Prognostic Value of Ki67 Percentage, WT-1 Expression and p16/CDKN2A Deletion in Diffuse Malignant Peritoneal Mesothelioma: A Single-Centre Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Histologic and Molecular Analysis
3.1.1. Histologic Evaluation
3.1.2. P16 Expression and CDKN2A Status
3.1.3. Analysis of Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Travis, W.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.J. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart; International Agency for Research on Cancer (IARCS): Lyon, France, 2015. [Google Scholar]
- Hodgson, J.T.; McElvenny, D.M.; Darnton, A.J.; Price, M.J.; Peto, J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br. J. Cancer 2005, 92, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; de Klerk, N.H.; Magnani, C.; Ferrante, D.; Berry, G.; Musk, A.W.; Merler, E. Mesothelioma risk after 40 years since first exposure to asbestos: A pooled analysis. Thorax 2014, 69, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viti, A.; Bertolaccini, L.; Terzi, A. Biologic therapy and gene therapy in the multimodality treatment of malignant pleural mesothelioma. Ann. Transl. Med. 2015, 3, 248. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Delmonte, A.; Burgio, M.A.; Verlicchi, A.; Puccetti, M.; Bravaccini, S.; Cravero, P.; Tumedei, M.M.; Diano, D.; Rossi, G.; et al. Impressive clinical response to anti-PD-1 therapy in epithelioid mesothelioma with high clonal PD-L1 expression and EML4-ALK rearrangement. Lung Cancer 2020, 142, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Sauter, J.L.; Nowak, A.K.; Kindler, H.L.; Gill, R.R.; Remy-Jardin, M.; Armato, S.G.; Fernandez-Cuesta, L.; Bueno, R.; Alcala, N.; et al. EURACAN/IASLC proposals for updating the histologic classification of pleural mesothelioma: Towards a more multidisciplinary approach. J. Thorac. Oncol. 