Relationship between Visual Perception and Microstructural Change of the Superior Longitudinal Fasciculus in Patients with Brain Injury in the Right Hemisphere: A Preliminary Diffusion Tensor Tractography Study
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Visual Perception Tests
2.3. MRI Acquisition
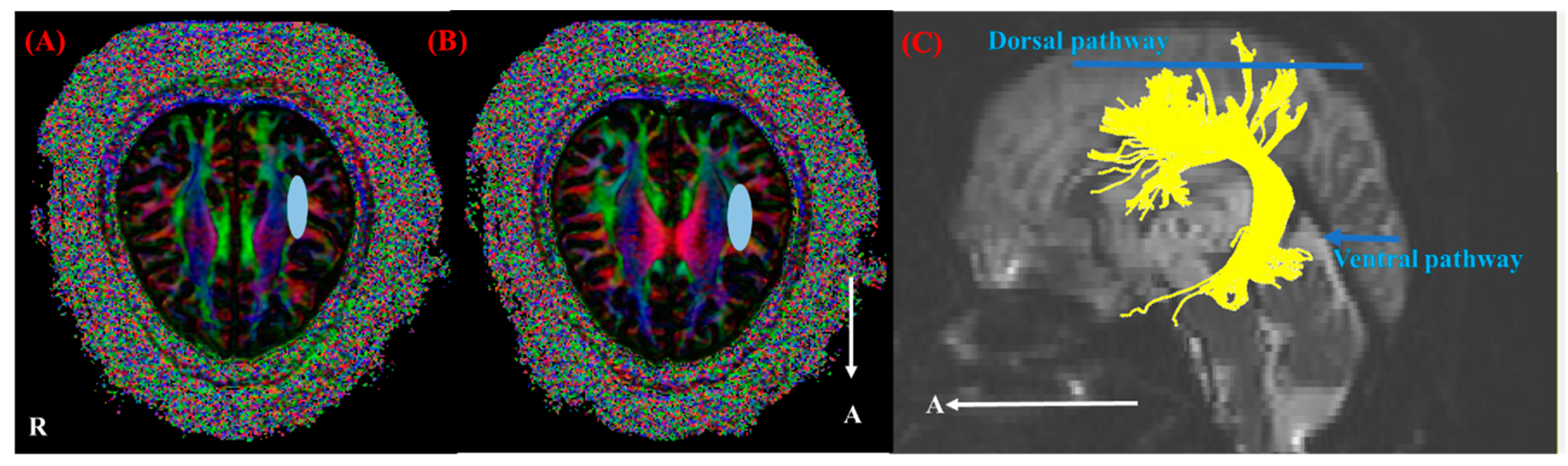
2.4. Fiber Tracking
2.5. Quantitative Analysis
2.6. Statistical Analysis
3. Results
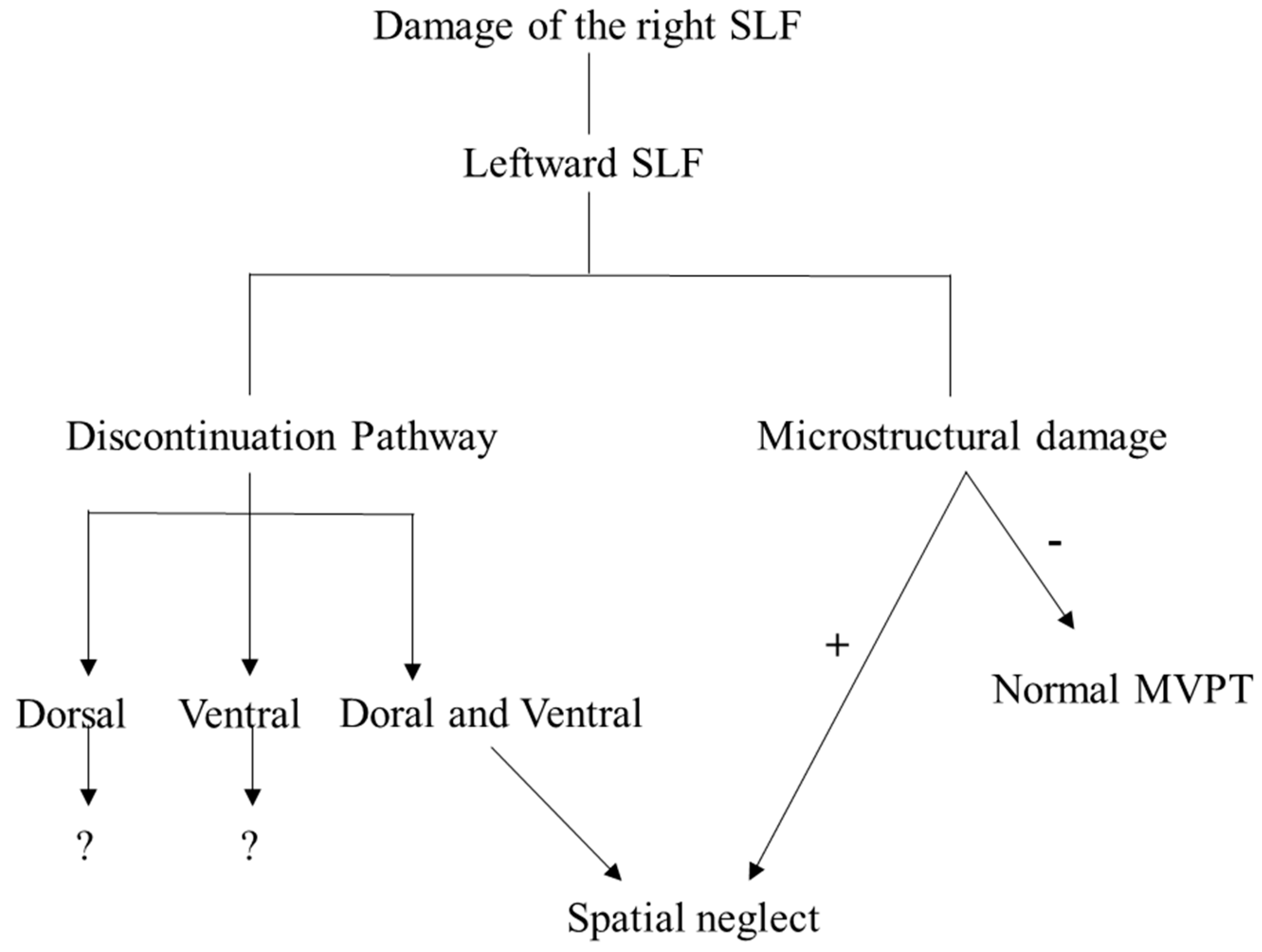
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SLF | Superior longitudinal fasciculus |
| DTI | Diffusion tensor imaging |
| MVPT | Motor-free visual perception test |
| ICH | Intracranial hemorrhage |
| H | Hemorrhage |
| I | Cerebral infarction |
| MCA | Middle cerebral artery |
| MRI | Magnetic resonance image |
| K-MMSE | Korean Mini-Mental Status Examination |
| GRE | T2-weighted gradient recalled echo |
| FA | Fractional anisotropy |
| TV | Tract volume |
| MD | Mean diffusivity |
| LI | Lateralization index |
| SD | Standard deviation |
References
- York, C.D.; Cermak, S.A. Visual Perception and Praxis in Adults After Stroke Christine. Am. J. Occup. Ther. 1995, 49, 543–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corballis, P.M. Visuospatial processing and the right-hemisphere interpreter. Brain Cogn. 2003, 53, 171–176. [Google Scholar] [CrossRef]
- Han, A.R.; Kim, D.Y.; Choi, T.W.; Moon, H.I.; Ryu, B.J.; Yang, S.N.; Pyun, S.B. Characteristics of visual-perceptual function measured by the motor-free visual perception test-3 in Korean adults. Ann. Rehabil. Med. 2014, 38, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Jang, W.H. The allocentric neglect due to injury of the inferior fronto-occipital fasciculus in a stroke patient. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Ffytche, D.H.; Blom, J.D.; Catani, M. Disorders of visual perception. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1280–1287. [Google Scholar] [CrossRef]
