Design, Validity, and Reliability of a New Test, Based on an Inertial Measurement Unit System, for Measuring Cervical Posture and Motor Control in Children with Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Individuals
2.2. Cervical Motor Control Test Development and Application
2.3. Funcional Assessment
2.4. Statistical Analysis
2.4.1. Validity Analysis
2.4.2. Reliability Analysis
3. Results
3.1. Construct Validity
3.2. Concurrent Validity
3.3. Test–Retest Reliability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bax, M.; Goldstein, M.; Rosenbaum, P.; Leviton, A.; Paneth, N.S.; Dan, B.; Jacobsson, B.; Damiano, D. Proposed definition and classification of cerebral palsy, April 2005. Dev. Med. Child Neurol. 2005, 47, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr. Dis. Treat. 2020, 16, 1505–1518. [Google Scholar] [CrossRef]
- Odding, E.; Roebroeck, M.E.; Stam, H.J. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disabil. Rehabilit. 2006, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, J.; Mohangoo, A.; Delnord, M. European Perinatal Health Report: Health and Care of Pregnant Women and Babies in Europe in 2010; Euro-Peristat: Paris, France, 2013. [Google Scholar]
- Winter, S.; Autry, A.; Boyle, C.; Yeargin-Allsopp, M. Trends in the Prevalence of Cerebral Palsy in a Population-Based Study. Pediatrics 2002, 110, 1220–1225. [Google Scholar] [CrossRef]
- Blair, E.M. Epidemiology of the Cerebral Palsies. Orthop. Clin. N. Am. 2010, 41, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Verma, I. Cerebral palsy in children: An overview. J. Clin. Orthop. Trauma 2012, 3, 77–81. [Google Scholar] [CrossRef]
- Pavão, S.L.; Dos Santos, A.N.; Woollacott, M.H.; Rocha, N.A.C.F. Assessment of postural control in children with cerebral palsy: A review. Res. Dev. Disabil. 2013, 34, 1367–1375. [Google Scholar] [CrossRef][Green Version]
- Papageorgiou, E.; Simon-Martinez, C.; Molenaers, G.; Ortibus, E.; Van Campenhout, A.; Desloovere, K. Are spasticity, weakness, selectivity, and passive range of motion related to gait deviations in children with spastic cerebral palsy? A statistical parametric mapping study. PLoS ONE 2019, 14, e0223363. [Google Scholar] [CrossRef]
- Redstone, F.; West, J.F. The importance of postural control for feeding. Pediatr. Nurs. 2004, 30, 97–100. [Google Scholar]
- Velasco, M.A.; Raya, R.; Muzzioli, L.; Morelli, D.; Otero, A.; Iosa, M.; Cincotti, F.; Rocon, E. Evaluation of cervical posture improvement of children with cerebral palsy after physical therapy based on head movements and serious games. Biomed. Eng. Online 2017, 16, 74. [Google Scholar] [CrossRef]
- A Gresty, M.; Halmagyi, G.M. Abnormal head movements. J. Neurol. Neurosurg. Psychiatry 1979, 42, 705–714. [Google Scholar] [CrossRef]
- Holt, K.G.; Ratcliffe, R.; Jeng, S.F. Head Stability in Walking in Children With Cerebral Palsy and in Children and Adults Without Neurological Impairment. Phys. Ther. 1999, 79, 1153–1162. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- McDowell, B. The Gross Motor Function Classification System--expanded and revised. Dev. Med. Child Neurol. 2008, 50, 725. [Google Scholar] [CrossRef] [PubMed]
- Raya, R.; Rocon, E.; Ceres, R.; Harlaar, J.; Geytenbeek, J.J.M. Characterizing Head Motor Disorders to Create Novel Interfaces for People with Cerebral Palsy: Creating an Alternative Communication Channel by Head Motion. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June—1 July 2011; Volume 2011, p. 5975409. [Google Scholar]
- Ronen, G.M.; Fayed, N.; Rosenbaum, P.L. Outcomes in pediatric neurology: A review of conceptual issues and recommendations the 2010 Ronnie Mac Keith Lecture. Dev. Med. Child Neurol. 2011, 53, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.A.; Raya, R.; Ceres, R.; Clemotte, A.; Bedia, A.R.; Franco, T.G.; Rocon, E. Positive and Negative Motor Signs of Head Motion in Cerebral Palsy: Assessment of Impairment and Task Performance. IEEE Syst. J. 2014, 10, 1–7. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.; Galán-Mercant, A.; Williams, J.M. The use of inertial sensors system for human motion analysis. Phys. Ther. Rev. 2010, 15, 462–473. [Google Scholar] [CrossRef]
