Fold-Over Oversampling Effects in the Measurements of Cerebral Cerebrospinal Fluid and Blood Flows with 2D Cine Phase-Contrast MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theory
2.2. Image Acquisition
2.3. Image Processing and Analysis
3. Results
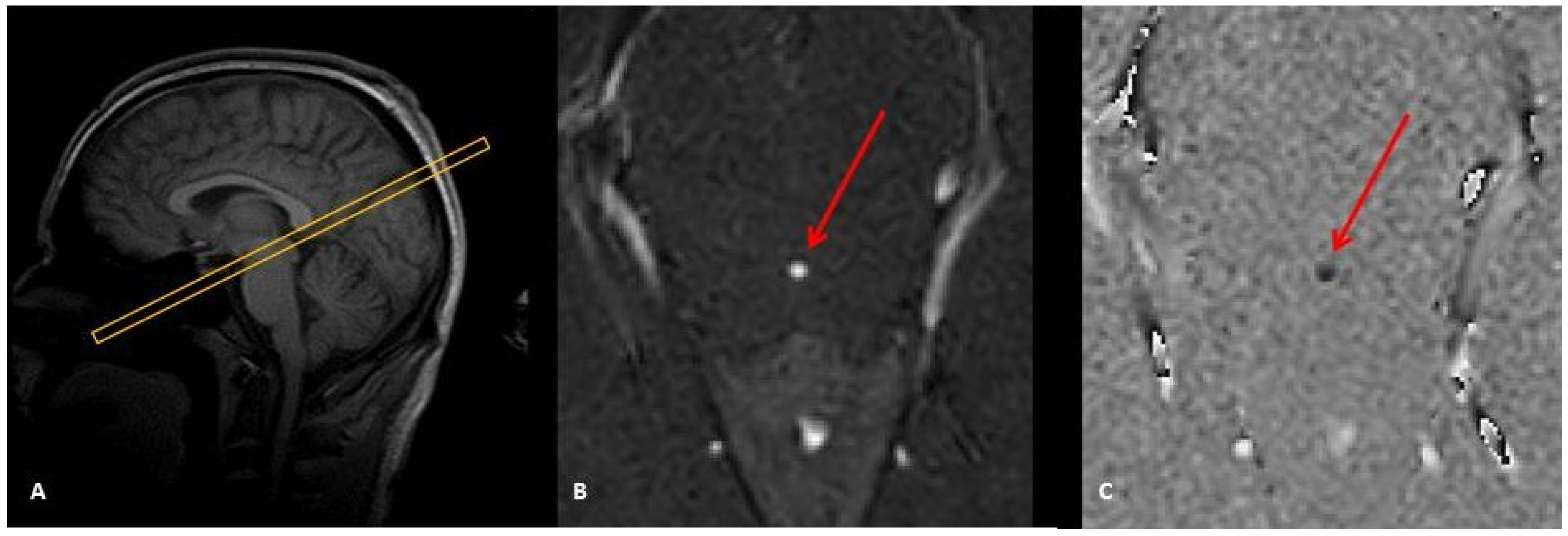
3.1. Section Area
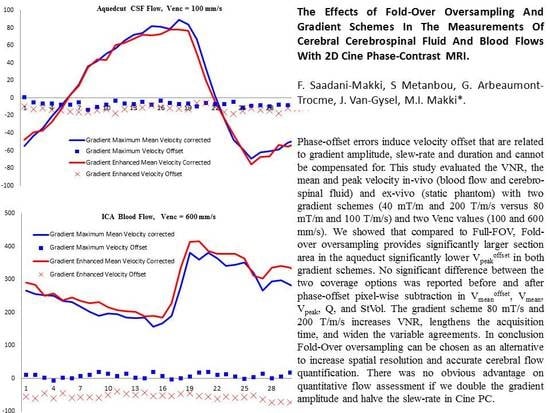
3.2. Velocity-to-Noise Ratio (VNR)
3.3. Vpeak, Vpeakoffset, and Vpeakcorr
3.4. Vmean, Vmeanoffset, and Vmeancorr
3.5. Vmin, Vminoffset, and Vmincorr
3.6. QNet and QNetcorr
3.7. SVol, and SVolcorr
3.8. Bland–Altman
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cine-PC | Cine Phase Contrast |
| CSF | Cerebrospinal Fluid |
| Venc | Velocity Encoding |
| VNR | Velocity-to-Noise Ratio |
| Vmean | mean flow velocity |
| Vmeancorr | corrected Vmean |
| QNet | Net Flow |
| QNetcorr | corrected QNet; |
| SVol | Stroke Volume |
| SVolcorr | corrected SVol; |
| ICA | Internal Carotid Artery; |
| FOV | Field-Of-View. |
References
- Bradley, W.G.; Scalzo, D.; Queralt, J.; Nitz, W.N.; Atkinson, D.J.; Wong, P. Normal-pressure hydrocephalus: Evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996, 198, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capel, C.; Makki, M.; Gondry-Jouet, C.; Bouzerar, R.; Courtois, V.; Krejpowicz, B.; Baledent, O. Insights Into Cerebrospinal Fluid and Cerebral Blood Flows in Infants and Young Children. J. Child Neurol. 2013, 29, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Tain, R.-W.; Bagci, A.M.; Lam, B.L.; Sklar, E.M.; Ertl-Wagner, B.; Alperin, N. Determination of cranio-spinal canal compliance distribution by MRI: Methodology and early application in idiopathic intracranial hypertension. J. Magn. Reson. Imaging 2011, 34, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, H.; Yazici, Z.; Hakyemez, B.; Erdogan, C.; Parlak, M.; Yıldız, H. Evaluation of CSF flow patterns of posterior fossa cystic malformations using CSF flow MR imaging. Neuroradiology 2006, 48, 595–605. [Google Scholar] [CrossRef]