- Vallar, G.; Bello, L.; Bricolo, E.; Castellano, A.; Casarotti, A.; Falini, A.; Riva, M.; Fava, E.; Papagno, C. Cerebral correlates of visuospatial neglect: A direct cerebral stimulation study. Hum. Brain Mapp. 2014, 35, 1334–1350. [Google Scholar] [CrossRef]
- Cubelli, R. Definition: Spatial neglect. Cortex 2017, 92, 320–321. [Google Scholar] [CrossRef]
- Bartolomeo, P.; D’Erme, P.; Perri, R.; Gainotti, G. Perception and action in hemispatial neglect. Neuropsychologia 1998, 36, 227–237. [Google Scholar] [CrossRef]
- Colarusso, R.P.; Hammill, D.D. Motor-Free Visual Perception Test; Academic Therapy Publications: Novato, CA, USA, 1972. [Google Scholar]
- Kamali, A.; Flanders, A.E.; Brody, J.; Hunter, J.V.; Hasan, K.M. Tracing Superior Longitudinal Fasciculus Connectivity in the Human Brain using High Resolution Diffusion Tensor Tractography. Brain Struct. Funct. 2014, 219, 269–281. [Google Scholar] [CrossRef]
- Urger, S.E.; De Bellis, M.D.; Hooper, S.R.; Woolley, D.P.; Chen, S.D.; Provenzale, J. The superior longitudinal fasciculus in typically developing children and adolescents: Diffusion tensor imaging and neuropsychological correlates. J. Child. Neurol. 2014, 30, 9–20. [Google Scholar] [CrossRef]
- Shinoura, N.; Suzuki, Y.; Yamada, R.; Tabei, Y.; Saito, K.; Yagi, K. Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia 2009, 47, 2600–2603. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lee, A.Y.; Shin, S.M. Injury of the arcuate fasciculus in the dominant hemisphere in patients with mild traumatic brain injury: A retrospective cross-sectional study. Medicine 2016, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lee, J.; Yeo, S.S. Central post-stroke pain due to injury of the spinothalamic tract in patients with cerebral infarction: A diffusion tensor tractography imaging study. Neural Regen. Res. 2017, 12, 2021–2024. [Google Scholar] [PubMed]
- Park, C.; Ryu, H.; Kim, C.; Joa, K.; Kim, M. Injury of Corticospinal Tract in a Patient with Subarachnoid Hemorrhage as Determined by Di ff usion Tensor Tractography: A Case Report. Brain Sci. 2020, 10, 177. [Google Scholar] [CrossRef]