- Kim, M.; Kim, B.H.; Jo, S. Quantitative Evaluation of a Low-Cost Noninvasive Hybrid Interface Based on EEG and Eye Movement. IEEE Trans. Neural Syst. Rehabilit. Eng. 2014, 23, 159–168. [Google Scholar] [CrossRef]
- Carcreff, L.; Gerber, C.N.; Paraschiv-Ionescu, A.; De Coulon, G.; Newman, C.J.; Armand, S.; Aminian, K. What is the Best Configuration of Wearable Sensors to Measure Spatiotemporal Gait Parameters in Children with Cerebral Palsy? Sensors 2018, 18, 394. [Google Scholar] [CrossRef]
- Haberfehlner, H.; Goudriaan, M.; Bonouvrié, L.A.; Jansma, E.P.; Harlaar, J.; Vermeulen, R.J.; Van Der Krogt, M.M.; Buizer, A.I. Instrumented assessment of motor function in dyskinetic cerebral palsy: A systematic review. J. Neuroeng. Rehabilit. 2020, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Valera, I.C.; Cuesta-Vargas, A.; Garrido-Castro, J.L.; Gardiner, P.; López-Medina, C.; Machado, P.; Condell, J.; Connolly, J.; Williams, J.M.; Muñoz-Esquivel, K.; et al. Measuring Spinal Mobility Using an Inertial Measurement Unit System: A Validation Study in Axial Spondyloarthritis. Diagnostics 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Paulis, W.; Horemans, H.L.; Brouwer, B.S.; Stam, H.J. Excellent test–retest and inter-rater reliability for Tardieu Scale measurements with inertial sensors in elbow flexors of stroke patients. Gait Posture 2011, 33, 185–189. [Google Scholar] [CrossRef]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.A.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabilit. 2012, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Delrobaei, M.; Memar, S.; Pieterman, M.; Stratton, T.W.; McIsaac, K.; Jog, M. Towards remote monitoring of Parkinson’s disease tremor using wearable motion capture systems. J. Neurol. Sci. 2018, 384, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.J.; Jacobs, J.V.; Lomond, K.V.; Henry, S.M. Detection of postural sway abnormalities by wireless inertial sensors in minimally disabled patients with multiple sclerosis: A case-control study. J. Neuroeng. Rehabilit. 2015, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Spain, R.I.; Mancini, M.; Horak, F.B.; Bourdette, D. Body-worn sensors capture variability, but not decline, of gait and balance measures in multiple sclerosis over 18 months. Gait Posture 2014, 39, 958–964. [Google Scholar] [CrossRef]
- Noort, J.V.D.; Harlaar, J.; Scholtes, V. O068 Inertial sensing improves clinical spasticity assessment. Gait Posture 2008, 28, S47. [Google Scholar] [CrossRef]
- Noort, J.C.V.D.; Ferrari, A.; Cutti, A.G.; Becher, J.G.; Harlaar, J. Gait analysis in children with cerebral palsy via inertial and magnetic sensors. Med. Boil. Eng. 2012, 51, 377–386. [Google Scholar] [CrossRef]
- Carmona-Pérez, C.; Garrido-Castro, J.L.; Vidal, F.T.; Alcaraz-Clariana, S.; García-Luque, L.; Alburquerque-Sendín, F.; Rodrigues-De-Souza, D.P. Concurrent Validity and Reliability of an Inertial Measurement Unit for the Assessment of Craniocervical Range of Motion in Subjects with Cerebral Palsy. Diagnostics 2020, 10, 80. [Google Scholar] [CrossRef]
- Hislop, H.; Avers, D.; Brown, M. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination and Performance Testing, 9th ed.; Elsevier: St. Louis, MI, USA, 2013. [Google Scholar]
- Manikowska, F.; Chen, B.P.-J.; Józwiak, M.; Lebiedowska, M.K. Validation of Manual Muscle Testing (MMT) in children and adolescents with cerebral palsy. Neurorehabilitation 2018, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Cole, T.J. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl. Health Stat. Rep. 2013, 11, 1–3. [Google Scholar]
- Vitrikas, K.; Dalton, H.; Breish, D. Cerebral Palsy: An Overview. Am. Fam. Physician 2020, 101, 213–220. [Google Scholar] [PubMed]
- National Institute of Neurological Disorders and Stroke. Cerebral Palsy: Hope Through Research; Office of Communications and Public Liaison, National Institute of Neurological Disorders Stroke, National Institute of Health, Eds.; National Institute of Neurological Disorders and Stroke: Bethesda, MD, USA, 2013.