- Zarrinkoob, L.; Ambarki, K.; Wåhlin, A.; Birgander, R.; Eklund, A.; Malm, J. Blood flow distribution in cerebral arteries. J. Cereb. Blood Flow Metab. 2015, 35, 648–654. [Google Scholar] [CrossRef] [Green Version]
- McRobbie, D.W.; Moore, E.A.; Graves, M.J.; Prince, M.R. MRI from Picture to Proton; Cambridge University Press: Cambridge, UK, 2003; ISBN 0-521-52319-2. [Google Scholar]
- Gatehouse, P.; Rolf, M.P.; Graves, M.J.; Hofman, M.B.; Totman, J.; Werner, B.; Quest, R.; Liu, Y.; Von Spiczak, J.; Dieringer, M.; et al. Flow measurement by cardiovascular magnetic resonance: A multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J. Cardiovasc. Magn. Reson. 2010, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Winkelmann, R.; Börnert, P.; Nehrke, K.; Dössel, O. Efficient foldover suppression using SENSE. Magn. Reson. Mater. Physics, Boil. Med. 2004, 18, 63–68. [Google Scholar] [CrossRef]
- He, J.J.; Sandino, C.; Zeng, D.; Vasanawala, S.; Cheng, J. Deep Spatiotemporal Phase Unwrapping of Phase-Contrast MRI Data. In Proceedings of the 27th ISMRM Annual Meeting & Exhibition, Montréal, QC, Canada, 11–16 May 2019. [Google Scholar]
- Haacke, E.M.; Brown, R.W.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design; John Wiley & Sons Inc.: New York, NY, USA, 1999; ISBN 0-471-35128-8. [Google Scholar]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Gorter, T.M.; Van Melle, J.P.; Freling, H.G.; Ebels, T.; Bartelds, B.; Pieper, P.G.; Berger, R.M.F.; Van Veldhuisen, D.J.; Willems, T.P. Pulmonary regurgitant volume is superior to fraction using background-corrected phase contrast MRI in determining the severity of regurgitation in repaired tetralogy of Fallot. Int. J. Cardiovasc. Imaging 2015, 31, 1169–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salfity, M.F.; Huntley, J.; Graves, M.J.; Marklund, O.; Cusack, R.; Beauregard, D. Extending the dynamic range of phase contrast magnetic resonance velocity