Association between Dyslipidemia and Chronic Rhinosinusitis in a Korean Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dyslipidemia (Exposure)
2.3. Chronic Rhinosinusitis (Outcome)
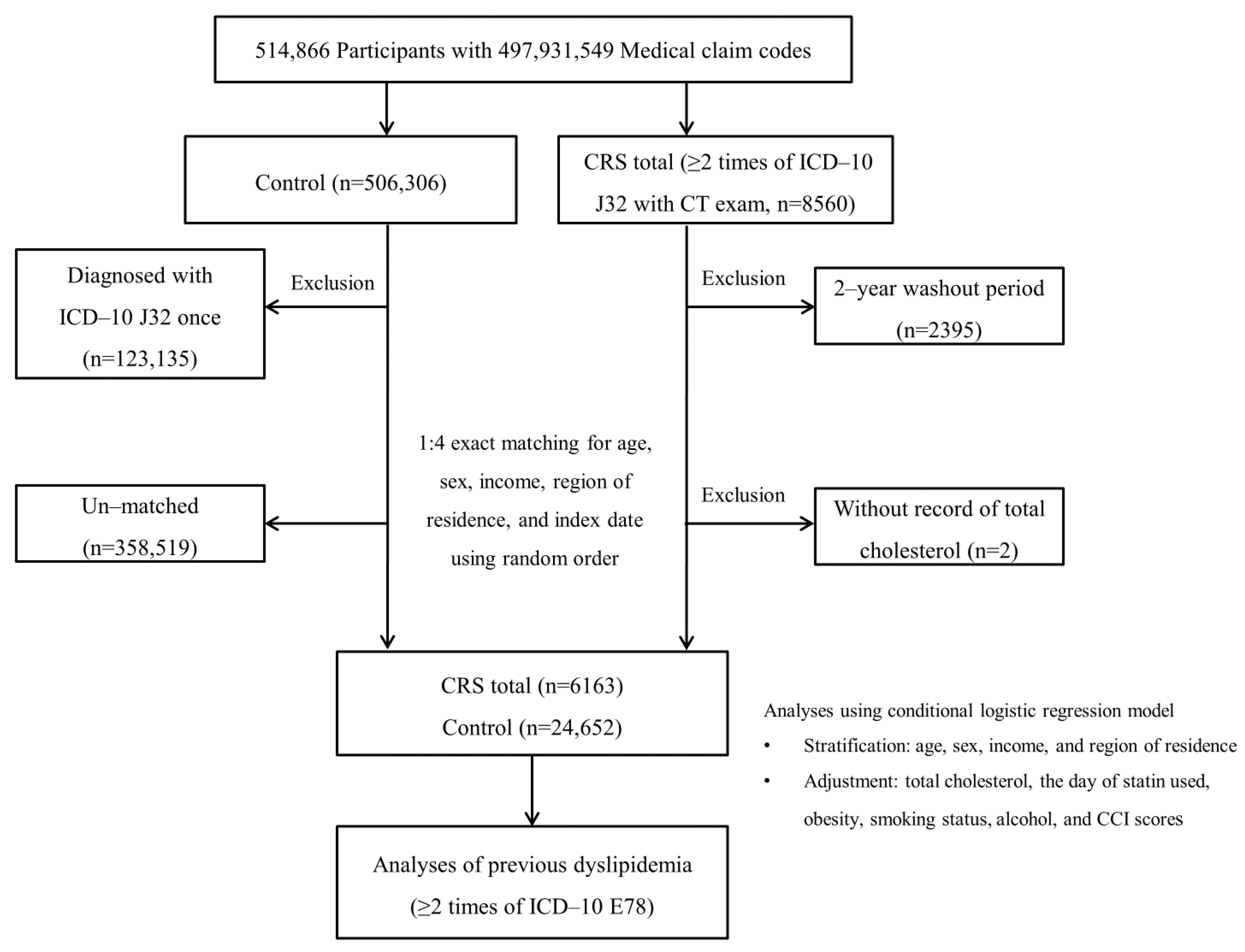
2.4. Participant Selection
2.5. Covariates
2.6. Statistics
3. Results
3.1. General Characteristics
3.2. Association between CRS and Dyslipidemia
3.3. Subgroup Analyses According to the Age and Sex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poddighe, D.; Brambilla, I.; Licari, A.; Marseglia, G.L. Pediatric rhinosinusitis and asthma. Respir. Med. 2018, 141, 94–99. [Google Scholar] [CrossRef]
- Ahern, S.; Cervin, A. Inflammation and Endotyping in Chronic Rhinosinusitis-A Paradigm Shift. Medicina 2019, 55, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poddighe, D.; Vangelista, L. Staphylococcus aureus Infection and Persistence in Chronic Rhinosinusitis: Focus on Leukocidin ED. Toxins 2020, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Beule, A. Epidemiology of chronic rhinosinusitis, selected risk factors, comorbidities, and economic burden. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2015, 14, 11. [Google Scholar]
- Jarvis, D.; Newson, R.; Lotvall, J.; Hastan, D.; Tomassen, P.; Keil, T.; Gjomarkaj, M.; Forsberg, B.; Gunnbjornsdottir, M.; Minov, J. Asthma in adults and its association with chronic rhinosinusitis: The GA2LEN survey in Europe. Allergy 2012, 67, 91–98. [Google Scholar] [CrossRef]
- Kelemence, A.; Abadoglu, O.; Gumus, C.; Berk, S.; Epozturk, K.; Akkurt, I. The frequency of chronic rhinosinusitis/nasal polyp in COPD and its effect on the severity of COPD. COPD 2011, 8, 8–12. [Google Scholar] [CrossRef]
- Wang, P.-C.; Lin, H.-C.; Kang, J.-H. Chronic rhinosinusitis confers an increased risk of acute myocardial infarction. Am. J. Rhinol. Allergy 2013, 27, e178–e182. [Google Scholar] [CrossRef]
- Bhattacharyya, N. Associations between obesity and inflammatory sinonasal disorders. Laryngoscope 2013, 123, 1840–1844. [Google Scholar] [CrossRef]
- Kabeya, Y.; Kato, K.; Tomita, M.; Katsuki, T.; Oikawa, Y.; Shimada, A. Association between diabetes and increased prevalence of paranasal sinus disease: A cross-sectional study in Japanese adults. J. Epidemiol. 2015, 25, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Dales, R.; Chen, Y.; Lin, M. Chronic sinusitis and arterial hypertension in a national population health survey. Int. J. Cardiol. 2006, 107, 230–234. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory Data Repository. 2008. Available online: https://apps.who.int/gho/data/view.main.2570?lang=en (accessed on 20 October 2020).