- Numanoğlu, A. Intraobserver reliability of modified Ashworth scale and modified Tardieu scale in the assessment of spasticity in children with cerebral palsy. Acta Orthop. Traumatol. Turc. 2012, 46, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.P. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. In Classic Papers in Orthopaedics; Springer Science and Business Media LLC: Berlin, Germany, 2013; Volume 67, pp. 415–417. [Google Scholar]
- Haik, M.N.; Alburquerque-Sendín, F.; Camargo, P.R. Reliability and Minimal Detectable Change of 3-Dimensional Scapular Orientation in Individuals With and Without Shoulder Impingement. J. Orthop. Sports Phys. Ther. 2014, 44, 341–349. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Askari, S.; Kirby, R.L.; Parker, K.; Thompson, K.; O’Neill, J. Wheelchair Propulsion Test: Development and Measurement Properties of a New Test for Manual Wheelchair Users. Arch. Phys. Med. Rehabilit. 2013, 94, 1690–1698. [Google Scholar] [CrossRef]
- Bartlett, D.J.; Palisano, R.J. Physical Therapists’ Perceptions of Factors Influencing the Acquisition of Motor Abilities of Children With Cerebral Palsy: Implications for Clinical Reasoning. Phys. Ther. 2002, 82, 237–248. [Google Scholar] [CrossRef]
- Bartlett, D.J.; Palisano, R.J. A Multivariate Model of Determinants of Motor Change for Children With Cerebral Palsy. Phys. Ther. 2000, 80, 598–614. [Google Scholar] [CrossRef]
- Li, X.; Gonzalez-Navas, C.; Garrido-Castro, J.L. Fiabilidad y validez de la medida de la movilidad cervical en pacientes con espondiloartritis axial utilizando un sensor inercial. Rehabilitación 2017, 51, 17–21. [Google Scholar] [CrossRef]
- Valera, I.A.; Perdigón, F.M.; Sánchez, I.M.; Navas, C.G.; Collantes-Estévez, E.; Garrido-Castro, J.L. Utilización de sensores inerciales para la evaluación de la movilidad espinal en pacientes con espondiloartritis axial. Rehabilitación 2018, 52, 100–106. [Google Scholar] [CrossRef]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved Scaling of the Gross Motor Function Measure for Children With Cerebral Palsy: Evidence of Reliability and Validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.; Rosenbaum, P.; Avery, L.M.; Lane, M. Gross Motor Function Measure (GMFM-66 and GMFM-88) User’s Manual; MacKeith Press: London, UK, 2002. [Google Scholar]
- Palisano, R.J.; E Hanna, S.; Rosenbaum, P.L.; Russell, D.J.; Walter, S.D.; Wood, E.P.; Raina, P.S.; E Galuppi, B. Validation of a Model of Gross Motor Function for Children With Cerebral Palsy. Phys. Ther. 2000, 80, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Vos-Vromans, D.C.W.M.; Ketelaar, M.; Gorter, J.W. Responsiveness of evaluative measures for children with cerebral palsy: The Gross Motor Function Measure and the Pediatric Evaluation of Disability Inventory. Disabil. Rehabilit. 2005, 27, 1245–1252. [Google Scholar] [CrossRef]
- Ferre-Fernández, M. Adaptación transcultural y propiedades psicométricas de la versión española del “Gross Motor Function Measure-88” (GMFM-88-SP). Ph.D. Thesis, Universidad Católica de Murcia, Murcia, Spain, 2019. [Google Scholar]