imaging using advanced higher-dimensional phase unwrapping algorithms. J. R. Soc. Interface 2005, 3, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Holland, B.J.; Printz, B.F.; Lai, W.W. Baseline correction of phase-contrast images in congenital cardiovascular magnetic resonance. J. Cardiovas. Magn. Reson. 2010, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyverfeldt, P.; Sigfridsson, A.; Knutsson, H.; Ebbers, T. A novel MRI framework for the quantification of any moment of arbitrary velocity distributions. Magn. Reson. Med. 2010, 65, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.Z.; Burger, P.; Kilner, P.J.; Karwatowski, S.P.; Firmin, D.N. Dynamic range extension of cine velocity measurements using motion registered spatiotemporal phase unwrapping. J. Magn. Reson. Imaging 1996, 6, 495–502. [Google Scholar] [CrossRef]
- Giese, D.; Haeberlin, M.; Barmet, C.; Pruessmann, K.P.; Schaeffter, T.; Kozerke, S. Analysis and correction of background velocity offsets in phase-contrast flow measurements using magnetic field monitoring. Magn. Reson. Med. 2011, 67, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.K.; Zheng, J.J.; Zhao, L.; Hao, X.Z.; Zhang, X.X.; Tian, J.Q.; Zheng, K.; Yang, Y.M. Reversed aqueductal cerebrospinal fluid net flow in idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2017, 136, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ringstad, G.; Emblem, K.E.; Eide, P.K. Phase-contrast magnetic resonance imaging reveals net retrograde aqueductal flow in idiopathic normal pressure hydrocephalus. J. Neurosurg. 2016, 124, 1850–1857. [Google Scholar] [CrossRef] [Green Version]
- Qvarlander, S.; Ambarki, K.; Wåhlin, A.; Jacobsson, J.; Birgander, R.; Malm, J.; Eklund, A. Cerebrospinal fluid and blood flow patterns in idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2017, 135, 576–584. [Google Scholar] [CrossRef]