- Surbeck, W.; Hänggi, J.; Scholtes, F.; Viher, P.V.; Schmidt, A.; Stegmayer, K.; Studerus, E.; Lang, U.E.; Riecher-Rössler, A.; Strik, W.; et al. Anatomical integrity within the inferior fronto-occipital fasciculus and semantic processing deficits in schizophrenia spectrum disorders. Schizophr. Res. 2020, 218, 267–275. [Google Scholar] [CrossRef]
- Shinoura, N.; Suzuki, Y.; Tsukada, M.; Katsuki, S.; Yamada, R.; Tabei, Y.; Saito, K.; Yagi, K. Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase 2007, 13, 127–130. [Google Scholar] [CrossRef]
- Madhavan, K.M.; McQueeny, T.; Howe, S.R.; Shear, P.; Szaflarski, J. Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res. 2014, 1562, 11–22. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P.; Lee, S.J. Diffusion Tensor Tractography Studies of Central Post-stroke Pain Due to the Spinothalamic Tract Injury: A mini-review. Front. Neurol. 2019, 10, 10–15. [Google Scholar] [CrossRef]
- Keser, Z.; Sebastian, R.; Hasan, K.M.; Hillis, A.E. Right Hemispheric Homologous Language Pathways Negatively Predicts Poststroke Naming Recovery. Stroke 2020, 51, 1002–1005. [Google Scholar] [CrossRef]
- Hosomi, A.; Nagakane, Y.; Yamada, K.; Kuriyama, N.; Mizuno, T.; Nishimura, T.; Nakagawa, M. Assessment of arcuate fasciculus with diffusion-tensor tractography may predict the prognosis of aphasia in patients with left middle cerebral artery infarcts. Neuroradiology 2009, 51, 549–555. [Google Scholar] [CrossRef]
- Vernooij, M.W.; Smits, M.; Wielopolski, P.A.; Houston, G.C.; Krestin, G.P.; van der Lugt, A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: A combined fMRI and DTI study. Neuroimage 2007, 35, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.Y.; Youn, J.C.; Park, J.H.; Park, I.S.; Jeong, J.W.; Lee, W.H.; Lee, S.B.; Park, Y.S.; Jhoo, J.H.; Lee, D.Y.; et al. The severe cognitive impairment rating scale—An instrument for the assessment of cognition in moderate to severe dementia patients. Dement. Geriatr. Cogn. Disord. 