- Harvey, A. The Gross Motor Function Measure (GMFM). J. Physiother. 2017, 63, 187. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Sheng, M.; Bowen, R.E.; Scaduto, A.A.; Kay, R.M.; Otsuka, N.Y.; Hara, R.; Chan, L.S. Concurrent and Discriminant Validity of Spanish Language Instruments for Measuring Functional Health Status. J. Pediatr. Orthop. 2008, 28, 199–212. [Google Scholar] [CrossRef]
- Bascones, M.G.; Riaño, M.O.A.; Badillo, A.Á. Adaptación transcultural y versión española de la Escala de Discapacidad Pediatric Evaluation of Disability Inventory (PEDI). Ph.D. Thesis, Universdad Complutense, Madrid, Spain, 2013. [Google Scholar]
- Dancey, C.; Reidy, J. Statistics without Maths for Psychology, 7th ed.; Pearson Education: London, UK, 2017. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Donoghue, D.; Stokes, E. Physiotherapy Research and Older People (PROP) group How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J. Rehabilitation Med. 2009, 41, 343–346. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.Y. How to Assess the Reliability of Measurements in Rehabilitation. Am. J. Phys. Med. Rehabilit. 2005, 84, 719–723. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM&R 2018, 10, S220–S232. [Google Scholar] [CrossRef]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. Neuroeng. Rehabilit. 2014, 11, 158. [Google Scholar] [CrossRef]
- Costa, V.; Ramírez, Ó.; Otero, A.; Muñoz-García, D.; Uribarri, S.; Raya, R. Validity and reliability of inertial sensors for elbow and wrist range of motion assessment. PeerJ 2020, 8, e9687. [Google Scholar] [CrossRef]
- Ferre-Fernández, M.; Murcia-González, M.A.; Espinosa, M.D.B.; Ríos-Díaz, J. Measures of Motor and Functional Skills for Children With Cerebral Palsy. Pediatr. Phys. Ther. 2020, 32, 12–25. [Google Scholar] [CrossRef]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.-S. Validity and Reliability of Wearable Sensors for Joint Angle Estimation: A Systematic Review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef]
- Cuccia, M.A.; CaraDonna, C. The relationship between the stomatognathic system and body posture. Clinics 2009, 64, 61–66. [Google Scholar] [CrossRef]
- Stack, B.; Sims, A. The Relationship Between Posture and Equilibrium and the Auriculotemporal Nerve In Patients with Disturbed Gait and Balance. CRANIO® 2009, 27, 248–260. [Google Scholar] [CrossRef]
- Wolff, A.; Sama, A.; Lenhoff, M.; Daluiski, A. The use of wearable inertial sensors effectively quantify arm asymmetry during gait in children with unilateral spastic cerebral palsy. J. Hand Ther. 2020. [Google Scholar] [CrossRef]
- Carcreff, L.; Paraschiv-Ionescu, A.; Gerber, C.N.; Newman, C.J.; Armand, S.; Aminian, K. A Personalized Approach to Improve Walking Detection in Real-Life Settings: Application to Children with Cerebral Palsy. Sensors 2019, 19, 5316. [Google Scholar] [CrossRef]
- Choi, S.; Shin, Y.B.; Kim, S.-Y.; Kim, J. A novel sensor-based assessment of lower limb spasticity in children with cerebral palsy. J. Neuroeng. Rehabilit. 2018, 15, 45. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Tang, L.; Cao, S.; Zhang, X.; Chen, X. Quantitative assessment of lower limbs gross motor function in children with cerebral palsy based on surface EMG and inertial sensors. Med. Boil. Eng. 2019, 58, 101–116. [Google Scholar] [CrossRef]