- Yoshida, K.; Takahashi, H.; Saijo, M.; Ueguchi, T.; Tanaka, H.; Fujita, N.; Murase, K. Phase-contrast MR studies of CSF flow rate in the cerebral aqueduct and cervical subarachnoid space with correlation-based segmentation. Magn. Reson. Med. Sci. 2009, 8, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Wåhlin, A.; Ambarki, K.; Hauksson, J.; Birgander, R.; Malm, J.; Eklund, A. Phase contrast MRI quantification of pulsatile volumes of brain arteries, veins, and cerebrospinal fluids compartments: Repeatability and physiological interactions. J. Magn. Reson. Imaging 2011, 35, 1055–1062. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Chung, H.-W.; Chen, M.-Y.; Giiang, L.-H.; Chin, S.-C.; Lee, C.-S.; Chen, C.-Y.; Liu, Y.-J. Supratentorial Cerebrospinal Fluid Production Rate in Healthy Adults: Quantification with Two-dimensional Cine Phase-Contrast MR Imaging with High Temporal and Spatial Resolution. Radiology 2004, 233, 603–608. [Google Scholar] [CrossRef]
- Pruitt, A.A.; Jin, N.; Liu, Y.; Simonetti, O.P.; Ahmad, R. A method to correct background phase offset for phase-contrast MRI in the presence of steady flow and spatial wrap-around artifact. Magn. Reson. Med. 2018, 81, 2424–2438. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Ryan, K.L.; Rickards, C.; Glorsky, S.L.; Idris, A.H.; Yannopoulos, D.; Metzger, A.; Lurie, K.G. Optimizing the Respiratory Pump: Harnessing Inspiratory Resistance to Treat Systemic Hypotension. Respir. Care 2011, 56, 846–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagshul, M.E.; Eide, P.K.; Madsen, J. The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 2011, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
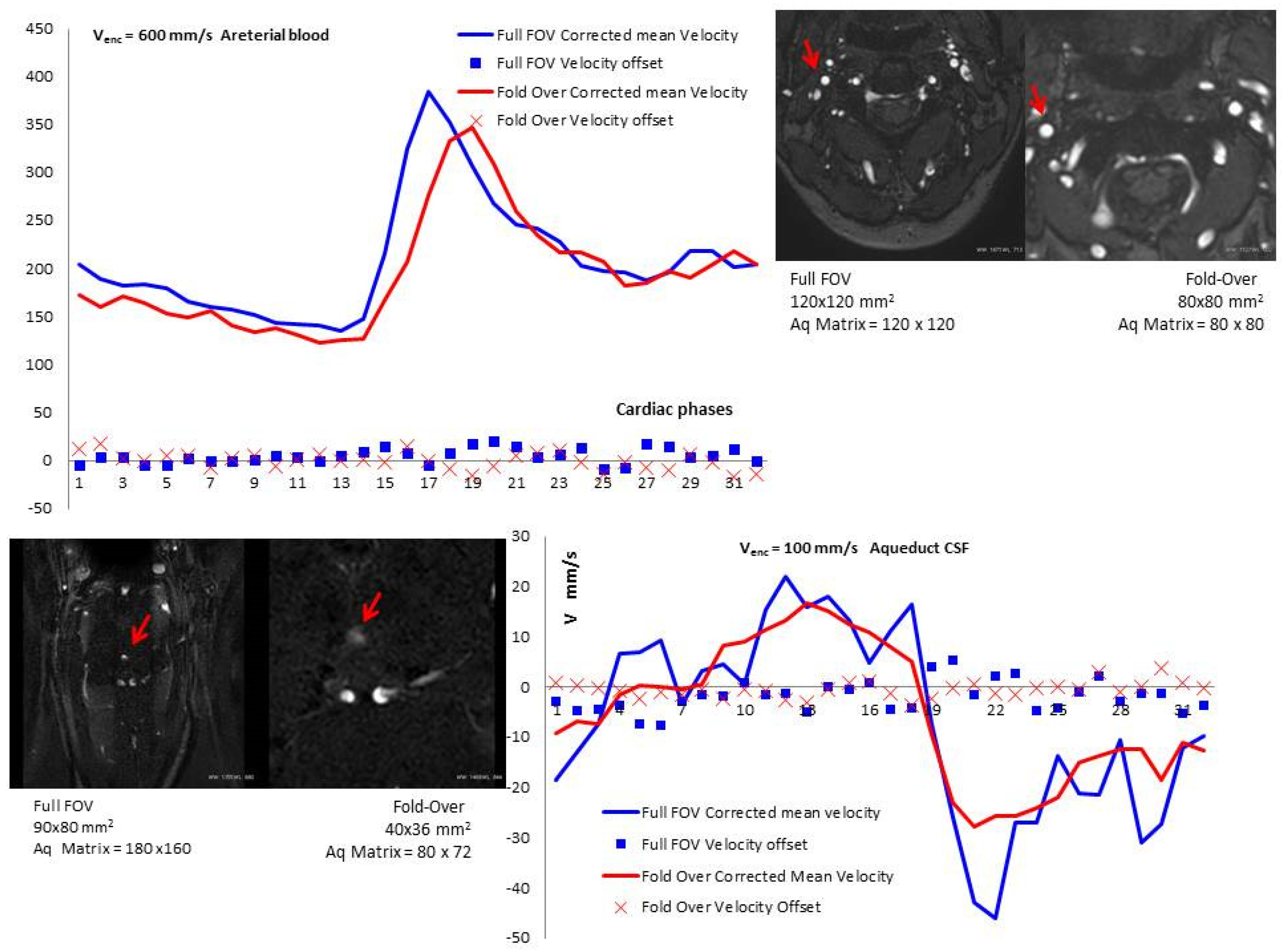


| Venc = 600 mm/s | Venc = 100 mm/s | |||
|---|---|---|---|---|
| Full-FOV | Fold-Over | Full-FOV | Fold-Over | |
| TR/TE [ms] | 10/6.2 | 10/6.2 | 14/8.8 | 15/8.1 |
| FOV [mm2] | 120 × 120 | 90 × 80 | 90 × 80 | 40 × 40 |
| Acquisition Matrix | 120 × 120 | 80 × 80 | 180 × 160 | 80 × 72 |
| Acquisition Pixel [mm2] | 1 × 1 | 1 × 1 | 0.5 × 0.5 | 0.5 × 0.5 |