- The Korean Society of Lipid and Atherosclerosis, Dyslipidemia Fact Sheets in Korea 2020, Seoul. 2020. Available online: https://www.lipid.or.kr/bbs/?code=fact_sheet (accessed on 20 October 2020).
- Wilson, J.H.; Payne, S.C.; Fermin, C.E.R.; Churnin, I.; Qazi, J.; Mattos, J.L. Statin use protective for chronic rhinosinusitis in a nationally representative sample of the United States. Laryngoscope 2020, 130, 848–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucca, C.; Marsico, A.; Panaro, E.; Bigo, P.; Brussino, L. Statins and nasal polyps. Ann. Intern. Med. 2005, 142, 310–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, B.; Lim, H.; Kim, M.; Kong, I.G.; Choi, H.G. Gastroesophageal reflux disease increases the risk of chronic rhinosinusitis: A nested case-control study using a national sample cohort. Int. Forum. Allergy Rhinol. 2019, 9, 357–362. [Google Scholar] [CrossRef]
- Ryu, G.; Min, C.; Park, B.; Choi, H.G.; Mo, J.-H. Bidirectional association between asthma and chronic rhinosinusitis: Two longitudinal follow-up studies using a national sample cohort. Sci. Rep. 2020, 10, 9589. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for the Western Pacific. In The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Chung, S.D.; Chen, P.Y.; Lin, H.C.; Hung, S.H. Comorbidity profile of chronic rhinosinusitis: A population-based study. Laryngoscope 2014, 124, 1536–1541. [Google Scholar] [CrossRef]
- Lee, E.J.; Hwang, H.J.; Jung, C.M.; Kim, M.K.; Kang, M.S.; Kim, K.-S. The relationship between chronic rhinosinusitis and metabolic syndrome. Am. J. Rhinol. Allergy 2017, 31, 222–227. [Google Scholar] [CrossRef]
- Wattanachayakul, P.; Rujirachun, P.; Ungprasert, P. Risk of stroke among patients with chronic rhinosinusitis: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the Interheart study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MB, S.K.; Ganz, P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am. J. Cardiol. 1997, 80, 11I–16I. [Google Scholar]
- Zeiher, A.M.; Drexler, H.; Saurbier, B.; Just, H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J. Clin. Investig. 1993, 92, 652–662. [Google Scholar] [CrossRef] [Green Version]
- Celermajer, D.S.; Sorensen, K.E.; Bull, C.; Robinson, J.; Deanfield, J.E. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J. Am. Coll. Cardiol. 1994, 24, 1468–1474. [Google Scholar] [CrossRef]
- Anderson, T.J.; Meredith, I.T.; Charbonneau, F.O.; Yeung, A.C.; Frei, B.; Selwyn, A.P.; Ganz, P. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation 1996, 93, 1647–1650. [Google Scholar] [CrossRef]