2008, 25, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Jo, S.A.; Jo, I.; Kim, E.; Park, M.H.; Kang, Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: Demographic influence and population-based norms (the AGE study). Arch. Gerontol. Geriatr. 2008, 47, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.G.; Chun, M.H.; Do, K.H.; Sung, E.J.; Kwon, Y.G.; Kim, D.Y. The effect of transcranial direct current stimulation on neglect syndrome in stroke patients. Ann. Rehabil. Med. 2016, 40, 223–229. [Google Scholar] [CrossRef]
- Oswanski, M.F.; Sharma, O.P.; Raj, S.S.; Vassar, L.A.; Woods, K.L.; Sargent, W.M.; Pitock, R.J. Evaluation of two assessment tools in predicting driving ability of senior drivers. Am. J. Phys. Med. Rehabil. 2007, 86, 190–199. [Google Scholar] [CrossRef]
- Liang, L.; Korogi, Y.; Sugahara, T.; Shigematsu, Y.; Okuda, T.; Ikushima, I.; Takahashi, M. Detection of intracranial hemorrhage with susceptibility-weighted MR sequences. Am. J. Neuroradiol. 1999, 20, 1527–1534. [Google Scholar]
- Lense, M.D.; Dankner, N.; Pryweller, J.R.; Thornton-Wells, T.A.; Thornton-Wells, T.A. Neural Correlates of Amusia in Williams Syndrome. Brain Sci. 2014, 4, 594–612. [Google Scholar] [CrossRef]
- Caverzasi, E.; Hervey-Jumper, S.L.; Jordan, K.M.; Lobach, I.V.; Li, J.; Panara, V.; Racine, C.A.; Sankaranarayanan, V.; Amirbekian, B.; Papinutto, N.; et al. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. J. Neurosurg. 2016, 125, 33–45. [Google Scholar] [CrossRef]
- Hebart, M.N.; Hesselmann, G. What visual information is processed in the human dorsal stream? J. Neurosci. 2012, 32, 8107–8109. [Google Scholar] [CrossRef]
- Wright, A.K.; Theilmann, R.J.; Ridgway, S.H.; Scadeng, M. Diffusion tractography reveals pervasive asymmetry of cerebral white matter tracts in the bottlenose dolphin (Tursiops truncatus). Brain Struct. Funct. 2018, 223, 1697–1711. [Google Scholar] [CrossRef]