| Reconstruction Matrix | 240 × 240 | 128 × 128 | 288 × 288 | 128 × 128 |
| Reconstruction Pixel [mm2] | 0.5 × 0.5 | 0.3 × 0.3 | 0.3 × 0.3 | 0.3 × 0.3 |
| Oversampling [mm x mm] | No | 35 × 35 | No | 70 × 70 |
| BW/pixel [Hz] | 191 | 191 | 145 | 144 |
| Acquisition time | 1′ 17” | 1′ 27” | 1′ 43” | 3′ 49” |
| Aqueduct CSF (Venc = 100 mm/s) | ICA Blood (Venc = 600 mm/s) | |||||
|---|---|---|---|---|---|---|
| Native | Corrected | p | Native | Corrected | p | |
| Full-FOV | ||||||
| Mean Velocity (mm.s−1) | −0.86 ± 6.89 | −2.03 ± 5.14 | 0.59 | 214 ± 30 | 223 ± 24 | 0.05 |
| Peak Velocity (mm.s−1) | 57.38 ± 20.15 | 54.42 ± 17.89 | 0.45 | 356 ± 45 | 366 ± 35 | 0.12 |
| Minimum Velocity (mm.s−1) | −33.31 ± 58.24 | −62.88 ± 21.36 | 0.11 | 135 ± 23 | 141 ± 18 | 0.16 |
| Net Flow volume (μL.s−1) | −10.98 ± 16 | −2.48 ± 12.24 | 0.07 | 4200 ± 562 | 4321 ± 537 | 0.07 |
| Stroke Volume (mL) | 6.36 ± 29.65 | 8.24 ± 28.97 | 0.26 | 1631 ± 837 | 1684 ± 847 | 0.05 |
| Fold-Over | ||||||
| Mean Velocity (mm.s−1) | −3.13 ± 4.19 | −0.74 ± 5.49 | 0.009 | 208 ± 28 | 214 ± 22 | 0.31 |
| Peak Velocity (mm.s−1) | 33.35 ± 31.81 | 43.68 ± 15.74 | 0.22 | 346 ± 34 | 355 ± 28 | 0.17 |
| Minimum Velocity (mm.s−1) | −42.78 ± 32.21 | −47.96 ± 19.36 | 0.50 | 131 ± 23 | 134 ± 24 | 0.64 |
| Net Flow volume (μL.s−1) | −10.48 ± 14.73 | 5.85 ± 17.50 | 0.02 | 4103 ± 500 | 4196 ± 560 | 0.31 |
| Stroke Volume (mL) | 38.66 ± 25.62 | 36.94 ± 26.90 | 0.30 | 1526 ± 340 | 1553 ± 340 | 0.45 |
| Aqueduct CSF (Venc = 100 mm/s) | ICA Blood (Venc = 600 mm/s) | |||||
|---|---|---|---|---|---|---|
| Full-FOV | Fold-Over | p | Full-FOV | Fold-Over | p | |
| VNR | 1.54 ± 1.99 | 2.58 ± 1.94 | 0.25 | 1.78 ± 0.82 | 2.49 ± 0.96 | 0.09 |
| Vmeanoffset (mm.s−1) | −2.01 ± 3.17 | −3.33 ± 3.03 | 0.36 | −8.6 ± 12.4 | −5.6 ± 17.1 | 0.66 |
| Vpeakoffset (mm.s−1) | 3.08 ± 2.65 | 0.4 ± 2.4 | <0.01 | 9.5 ± 12.0 | 8.8 ± 14.2 | 0.91 |
| VminOff (mm.s−1) | −7.03 ± 4.95 | −6.75 ± 4.31 | 0.82 | −22.5 ± 13.7 | −22.3 ± 20.3 | 0.84 |