2020, 15, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Moolgavkar, S.H.; Meza, R.; Turim, J. Pleural and peritoneal mesotheliomas in SEER: Age effects and temporal trends, 1973-2005. Cancer Causes Control 2009, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Deraco, M.; Elias, D.; Glehen, O.; Levine, E.A.; Moran, B.J.; Morris, D.L.; Chua, T.C.; Piso, P.; Sugarbaker, P.H.; et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database. Cancer 2011, 117, 1855–1863. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Taub, R.N.; Hesdorffer, M.; Hibshoosh, H.; Chabot, J.A.; Keohan, M.L.; Alsberry, R.; Alexis, D.; Powell, C.A. P16 loss and mitotic activity predict poor survival in patients with peritoneal malignant mesothelioma. Clin. Cancer Res. 2005, 11, 3303–3308. [Google Scholar] [CrossRef] [Green Version]
- Scattone, A.; Serio, G.; Marzullo, A.; Nazzaro, P.; Corsi, F.; Cocca, M.P.; Mattoni, M.; Punzi, A.; Gentile, M.; Buonadonna, A.L.; et al. High Wilms’ tumour gene (WT1) expression and low mitotic count are independent predictors of survival in diffuse malignant peritoneal mesothelioma. Histopathology 2012, 60, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Brich, S.; Bozzi, F.; Perrone, F.; Tamborini, E.; Cabras, A.D.; Deraco, M.; Stacchiotti, S.; Dagrada, G.P.; Pilotti, S. Fluorescence in situ hybridization (FISH) provides estimates of minute and interstitial BAP1, CDKN2A, and NF2 gene deletions in peritoneal mesothelioma. Mod. Pathol. 2020, 33, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Suzuki, K.; Colovos, C.; Sima, C.S.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod. Pathol. 2012, 25, 260–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacic, S.; Kothmaier, H.; Land, S.; Shuai, Y.; Halbwedl, I.; Morbini, P.; Murer, B.; Comin, C.; Galateau-Salle, F.; Demirag, F.; et al. Prognostic significance of p16/CDKN2A loss in pleural malignant mesotheliomas. Virchows Arch. 2008, 453, 627–635. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Paz-Ares, L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur. J. Surg. Oncol. 2006, 32, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Serio, G.; Pezzuto, F.; Marzullo, A.; Scattone, A.; Cavone, D.; Punzi, A.; Fortarezza, F.; Gentile, M.; Buonadonna, A.L.; Barbareschi, M.; et al. Peritoneal mesothelioma with residential asbestos exposure. Report of a case with long survival (seventeen years) analyzed by cgh-array. Int. J. Mol. Sci. 2017, 18, 1818. [Google Scholar] [CrossRef] [Green Version]
- Shavelle, R.; Vavra-Musser, K.; Lee, J.; Brooks, J. Life expectancy in pleural and peritoneal mesothelioma. Lung Cancer Int. 2017, 2017, 2782590. [Google Scholar] [CrossRef] [Green Version]
- Pillai, K.; Pourgholami, M.H.; Chua, T.C.; Morris, D.L. Ki67-BCL2 index in prognosis of malignant peritoneal mesothelioma. Am. J. Cancer Res. 2013, 3, 411–423. [Google Scholar]
- Pillai, K.; Pourgholami, M.H.; Chua, T.C.; Morris, D.L. Prognostic significance of Ki67 expression in malignant peritoneal mesothelioma. Am. J. Clin. Oncol. 2015, 38, 388–394. [Google Scholar] [CrossRef]
- Kusamura, S.; Torres Mesa, P.A.; Cabras, A.; Baratti, D.; Deraco, M. The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann. Surg. Oncol. 2016, 23, 1468–1473. [Google Scholar] [CrossRef]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 14, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Kumar-Singh, S.; Segers, K.; Rodeck, U.; Backhovens, H.; Bogers, J.; Weyler, J.; Van Broeckhoven, C.; Van Marck, E. WT1 mutation in malignant mesothelioma and WT1 immunoreactivity in relation to p53 and growth factor receptor expression, cell-type transition, and prognosis. J. Pathol. 1997, 181, 67–74. [Google Scholar] [CrossRef]