- Sreedharan, R.M.; Menon, A.C.; James, J.S.; Kesavadas, C.; Thomas, S.V. Arcuate fasciculus laterality by diffusion tensor imaging correlates with language laterality by functional MRI in preadolescent children. Neuroradiology 2015, 57, 291–297. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P. Differences of the medial lemniscus and spinothalamic tract according to the cortical termination areas: A diffusion tensor tractography study. Somatosens. Mot. Res. 2015, 32, 67–71. [Google Scholar] [CrossRef]
- Santillo, A.F.; Mårtensson, J.; Lindberg, O.; Nilsson, M.; Manzouri, A.; Landqvist Waldö, M.; van Westen, D.; Wahlund, L.O.; Lätt, J.; Nilsson, C. Diffusion Tensor Tractography versus Volumetric Imaging in the Diagnosis of Behavioral Variant Frontotemporal Dementia. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Park, C.-H.; Kim, S.-H.; Jung, H.-Y. Diffusion-Tensor-Tractography-Based Diagnosis for Injury of Corticospinal Tract in a Patient with Hemiplegia Following Traumatic Brain Injury. Diagnostics 2020, 10, 156. [Google Scholar] [CrossRef]
- Clark, K.A.; Nuechterlein, K.H.; Asarnow, R.F.; Hamilton, L.S.; Phillips, O.R.; Hageman, N.S.; Woods, R.P.; Alger, J.R.; Toga, A.W.; Narr, K.L. Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. J. Psychiatr. Res. 2011, 45, 980–988. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, Y.S. Diagnosis of conversion disorder using diffusion tensor tractography and transcranial magnetic stimulation in a patient with mild traumatic brain injury. Diagnostics 2019, 9, 155. [Google Scholar] [CrossRef]
- Jang, S.H.; Yi, J.H.; Kwon, H.G. Injury of the inferior cerebellar peduncle in patients with mild traumatic brain injury: A diffusion tensor tractography study. Brain Inj. 2016, 30, 1271–1275. [Google Scholar] [CrossRef]
- De Schotten, M.T.; Dell’Acqua, F.; Forkel, S.J.; Simmons, A.; Vergani, F.; Murphy, D.G.M.; Catani, M. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011, 14, 1245–1246. [Google Scholar] [CrossRef]