| Vmean (mm.s−1) | −0.9 ± 6.9 | −3.1 ± 4.2 | 0.39 | 214 ± 30 | 209 ± 29 | 0.66 |
| Vpeak (mm.s−1) | 57.4 ± 20.2 | 33.4 ± 31.8 | 0.06 | 357 ± 46 | 346 ± 34 | 0.58 |
| Vmin (mm.s−1) | −33.31 ± 58.24 | −42.78 ± 32.21 | 0.64 | 135 ± 23 | 131 ± 23 | 0.47 |
| Vmeancorr (mm.s−1) | −2.0 ± 5.1 | −0.7 ± 5.5 | 0.58 | 223 ± 24 | 214 ± 22 | 0.41 |
| Vpeakcorr (mm.s−1) | 54.4 ± 17.9 | 43.7 ± 15.7 | 0.17 | 365 ± 35 | 355 ± 28 | 0.47 |
| Vmincorr (mm.s−1) | −62.88 ± 21.36 | −47.96 ± 19.35 | 0.08 | 141 ± 18 | 134 ± 24 | 0.15 |
| QNet (μL.s−1) | −10.98 ± 16.00 | −10.48 ± 14.73 | 0.94 | 4200 ± 562 | 4104 ± 500 | 0.69 |
| QNetcorr (μL.s−1) | −2.48 ± 12.24 | 5.85 ± 17.50 | 0.23 | 4321 ± 538 | 4196 ± 560 | 0.62 |
| Section Area (mm2) | 2.86 ± 0.98 | 3.47 ± 1.30 | <0.01 | 19.78 ± 2.73 | 19.90 ± 2.86 | 0.83 |
| StVol (mL) | 37.79 ± 28.3 | 40.56 ± 30.95 | 0.52 | 1632 ± 837 | 1526 ± 340 | 0.67 |
| SVolcorr (mL) | 41.58 ± 29.64 | 40.15 ± 31.18 | 0.86 | 1685 ± 848 | 1554 ± 340 | 0.59 |
| Fold-Over Versus Full-FOV | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aqueduct CSF | Internal Carotid Artery Blood | |||||||||||
| Bland–Altman | Pearson | Bland–Altman | Pearson | |||||||||
| M | L | U | B | r | p | M | L | U | B | r | p | |
| Vmeanoffset (mm.s−1) | 1.3 | −3.0 | 5.6 | −13.3 | 0.75 | 0.012 | −3.0 | −21.5 | 15.5 | 0.5 | 0.84 | 0.002 |
| Vmean (mm.s−1) | 2.3 | −15.5 | 20.0 | 6.7 | −0.29 | 0.41 | 5.8 | −29.7 | 41.3 | 0.2 | 0.81 | 0.004 |
| Vmeancorr (mm.s−1) | −1.3 | −11.45 | 8.88 | 0.90 | 0.52 | 0.12 | 8.7 | −30.4 | 47.9 | 0.3 | 0.63 | 0.048 |
| Vpeakoffset (mm.s−1) | 2.7 | −0.4 | 5.8 | 1.8 | 0.81 | 0.005 | 0.7 | −20.2 | 21.6 | −0.9 | 0.68 | 0.030 |
| Vpeak (mm.s−1) | 14.7 | −14.2 | 14.6 | 4.3 | 0.52 | 0.12 | 10.1 | −57.6 | 77.8 | 0.3 | 0.66 | 0.039 |
| Vpeakcorr (mm.s−1) | 10.7 | −4.7 | 26.2 | 2.4 | 0.90 | <0.001 | 10.4 | −63.2 | 84.0 | 0.3 | 0.31 | 0.39 |
| VminOff (mm.s−1) | −0.3 | −8.0 | 7.5 | 0.001 | 0.64 | 0.045 | −0.2 | −20.9 | 20.6 | 0.1 | 0.88 | <0.001 |