- Cedrés, S.; Montero, M.A.; Zamora, E.; Martínez, A.; Martínez, P.; Fariñas, L.; Navarro, A.; Torrejon, D.; Gabaldon, A.; Ramon, Y.; et al. Expression of Wilms’ tumor gene (WT1) is associated with survival in malignant pleural mesothelioma. Clin. Transl. Oncol. 2014, 16, 776–782. [Google Scholar] [CrossRef]
- Kettunen, E.; Savukoski, S.; Salmenkivi, K.; Böhling, T.; Vanhala, E.; Kuosma, E.; Anttila, S.; Wolff, H. CDKN2A copy number and p16 expression in malignant pleural mesothelioma in relation to asbestos exposure. BMC Cancer 2019, 19, 507. [Google Scholar] [CrossRef]
- Illei, P.B.; Ladanyi, M.; Rusch, V.W.; Zakowski, M.F. The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer 2003, 99, 51–56. [Google Scholar] [CrossRef]
- Chiosea, S.; Krasinskas, A.; Cagle, P.T.; Mitchell, K.A.; Zander, D.S.; Dacic, S. Diagnostic importance of 9p21 homozygous deletion in malignant mesotheliomas. Mod. Pathol. 2008, 21, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Krasinskas, A.M.; Bartlett, D.L.; Cieply, K.; Dacic, S. CDKN2A and MTAP deletions in peritoneal mesotheliomas are correlated with loss of p16 protein expression and poor survival. Mod. Pathol. 2010, 23, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive Genomic Analysis of Malignant Pleural Mesothelioma Identifies Recurrent Mutations, Gene Fusions and Splicing Alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, N.; Toyooka, S.; Yanai, H.; Soh, J.; Fujimoto, N.; Yamamoto, H.; Ichihara, S.; Kimura, K.; Ichimura, K.; Sano, Y.; et al. Frequent p16 inactivation by homozygous deletion or methylation is associated with a poor prognosis in Japanese patients with pleural mesothelioma. Lung Cancer 2008, 62, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Bahnassy, A.A.; Zekri, A.R.; Abou-Bakr, A.A.; El-Deftar, M.M.; El-Bastawisy, A.; Sakr, M.A.; El-Sherif, G.M.; Gaafar, R.M. Aberrant expression of cell cycle regulatory genes predicts overall and disease-free survival in malignant pleural mesothelioma patients. Exp. Mol. Pathol. 2012, 93, 154–161. [Google Scholar] [CrossRef]