- Martino, J.; Brogna, C.; Robles, S.G.; Vergani, F.; Duffau, H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 2010, 46, 691–699. [Google Scholar] [CrossRef]
- Herbet, G.; Zemmoura, I.; Duffau, H. Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Front. Neuroanat. 2018, 12, 1–15. [Google Scholar] [CrossRef]
- Turken, A.U.; Whitfield-Gabrieli, S.; Bammer, R.; Baldo, J.V.; Dronkers, N.F.; Gabrieli, J.D.E. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 2008, 42, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
| Patient Group (n = 8) | Control Group (n = 16) | |
|---|---|---|
| Age (years) | 52.50 ± 16.72 | 55.68 ± 6.61 |
| Sex (M/F) | 7/1 | 4/12 |
| MMSE (scores) | 24.88 ± 4.29 | |
| MVPT | ||
| Raw (scores) | 20.63 ± 9.35 | |
| Processing time (s) | 10.28 ± 7.88 | |
| Left response | 13.13 ± 8.76 | |
| DTI parameters of the right SLF | ||
| FA | 0.359 ± 0.091 | 0.504 ± 0.033 |
| TV | 2058.750 ± 1638.910 | 5203.88 ± 749.74 |
| MD (×10−3 mm2/s) | 0.854 ± 0.076 | 0.724 ± 0.301 |
| LI-TV | 0.515 ± 0.298 | −0.007 ± 0.144 |
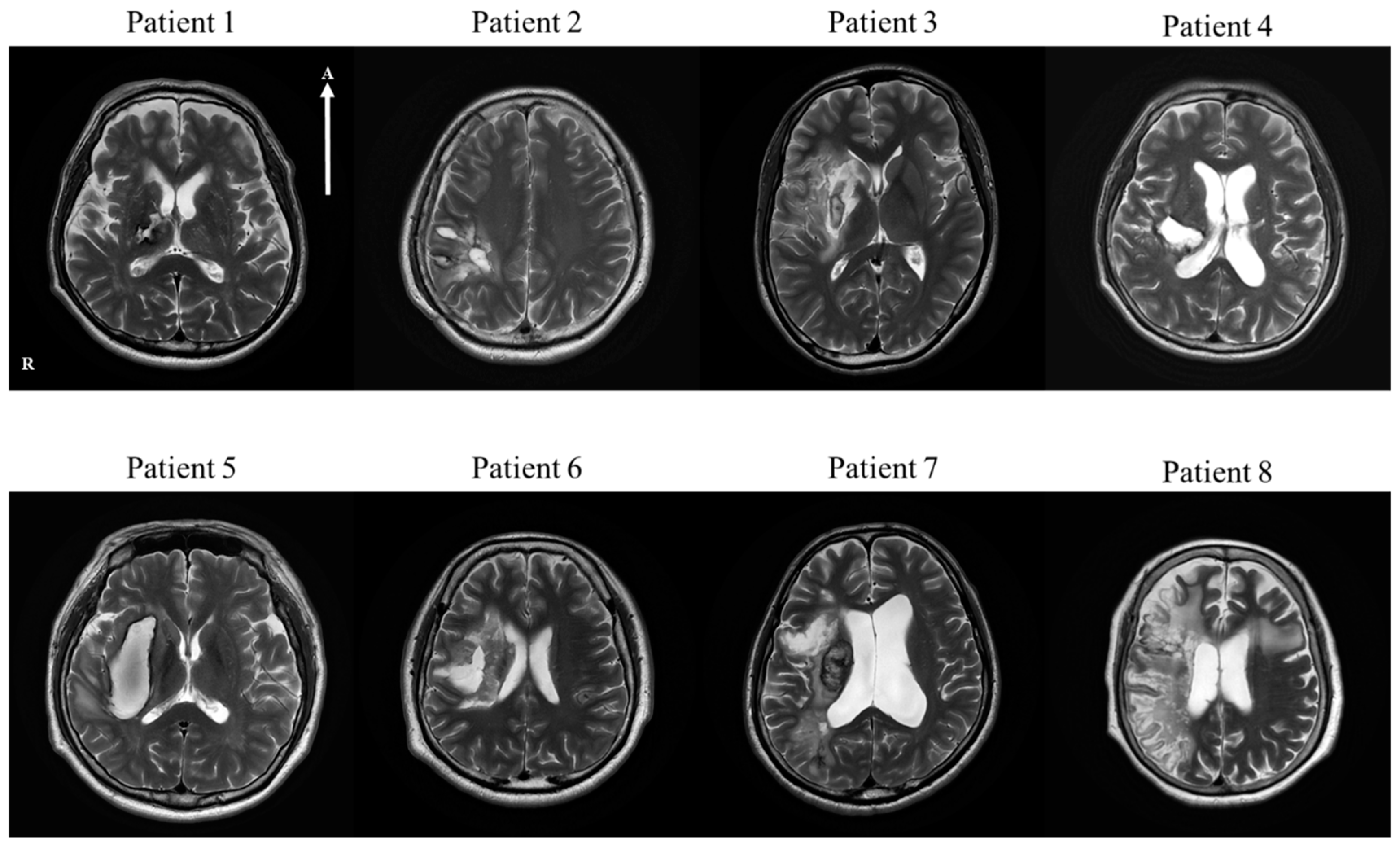
| No | Age | Sex | Lesion | Type | MMSE | Medical History | Operation | Medication for Stroke |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 68 | M | Thalamus and posterior limb of internal capsule, right | H | 23 | HTN | Craniotomy | |
| Patient 2 | 37 | M | Parietal lobe, right | H | 30 | Craniectomy and Cranioplasty | ||
| Patient 3 | 23 | M | Frontal lobe, insular cortex and basal ganglia, right | I | 28 | cilotazol, atovastatin | ||
| Patient 4 | 47 | M | Basal ganglia, frontoparietal lobe, right | H | 17 | HTN | Conservative treatment | |
| Patient 5 | 61 | M | Basal ganglia, right | H | 26 | HTN, MI | Conservative treatment | |
| Patient 6 | 48 | M | Basal ganglia, right | H | 28 | Craniotomy | ||
| Patient 7 | 67 | M | Frontal, parietal, temporal lobe and insula, right | I | 26 | Thrombectomy | aspirin, atorvastatin | |