- Walmsley, C.P.; Williams, S.A.; Grisbrook, T.L.; Elliott, C.; Imms, C.; Campbell, A. Measurement of Upper Limb Range of Motion Using Wearable Sensors: A Systematic Review. Sports Med. Open 2018, 4, 53. [Google Scholar] [CrossRef]
- Gerber, C.N.; Carcreff, L.; Paraschiv-Ionescu, A.; Armand, S.; Newman, C.J. Reliability of single-day walking performance and physical activity measures using inertial sensors in children with cerebral palsy. Ann. Phys. Rehabilit. Med. 2019. [Google Scholar] [CrossRef]
- Aroojis, A.; Sarathy, K.; Doshi, C. Clinical examination of children with cerebral palsy. Indian J. Orthop. 2019, 53, 35–44. [Google Scholar] [CrossRef]
- Chang, K.-V.; Wu, W.-T.; Chen, M.-C.; Chiu, Y.-C.; Han, D.; Chen, C.-C. Smartphone Application with Virtual Reality Goggles for the Reliable and Valid Measurement of Active Craniocervical Range of Motion. Diagnostics 2019, 9, 71. [Google Scholar] [CrossRef]
- Wichers, M.; Hilberink, S.; Roebroeck, M.; Van Nieuwenhuizen, O.; Stam, H. Motor impairments and activity limitations in children with spastic cerebral palsy: A Dutch population-based study. J. Rehabilit. Med. 2009, 41, 367–374. [Google Scholar] [CrossRef]

| CP Group (n = 24) | Control Group (n = 24) | p-Value | |
|---|---|---|---|
| Age (years) | 9.1 (3.0) | 8.8 (3.2) | 0.720 |
| Sex (women/men) | 15/9 | 15/9 | |
| Weight (kg) | 28.5 (12.9) | 33.2 (14.0) | 0.250 |
| Height (m) | 1.33 (0.22) | 1.35 (0.23) | 0.736 |
| BMI (Z-score) | −0.15 (1.19) | 0.08 (1.30) | 0.171 |
| GMFCS level (frequency) | I: 11; II: 4; III: 1; IV: 8 | - | - |
| Type of motor disorder (frequency) | Spastic: 20; Dyskinetic: 3; Ataxic: 0; Mixed: 1 | - | - |
| GMFM-88 | - | - | |
| Dimension A | 81.7 (24.3) | - | - |
| Dimension B | 73.5 (32.9) | ||
| Dimension C | 62.8 (39.4) | ||
| Dimension D | 54.2 (39.4) | ||
| Dimension E | 46.1 (38.3) | - | - |
| Total score | 63.6 (33.7) | - | - |
| PEDI | - | - | |
| Self-Care | 24.1 (13.52) | - | - |
| Mobility | 20.1 (12.8) | - | - |
| Social Function | 18.8 (7.3) | - | - |
| Total Score | 63.0 (32.1) | - | - |
| CP Group (n = 24) | Control Group (n = 24) | Mean Difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Movement characteristics | ||||
| Flexion-extension angle (°) | 8.59 (8.69) | 3.93 (2.88) | −4.66 (−8.56; −0.75) | 0.021 |
| Rotational angle (°) | 10.29 (11.30) | 2.57 (2.53) | −7.72 (−12.70; −2.75) | 0.004 |
| Lateral angle (°) | 7.16 (6.01) | 2.59 (2.36) | −4.57 (−7.32; −1.83) | 0.002 |
| Mean angle (°) | 8.68 (7.78) | 3.03 (2.20) | −5.65 (−9.11; −2.19) | 0.002 |
| Flexion-extension velocity (°/s) | 8.77 (9.92) | 4.85 (3.97) | −3.92 (−8.45; 0.61) | 0.087 |
| Rotational velocity (°/s) | 10.35 (14.64) | 4.24 (5.75) | −6.11 (−12.79; 0.57) | 0.071 |
| Lateral velocity (°/s) | 8.22 (9.25) | 4.72 (4.22) | −3.49 (−7.79; 0.81) | 0.107 |
| Mean velocity (°/s) | 9.11 (11.13) | 4.61 (4.56) | −4.51 (−9.61; 0.59) | 0.081 |
| Flexion-extension acceleration (°/s2) | 128.75 (157.13) | 107.92 (75.53) | −20.82 (−94.40; 52.75) | 0.568 |
| Rotational acceleration (°/s2) | 128.54 (175.01) | 91.48 (62.83) | −37.06 (−116.23; 42.11) | 0.345 |
| Lateral acceleration (°/s2) | 142.52 (159.44) | 121.98 (92.79) | −20.53 (−97.85; 56.78) | 0.593 |
| Mean acceleration (°/s2) | 133.27 (162.05) | 107.13 (75.02) | −26.14 (−101.60; 49.32) | 0.485 |
| Ellipse variables | ||||