- Chung, C.T.; Santos Gda, C.; Hwang, D.M.; Ludkovski, O.; Pintilie, M.; Squire, J.A.; Tsao, M.S. FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J. Clin. Pathol. 2010, 63, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Bueno, R.; Chen, C.; Gordon, G.; Heilig, E.; Kelsey, K. Alterations of the p16(INK4) locus in human malignant mesothelial tumors. Carcinogenesis 2002, 23, 1127–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantini, L.; Hassan, R.; Sterman, D.H.; Aerts, J.G.J.V. Emerging treatments for malignant pleural mesothelioma: Where are we heading? Front. Oncol. 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Number of Cases | Survival (Months) Mean ± SD (C.I. 95%) | p-Value | Multivariate Analysis |
|---|---|---|---|---|
| Histotype | ||||
| Epithelioid | 32 | 19.7 ± 3.3 (13.2–26.2) | ||
| Biphasic | 9 | 4.8 ± 1.8 (1.3–8.4) | 0.0001 * | n.s |
| Sarcomatoid | 4 | 2.7 ± 1 (0.7–4.7) | ||
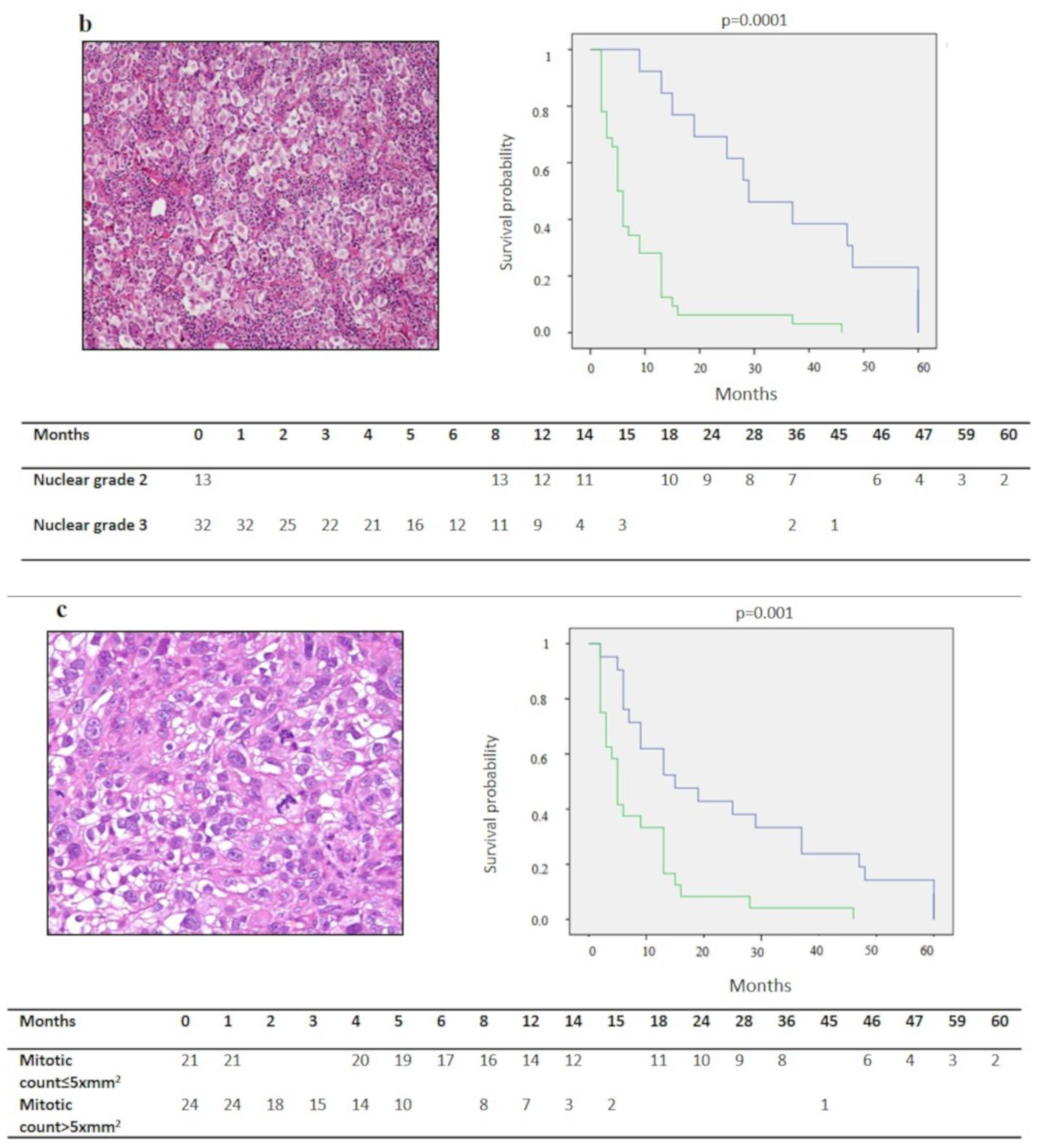
| Nuclear Grade | ||||
| 1 | 0 | |||
| 2 | 13 | 33.7 ± 5.2 (23.5–44) | 0.0001 * | n.s |
| 3 | 32 | 76 ± 5.6 (4.3–11.1) | ||
| Mitotic Count | ||||
| ≤5/10 x mm2 | 21 | 23.5 ± 20.5 (14.7–32.2) | 0.001 * | n.s |
| >5/10 x mm2 | 24 | 8 ± 10.1 (3.9–12.1) | ||
| Necrosis | ||||
| Absent | 27 | 17.3 ± 18.7 (10.3–24.4) | n.s | |
| Present | 18 | 12.1 ± 15.4 (4.9–19.2) | ||
| Inflammatory Infiltrate | ||||
| Absent | 11 | 15.5 ± 19.1 (4.2–26.8) | n.s | |
| Present | 34 | 15.1 ± 17.2 (9.3–21) | ||
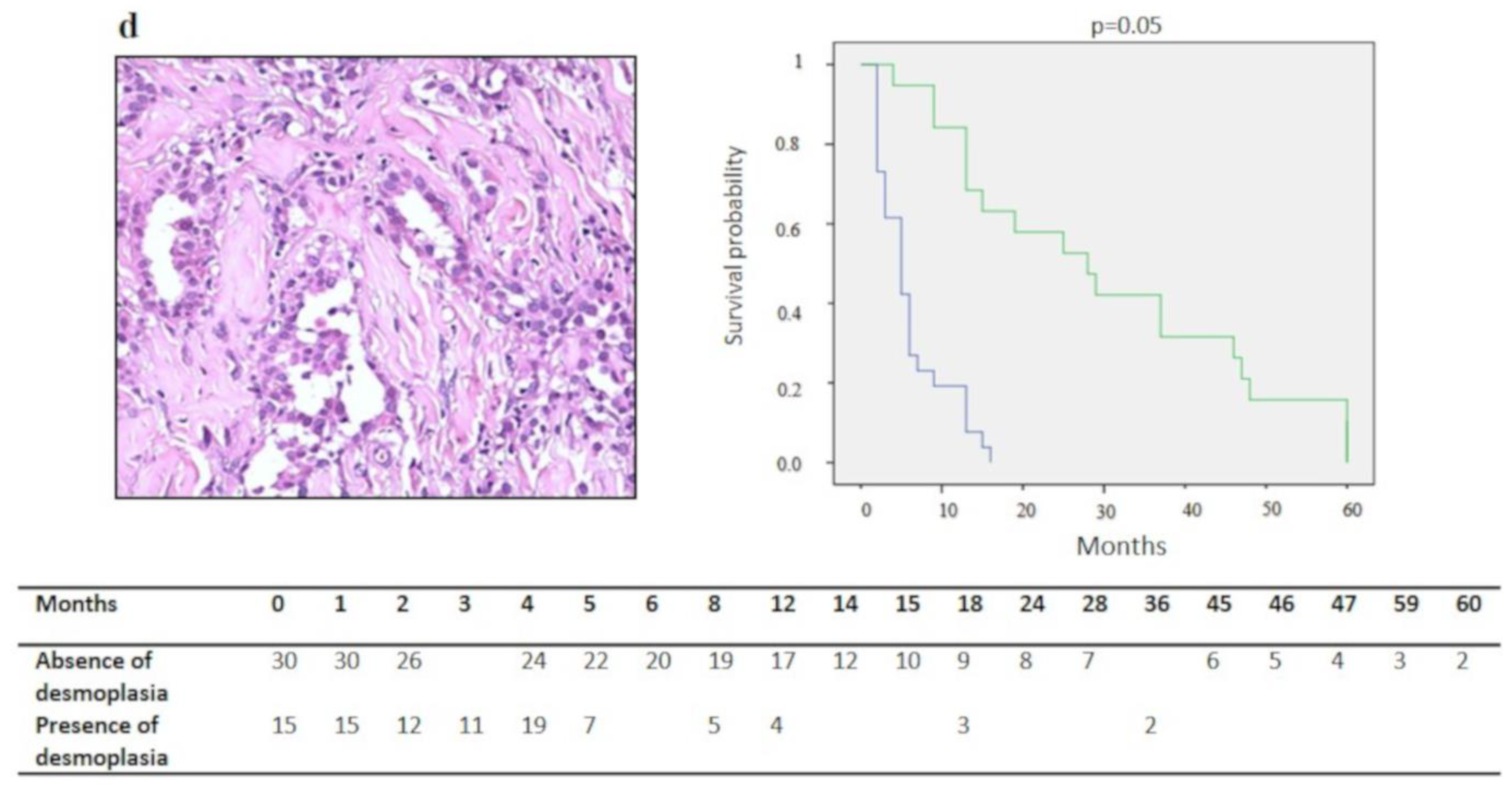
| Desmoplastic Stromal Reaction | ||||
| Absent | 30 | 18.2 ± 19.2 (11.3–25.1) | 0.05 * | n.s. |
| Present | 15 | 9.3 ± 11.7 (3.3–15.2) | ||
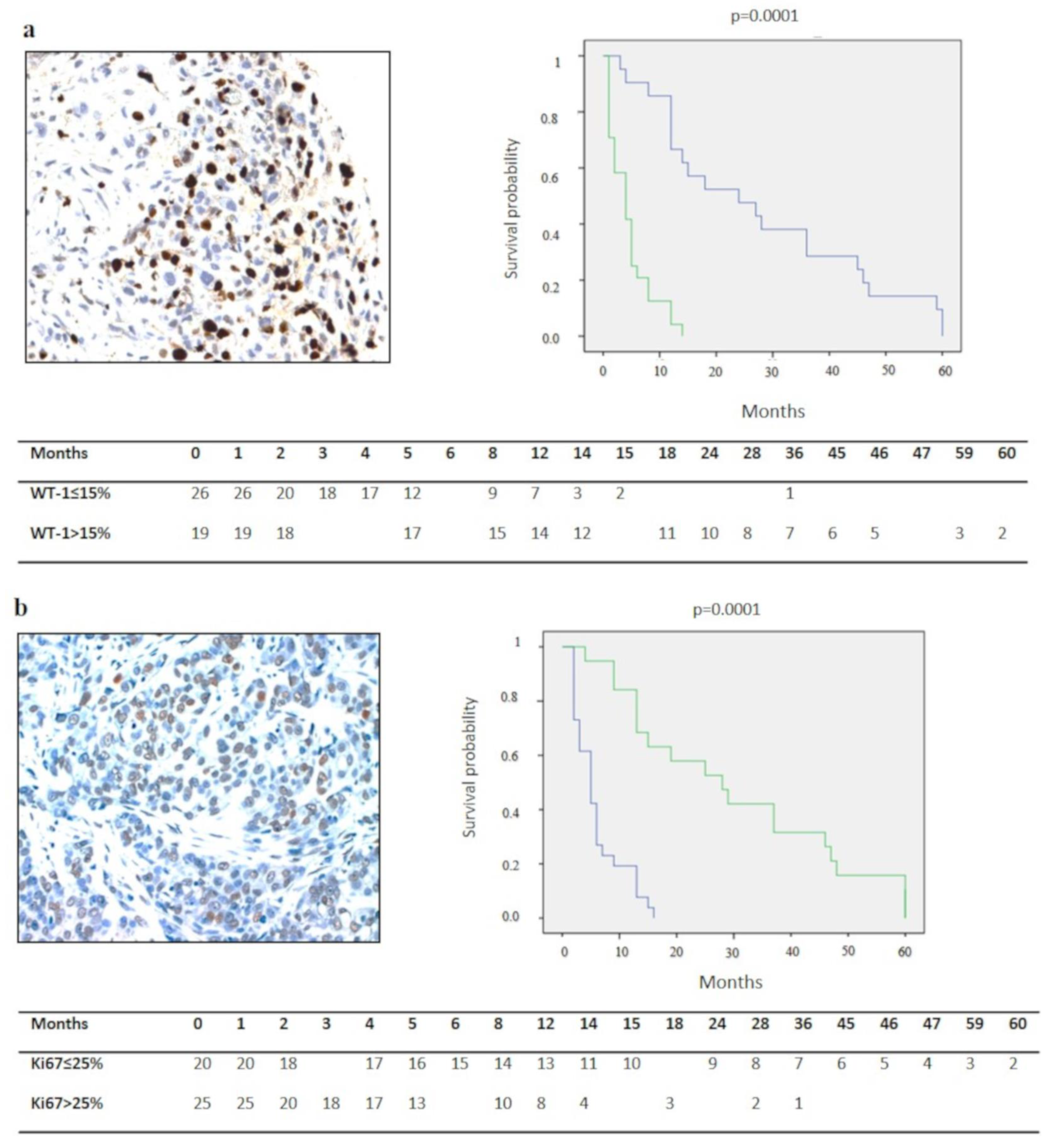
| Ki67 | ||||
| ≤25% | 21 | 26.14 ± 3.5 (2.7–33.3) | 0.0001 * | 0.01 * |
| >25% | 24 | 9.2 ± 1.5 (3.4–6.5) | ||
| WT-1 | ||||
| ≤15% | 26 | 5 ± 0.8 (3.4–6.7) | 0.0001 * | 0.001 * |
