. [24] | 84/M | III | CD3+, CD5+, CD7-, CD4+, | CD20+ variable | - | TRG clonal | R-CHOP | Progressive disease |
| Rahemtullah et al. [9] Patient 2 | 77/M | II | CD3+, CD5+, CD2+, CD7-, CD4+, CD8- | CD20+dim, CD79a-, PAX5- | - | TRG clonal | R plus chemotherapy including anthracycline | Alive at 4 months of treatment |
| Rahemtullah et al. [9] Patient 4 | 36/F | III | CD3+, CD5+, CD2+, CD7+, CD4+, CD8- | CD20+dim, CD79a-, CD19-, CD22- | ND | TRG and TRB clonal IGH and IGK polyclonal | R plus chemotherapy including anthracycline | Partial remission |
| Rahemtullah et al. [9] Patient 5 | 75/M | IV | CD3+, CD5-, CD2-, CD7+, CD4-, CD8- | CD20+strong, CD79a+, CD19+ PAX5- | + (rare positive cells) | TRG and TRB clonal IGH polyclonal | R plus chemotherapy including anthracycline | Partial remission |
| Makita et al. [26] * | 59/M | IV | CD3+, CD5+, CD7+, CD4-, CD8-, GrB+, TIA1+ | CD20+strong, CD79a-, PAX5- | ND | TRB clonal IGH polyclonal | R-CHOP | Progressive disease |
| Hirata et al. [22] | 74/M | III | CD3+, CD5+, CD2+, CD7+, CD4+, CD8- | CD20+ variable, CD79a-, CD19-, CD22- | - | TRG clonal IGH polyclonal | R | Stable disease |
| Cumiskey et al. [25] | 84/M | III | CD3+, CD5+, CD4+, CD8- | CD20+ strong, CD79a- | - | TCR (gene not specified) clonal IGH polyclonal | R-CEOP | Complete remission |
| Matnani et al. [16] | 75/M | IV | CD3+, CD5+/-, CD7+/-, CD4-, CD8- | CD20+ variable, CD19+, CD79a- | - | TRG monoclonal IGH polyclonal | R-CHOP | Complete remission |
| Kamata et al. [27] | 83/F | IV | CD3+, CD5+, CD4+, CD8- | CD20+ variable, CD79a-, PAX5- | - | TRG clonal IGH polyclonal | R-CHOP | Partial remission |
| Kakinoki et al. [19] | 44/M | IE | CD3+, CD5+, CD7+, CD4-, CD8- | CD20+, CD79a- variable, PAX5- | ND | TRG and TRB clonal IGH polyclonal | R-CHOP | Stable disease |
| Teshima et al. [21] * | 79/M | III | n/a | CD20+ | ND | TCR (gene not specified) clonal IGH polyclonal | R-CHOP | Partial remission |
| Shao et al. [20] | 65/M | III | CD3+ | CD20+, PAX5- | ND | TCR (gene not specified) clonal | R-pGEMOX | Partial remission |
| Mangogna et al. (our case) | 59/M | IV | CD3+, CD5+, CD4+, CD8-, TIA1+, GrB- | CD20+, CD79a+, PAX5- | + (rare positive cells < 1%) | TRG clonal IGH polyclonal IGK polyclonal | R-DHAP R-GEMOX | Progressive disease |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangogna, A.; Cox, M.C.; Ruco, L.; Lopez, G.; Belmonte, B.; Di Napoli, A. Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature. Diagnostics 2020, 10, 341. https://doi.org/10.3390/diagnostics10060341
Mangogna A, Cox MC, Ruco L, Lopez G, Belmonte B, Di Napoli A. Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature. Diagnostics. 2020; 10(6):341. https://doi.org/10.3390/diagnostics10060341
Chicago/Turabian StyleMangogna, Alessandro, Maria Christina Cox, Luigi Ruco, Gianluca Lopez, Beatrice Belmonte, and Arianna Di Napoli. 2020. "Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature" Diagnostics 10, no. 6: 341. https://doi.org/10.3390/diagnostics10060341
APA StyleMangogna, A., Cox, M. C., Ruco, L., Lopez, G., Belmonte, B., & Di Napoli, A. (2020). Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature. Diagnostics, 10(6), 341. https://doi.org/10.3390/diagnostics10060341