| Distance (°/s) | 11.61 (13.75) | 6.43 (4.86) | −5.18 (−11.39; 1.04) | 0.099 |
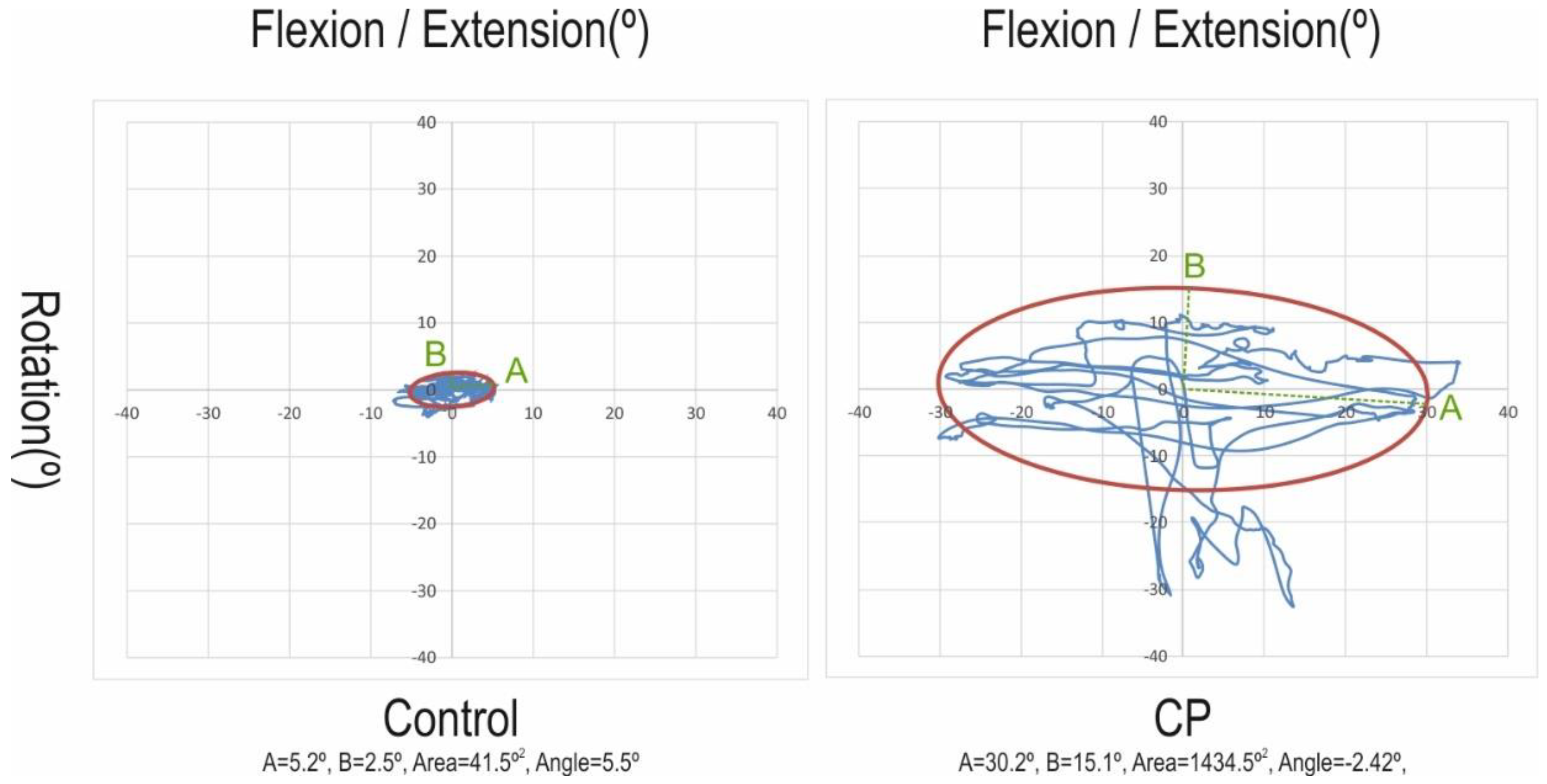
| Area (°2) | 857.18 (1374.52) | 91.33 (206.35) | −765.85 (−1364.98; −166.72) | 0.015 |
| Angle (°) | 7.01 (27.01) | 0.64 (21.80) | −6.38 (−20.59; 7.83) | 0.371 |
| A-dimension (°) | 12.34 (12.24) | 4.59 (3.32) | −7.75 (−13.18; −2.31) | 0.007 |
| B-dimension (°) | 13.25 (13.96) | 3.65 (5.11) | −9.60 (−15.92; −3.27) | 0.004 |
| Dimension A | Dimension B | Dimension C | Dimension D | Dimension E | GMFM-88 Total Score | Self-Care | Mobility | Social Function | PEDI Total Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Weight | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Height | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| BMI | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.414; 0.048 | n.s. | 0.596; 0.003 | 0.442; 0.035 |
| Flexion-extension angle | −0.738; <0.001 | −0.677; <0.001 | −0.615; 0.002 | −0.589; 0.003 | −0.626; 0.001 | −0.663; 0.001 | −0.723; <0.001 | −0.702; <0.001 | −0.590; 0.003 | −0.715; <0.001 |
| Rotational angle | −0.446; 0.033 | n.s. | n.s. | n.s. | −0.432; 0.040 | n.s. | −0.575; 0.004 | −0.472; 0.023 | −0.583; 0.004 | −0.561; 0.005 |
| Lateral angle | n.s. | n.s. | n.s. | n.s. | −0.465.026 | n.s. | −0.476; 0.022 | −0.419; 0.046 | −0.463; 0.026 | −0.472; 0.026 |
| Mean angle | −0.569; 0.005 | −0.523; 0.010 | −0.478; 0.021 | −0.451; 0.031 | −0.562; 0.005 | −0.530; 0.009 | −0.670; <0.001 | −0.597; 0.003 | −0.622; 0.002 | −0.660; 0.001 |
| Flexion-extension velocity | −0.516; 0.012 | −0.447; 0.032 | −0.415; 0.049 | −0.348 | −0.434; 0.034 | −0.439; 0.036 | −0.574; 0.004 | −0.479; 0.021 | −0.516; 0.012 | −0.549; 0.007 |
| Rotational velocity | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.500 | n.s. | −0.490 | n.s. |
| Lateral velocity | −0.557; 0.006 | −0.508; 0.013 | −0.467; 0.025 | −0.415; 0.049 | −0.480; 0.020 | −0.495; 0.016 | −0.621; 0.002 | −0.535; 0.009 | −0.573; 0.004 | −0.604; 0.002 |
| Mean velocity | −0.472; 0.023 | −0.414; 0.048 | n.s. | n.s. | −0.428; 0.041 | −0.416; 0.048 | −0.562; 0.005 | −0.462; 0.026 | −0.527; 0.010 | −0.539; 0.008 |
| Flexion-extension acceleration | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.427; 0.042 | n.s. | n.s. | n.s. |
| Rotational acceleration | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.442; 0.035 | n.s. | n.s. | −0.417; 0.046 |
| Lateral acceleration | −0.521; 0.011 | −0.472; 0.023 | −0.426; 0.042 | n.s. | n.s. | −0.443; 0.034 | −0.573; 0.004 | −0.477; 0.021 | −0.542; 0.008 | −0.553; 0.006 |
| Mean acceleration | −0.432; 0.039 | n.s. | n.s. | n.s. | n.s. | n.s. | −0.485; 0.019 | n.s. | −0.443; 0.034 | −0.459; 0.028 |
| Distance | −0.481; 0.020 | −0.423; 0.044 | n.s. | n.s. | −0.424; 0.044 | −0.418; 0.047 | −0.560; 0.005 | −0.462; 0.026 | −0.526; 0.010 | −0.538; 0.008 |
| Area | −0.578; 0.004 | −0.504; 0.014 | −0.461; 0.027 | n.s. | −0.477; 0.021 | −0.489; 0.018 | −0.640; 0.001 | −0.545; 0.007 | −0.606; 0.002 | −0.623; 0.001 |
| Angle | −0.417; 0.048 | −0.478; 0.021 | −0.429; 0.041 | −0.453; 0.030 | −0.439; 0.036 | −0.460; 0.027 | −0.470; 0.024 | −0.495; 0.016 | −0.516; 0.016 | −0.511; 0.016 |
| A-dimension | −0.711; <0.001 | −0.649; 0.001 | −0.595; 0.003 | −0.568; 0.005 | −0.639; 0.001 | −0.647; 0.001 | −0.754; <0.001 | −0.703; <0.001 | −0.700; <0.001 | −0.748; <0.001 |