| Patient 8 | 69 | F | Basal ganglia fronto-temporal lobe, right | I | 21 | AF | Thrombectomy | micronized rivaroxaban, atorvastatin |
| MVPT | ||||
|---|---|---|---|---|
| No | Age | Raw | Processing Time | Lt Response |
| Patient 1 | 68 | 18 * | 14.27 * | 21 |
| Patient 2 | 37 | 28 * | 4.89 * | 16 * |
| Patient 3 | 23 | 35 | 3.16 | 21 |
| Patient 4 | 47 | 25 * | 8.56 * | 16 * |
| Patient 5 | 61 | 26 * | 6.84 * | 20 |
| Patient 6 | 48 | 14 * | 27.00 * | 11 * |
| Patient 7 | 67 | 11 * | 13.23 * | 0 * |
| Patient 8 | 69 | 8 * | 4.27 | 0 * |
| No | Rt SLF | Lt SLF | LI-TV | |||||
|---|---|---|---|---|---|---|---|---|
| FA | TV | MD | FA | TV | MD | |||
| Patient 1 | 0.417 * | 3171.000 * | 0.830 * | 0.447 | 5970.000 | 0.783 | 0.306 * | |
| Patient 2 | 0.337 * | 610.000 * | 0.926 * | 0.492 | 7779.000 | 0.793 | 0.855 * | |
| Patient 3 | 0.505 | 4472.000 | 0.741 | 0.495 | 6334.000 | 0.739 | 0.172 * | |
| Patient 4 | 0.289 * | 970.000 * | 0.827 * | 0.450 | 3831.000 | 0.765 | 0.596 * | |
| Patient 5 | 0.446 | 3533.000 * | 0.828 * | 0.493 | 4642.000 | 0.745 | 0.136 * | |
| Patient 6 | 0.326 * | 652.000 * | 0.837 * | 0.484 | 4443.000 | 0.772 | 0.744 * | |
| Patient 7 | 0.283 * | 2127.000 * | 0.982 * | 0.479 | 6268.000 | 0.790 | 0.493 * | |
| Patient 8 | 0.255 * | 389.000 * | 0.831 * | 0.483 | 4438.000 | 0.769 | 0.839 * | |
| Avg | 0.359 | 2058.750 | 0.854 | 0.478 | 5463.125 | 0.761 | 0.515 | |
| SD | 0.091 | 1638.910 | 0.076 | 0.018 | 1278.060 | 0.155 | 0.298 | |
| Control | Avg | 0.504 | 5203.875 | 0.724 | 0.474 | 4996.625 | 0.749 | −0.007 |
| SD | 0.033 | 749.737 | 0.301 | 0.017 | 648.539 | 0.013 | 0.144 | |
| p-value | 0.000 a | 0.000 a | 0.000 a | 0.697 | 0.490 | 0.106 | 0.000 a |
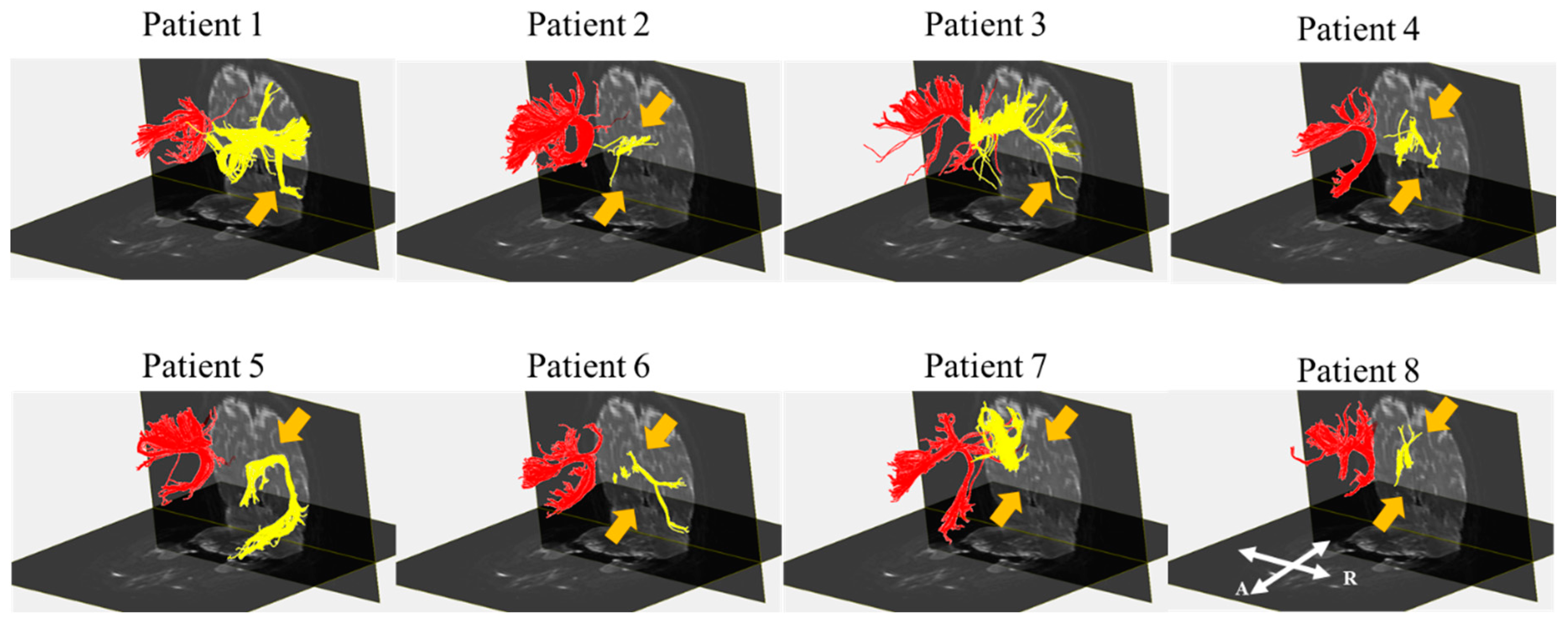
| MVPT | Discontinuation Pathway | DTI Parameter of Rt SLF | DTI Parameter of Lt SLF | LI | |||
|---|---|---|---|---|---|---|---|
| Raw | Processing time | Lt response | |||||
| Patient 1 | Abnormal | Abnormal | Normal | Vental | Abnormal | Normal | Abnormal |