| Vmin (mm.s−1) | 15.5 | −111 | 142 | 71 | 0.15 | 0.68 | 3.2 | −23.4 | 29.8 | 0.1 | 0.82 | 0.003 |
| VminCorr (mm.s−1) | −14.9 | −61.6 | 31.8 | −3.9 | 0.32 | 0.37 | 7.0 | −20.9 | 34.9 | 0.4 | 0.80 | 0.005 |
| QNet (μL.s−1) | −0.5 | −26.9 | 25.9 | 0.2 | 0.62 | 0.06 | 97 | −428 | 621 | 2.1 | 0.85 | 0.002 |
| QNetcorr (μL.s−1) | −8.3 | −45.7 | 29.0 | 3.7 | 0.21 | 0.55 | 125 | −444 | 693 | 3.7 | 0.85 | 0.002 |
| SVol (mL) | 32.3 | −30.6 | 95.2 | −3.6 | 0.90 | <0.001 | 106 | −1361 | 1572 | −0.6 | 0.45 | 0.19 |
| SVolcorr (mL) | −28.7 | −89.7 | 32.3 | 0.9 | 0.68 | 0.030 | −131 | −1606 | 1343 | 1.0 | 0.47 | 0.17 |
| Area (mm2) | −0.6 | −1.7 | 0.5 | 0.1 | 0.91 | <0.001 | −0.1 | −3.61 | 3.36 | 0.001 | 0.80 | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadani-Makki, F.; Metanbou, S.; Arbeaumont-Trocme, G.; Van Gysel, J.; Makki, M.I. Fold-Over Oversampling Effects in the Measurements of Cerebral Cerebrospinal Fluid and Blood Flows with 2D Cine Phase-Contrast MRI. Diagnostics 2020, 10, 387. https://doi.org/10.3390/diagnostics10060387
Saadani-Makki F, Metanbou S, Arbeaumont-Trocme G, Van Gysel J, Makki MI. Fold-Over Oversampling Effects in the Measurements of Cerebral Cerebrospinal Fluid and Blood Flows with 2D Cine Phase-Contrast MRI. Diagnostics. 2020; 10(6):387. https://doi.org/10.3390/diagnostics10060387
Chicago/Turabian StyleSaadani-Makki, Fadoua, Serge Metanbou, Garance Arbeaumont-Trocme, Julien Van Gysel, and Malek I. Makki. 2020. "Fold-Over Oversampling Effects in the Measurements of Cerebral Cerebrospinal Fluid and Blood Flows with 2D Cine Phase-Contrast MRI" Diagnostics 10, no. 6: 387. https://doi.org/10.3390/diagnostics10060387
APA StyleSaadani-Makki, F., Metanbou, S., Arbeaumont-Trocme, G., Van Gysel, J., & Makki, M. I. (2020). Fold-Over Oversampling Effects in the Measurements of Cerebral Cerebrospinal Fluid and Blood Flows with 2D Cine Phase-Contrast MRI. Diagnostics, 10(6), 387. https://doi.org/10.3390/diagnostics10060387