| B-dimension | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.540; 0.008 | −0.423; 0.044 | −0.575; 0.004 | −0.525; 0.010 |
| Predictor Variables | B | Standard Error B | 95% CI | Β | t | p | R2 Adjusted | |
|---|---|---|---|---|---|---|---|---|
| GMGM-88 total score | Step 1 Flexion-extension angle | −2.569 | 0.633 | −3.885, −1.254 | −0.663 | −4.062 | 0.001 | 0.413 |
| Step 2 | 0.548 | |||||||
| Flexion-extension angle | −2.734 | 0.587 | −3.959, −1.508 | −0.706 | −4.653 | <0.001 | ||
| Age | −3.428 | 1.573 | −6.708, −0.147 | −0.331 | −2.180 | 0.041 | ||
| Step 3 | 0.638 | |||||||
| Flexion-extension angle | −2.607 | 0.542 | −3.741, −1.472 | −0.673 | −4.809 | <0.001 | ||
| Age | −8.634 | 2.791 | −14.485, −2.800 | −0.833 | −3.096 | 0.006 | ||
| Height | 100.671 | 46.130 | 4.121, 197.222 | 0.591 | 2.182 | 0.042 | ||
| PEDI total score | Step 1 | 0.560 | ||||||
| Area | −1.963 | 0.380 | −2.753, −1.173 | −0.748 | −5.170 | <0.001 | ||
| Step 2 | 0.653 | |||||||
| Area | −3.037 | 0.577 | −4.242, −1.833 | −1.158 | −5.261 | <0.001 | ||
| Rotational acceleration | 0.094 | 0.040 | 0.010, 0.178 | 0.511 | 2.322 | 0.031 | ||
| Step 3 | 0.790 | |||||||
| Area | −2.747 | 0.508 | −3.814, −1.681 | −1.047 | −5.412 | <0.001 | ||
| Rotational acceleration | 0.255 | 0.068 | 0.111, 0.398 | 1.387 | 3.721 | 0.002 | ||
| Age | −2.265 | 1.078 | −4.530, –0.001 | −0.229 | −2.102 | 0.050 | ||
| Step 4 | 0.833 | |||||||
| Area | −2.486 | 0.484 | −3.502, −1.470 | −0.947 | −5.139 | <0.001 | ||
| Rotational acceleration | 0.357 | 0.082 | 0.186, 0.529 | 1.946 | 4.373 | <0.001 | ||
| Age | −3.012 | 0.967 | −5.043, −0.981 | −0.304 | −3.116 | 0.006 | ||
| Distance | −3.837 | 1.158 | −6.270, −1.405 | −1.643 | −3.314 | 0.004 |
| Intra-Day Reliability | |||||
|---|---|---|---|---|---|
| Spatial Plane | Second Day Data (Standard Deviation) | ICC (95% CI) | SEM | MDC90 | |
| CP group (n = 24) | |||||
| Flexion-extension angle (°) | 8.29 (6.30) | 0.826 (0.579, 0.928) | 3.13 | 7.25 | |
| Rotational angle (°) | 8.50 (8.48) | 0.918 (0.804, 0.966) | 2.83 | 6.57 | |
| Lateral angle (°) | 6.67 (6.16) | 0.821 (0.566, 0.926) | 2.58 | 5.97 | |
| Mean angle (°) | 7.82 (6.22) | 0.923 (0.817, 0.968) | 1.94 | 4.51 | |
| Flexion-extension velocity (°/s) | 9.81 (9.00) | 0.921 (0.812, 0.967) | 2.66 | 6.16 | |
| Rotational velocity (°/s) | 11.71 (13.03) | 0.916 (0.799, 0.965) | 4.00 | 9.30 | |
| Lateral velocity (°/s) | 8.67 (8.21) | 0.889 (0.731, 0.954) | 2.91 | 6.75 | |
| Mean velocity (°/s) | 10.06 (9.88) | 0.919 (0.805, 0.966) | 2.99 | 6.93 | |
| Flexion-extension acceleration (°/s2) | 146.09 (153.44) | 0.914 (0.795, 0.964) | 45.54 | 105.61 | |
| Rotational acceleration (°/s2) | 138.06 (138.83) | 0.882 (0.714, 0.951) | 54.91 | 125.02 | |
| Lateral acceleration (°/s2) | 141.31 (127.34) | 0.854 (0.645, 0.939) | 54.79 | 127.07 | |
| Mean acceleration (°/s2) | 141.82 (137.58) | 0.892 (0.739, 0.955) | 49.23 | 114.19 | |
| Distance (°/s) | 11.83 (11.20) | 0.929 (0.829, 0.971) | 3.32 | 7.71 | |
| Area (°2) | 944.56 (1599.32) | 0.901 (0.761, 0.959) | 467.85 | 1085.09 | |
| Angle (°) | −2.48 (22.44) | 0.595 (0.334, 0.618) | 15.74 | 36.50 | |
| A-dimension (°) | 13.11 (10.67) | 0.770 (0.439, 0.905) | 5.49 | 12.74 | |