| >15% | 19 | 29.2 ± 4.3 (20.6–37.8) | ||
| P16 IHC | ||||
| Absent/≤5% | 23 | 7.7 ± 1.8 (4.1–11.3) | 0.001 * | 0.017 * |
| Present | 22 | 23.1 ± 4.4 (14.5–31.8) | ||
| P16 FISH | ||||
| No deletion | 8 | 22 ± 8.8 (4.6–39.3) | ||
| Heterozygous deletion | 14 | 23.8 ± 5.5 (13.9–33.8) | 0.004 * | |
| Homozygous deletion | 23 | 7.7 ± 1.8 (4.1–11.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzuto, F.; Serio, G.; Fortarezza, F.; Scattone, A.; Caporusso, C.; Punzi, A.; Cavone, D.; Pennella, A.; Marzullo, A.; Vimercati, L. Prognostic Value of Ki67 Percentage, WT-1 Expression and p16/CDKN2A Deletion in Diffuse Malignant Peritoneal Mesothelioma: A Single-Centre Cohort Study. Diagnostics 2020, 10, 386. https://doi.org/10.3390/diagnostics10060386
Pezzuto F, Serio G, Fortarezza F, Scattone A, Caporusso C, Punzi A, Cavone D, Pennella A, Marzullo A, Vimercati L. Prognostic Value of Ki67 Percentage, WT-1 Expression and p16/CDKN2A Deletion in Diffuse Malignant Peritoneal Mesothelioma: A Single-Centre Cohort Study. Diagnostics. 2020; 10(6):386. https://doi.org/10.3390/diagnostics10060386
Chicago/Turabian StylePezzuto, Federica, Gabriella Serio, Francesco Fortarezza, Anna Scattone, Concetta Caporusso, Alessandra Punzi, Domenica Cavone, Antonio Pennella, Andrea Marzullo, and Luigi Vimercati. 2020. "Prognostic Value of Ki67 Percentage, WT-1 Expression and p16/CDKN2A Deletion in Diffuse Malignant Peritoneal Mesothelioma: A Single-Centre Cohort Study" Diagnostics 10, no. 6: 386. https://doi.org/10.3390/diagnostics10060386
APA StylePezzuto, F., Serio, G., Fortarezza, F., Scattone, A., Caporusso, C., Punzi, A., Cavone, D., Pennella, A., Marzullo, A., & Vimercati, L. (2020). Prognostic Value of Ki67 Percentage, WT-1 Expression and p16/CDKN2A Deletion in Diffuse Malignant Peritoneal Mesothelioma: A Single-Centre Cohort Study. Diagnostics, 10(6), 386. https://doi.org/10.3390/diagnostics10060386