| Patient 2 | Abnormal | Abnormal | Abnormal | Ventral, Dorsal | Abnormal | Normal | Abnormal |
| Patient 3 | Normal | Normal | Normal | Vental | Normal | Normal | Abnormal |
| Patient 4 | Abnormal | Abnormal | Abnormal | Ventral, Dorsal | Abnormal | Normal | Abnormal |
| Patient 5 | Abnormal | Abnormal | Normal | Dorsal | Abnormal | Normal | Abnormal |
| Patient 6 | Abnormal | Abnormal | Abnormal | Ventral, Dorsal | Abnormal | Normal | Abnormal |
| Patient 7 | Abnormal | Abnormal | Abnormal | Ventral, Dorsal | Abnormal | Normal | Abnormal |
| Patient 8 | Abnormal | Normal | Abnormal | Ventral, Dorsal | Abnormal | Normal | Abnormal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Jeon, H.-E.; Park, C.-H. Relationship between Visual Perception and Microstructural Change of the Superior Longitudinal Fasciculus in Patients with Brain Injury in the Right Hemisphere: A Preliminary Diffusion Tensor Tractography Study. Diagnostics 2020, 10, 641. https://doi.org/10.3390/diagnostics10090641
Kim S-H, Jeon H-E, Park C-H. Relationship between Visual Perception and Microstructural Change of the Superior Longitudinal Fasciculus in Patients with Brain Injury in the Right Hemisphere: A Preliminary Diffusion Tensor Tractography Study. Diagnostics. 2020; 10(9):641. https://doi.org/10.3390/diagnostics10090641
Chicago/Turabian StyleKim, Su-Hong, Hyeong-Eun Jeon, and Chan-Hyuk Park. 2020. "Relationship between Visual Perception and Microstructural Change of the Superior Longitudinal Fasciculus in Patients with Brain Injury in the Right Hemisphere: A Preliminary Diffusion Tensor Tractography Study" Diagnostics 10, no. 9: 641. https://doi.org/10.3390/diagnostics10090641
APA StyleKim, S.-H., Jeon, H.-E., & Park, C.-H. (2020). Relationship between Visual Perception and Microstructural Change of the Superior Longitudinal Fasciculus in Patients with Brain Injury in the Right Hemisphere: A Preliminary Diffusion Tensor Tractography Study. Diagnostics, 10(9), 641. https://doi.org/10.3390/diagnostics10090641