| B-dimension (°) | 14.35 (16.72) | 0.941 (0.860, 0.976) | 3.73 | 8.64 | |
| Control group (n = 24) | |||||
| Flexion-extension angle (°) | 4.45 (4.49) | 0.652 (0.388, 0.850) | 2.18 | 5.04 | |
| Rotational angle (°) | 2.45 (2.38) | 0.894 (0.757, 0.954) | 0.80 | 1.86 | |
| Lateral angle (°) | 2.08 (1.64) | 0.774 (0.486, 0.901) | 0.95 | 2.21 | |
| Mean angle (°) | 2.99 (2.43) | 0.934 (0.849, 0.972) | 0.59 | 1.38 | |
| Flexion-extension velocity (°/s) | 4.13 (2.23) | 0.704 (0.332, 0.870) | 1.69 | 3.91 | |
| Rotational velocity (°/s) | 3.22 (3.48) | 0.839 (0.631, 0.930) | 1.85 | 4.29 | |
| Lateral velocity (°/s) | 4.02 (2.37) | 0.656 (0.321, 0.850) | 1.93 | 4.48 | |
| Mean velocity (°/s) | 3.79 (2.49) | 0.751 (0.437, 0.891) | 1.76 | 4.08 | |
| Flexion-extension acceleration (°/s2) | 98.09 (60.10) | 0.637 (0.365, 0.843) | 40.86 | 94.76 | |
| Rotational acceleration (°/s2) | 77.84 (55.80) | 0.578 (0.048, 0.816) | 38.53 | 89.37 | |
| Lateral acceleration (°/s2) | 105.27 (62.22) | 0.495 (0.000, 0.780) | 54.08 | 127.74 | |
| Mean acceleration (°/s2) | 93.74 (56.87) | 0.587 (0.263, 0.820) | 42.38 | 98.29 | |
| Distance (°/s) | 5.45 (2.86) | 0.522 (0.000, 0.791)) | 2.67 | 6.19 | |
| Area (°2) | 82.59 (214.80) | 0.927 (0.831, 0.968) | 56.89 | 131.96 | |
| Angle (°) | 3.02 (20.41) | 0.514 (0.000, 0.792) | 14.71 | 34.13 | |
| A-dimension (°) | 4.98 (3.58) | 0.944 (0.871, 0.976) | 0.82 | 1.89 | |
| B-dimension (°) | 3.01 (3.98) | 0.841 (0.637, 0.931) | 1.81 | 4.20 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Pérez, C.; Pérez-Ruiz, A.; Garrido-Castro, J.L.; Vidal, F.T.; Alcaraz-Clariana, S.; García-Luque, L.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F. Design, Validity, and Reliability of a New Test, Based on an Inertial Measurement Unit System, for Measuring Cervical Posture and Motor Control in Children with Cerebral Palsy. Diagnostics 2020, 10, 661. https://doi.org/10.3390/diagnostics10090661
Carmona-Pérez C, Pérez-Ruiz A, Garrido-Castro JL, Vidal FT, Alcaraz-Clariana S, García-Luque L, Rodrigues-de-Souza DP, Alburquerque-Sendín F. Design, Validity, and Reliability of a New Test, Based on an Inertial Measurement Unit System, for Measuring Cervical Posture and Motor Control in Children with Cerebral Palsy. Diagnostics. 2020; 10(9):661. https://doi.org/10.3390/diagnostics10090661
Chicago/Turabian StyleCarmona-Pérez, Cristina, Alberto Pérez-Ruiz, Juan L. Garrido-Castro, Francisco Torres Vidal, Sandra Alcaraz-Clariana, Lourdes García-Luque, Daiana Priscila Rodrigues-de-Souza, and Francisco Alburquerque-Sendín. 2020. "Design, Validity, and Reliability of a New Test, Based on an Inertial Measurement Unit System, for Measuring Cervical Posture and Motor Control in Children with Cerebral Palsy" Diagnostics 10, no. 9: 661. https://doi.org/10.3390/diagnostics10090661
APA StyleCarmona-Pérez, C., Pérez-Ruiz, A., Garrido-Castro, J. L., Vidal, F. T., Alcaraz-Clariana, S., García-Luque, L., Rodrigues-de-Souza, D. P., & Alburquerque-Sendín, F. (2020). Design, Validity, and Reliability of a New Test, Based on an Inertial Measurement Unit System, for Measuring Cervical Posture and Motor Control in Children with Cerebral Palsy. Diagnostics, 10(9), 661. https://doi.org/10.3390/diagnostics10090661