- Asher, B.F.; Guilford, F.T. Oxidative stress and low glutathione in common ear, nose, and throat conditions: A systematic review. Altern. Ther. Health Med. 2016, 22, 44–50. [Google Scholar]
- Lam, K.; Schleimer, R.; Kern, R.C. The etiology and pathogenesis of chronic rhinosinusitis: A review of current hypotheses. Curr. Allergy Asthma Rep. 2015, 15, 41. [Google Scholar] [CrossRef] [Green Version]
- Stokes, K.Y.; Cooper, D.; Tailor, A.; Granger, D.N. Hypercholesterolemia promotes inflammation and microvascular dysfunction: Role of nitric oxide and superoxide. Free Radic. Biol. Med. 2002, 33, 1026–1036. [Google Scholar] [CrossRef]
- Al-Shawwa, B.; Al-Huniti, N.; Titus, G.; Abu-Hasan, M. Hypercholesterolemia is a potential risk factor for asthma. J. Asthma. 2006, 43, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, G.; Lind, P.; Hedblad, B.; Stavenow, L.; Janzon, L.; Lindgarde, F. Effects of cholesterol and inflammation-sensitive plasma proteins on incidence of myocardial infarction and stroke in men. Circulation 2002, 105, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Park, D.C.; Kim, S.H.; Yeo, S.G. Role of obesity in otorhinolaryngologic diseases. Curr. Allergy Asthma Rep. 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Chapman, R.; Grandy, S.; Group, S.I. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: Comparison of data from two national surveys. Int. J. Clin. Pract. 2007, 61, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China National Stroke Screening and prevention project (CNSSPP). BMC Public Health 2019, 19, 1500. [Google Scholar] [CrossRef]
- Surendar, J.; Mohan, V.; Rao, M.M.; Babu, S.; Aravindhan, V. Increased levels of both Th1 and Th2 cytokines in subjects with metabolic syndrome (CURES-103). Diabetes Technol. Ther. 2011, 13, 477–482. [Google Scholar] [CrossRef]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H.; Khan, Z.S.; Tesfa, L.; Hall, C.B.; Macian, F. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am. J. Respir Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.K.; Zhou, X.; Strandvik, B.; Hansson, G.K. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand. J. Immunol. 2004, 59, 285–293. [Google Scholar] [CrossRef]
- Zhou, X.; Paulsson, G.; Stemme, S.; Hansson, G.K. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Investig. 1998, 101, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Total Participants | ||
|---|---|---|---|
| CRS total | Control | p-Value | |
| Age (years old), n (%) | 1.000 | ||
| 40–44 | 232 (3.8) | 928 (3.8) | |
| 45–49 | 972 (15.8) | 3888 (15.8) | |
| 50–54 | 1339 (21.7) | 5356 (21.7) | |
| 55–59 | 1303 (21.1) | 5212 (21.1) | |
| 60–64 | 986 (16.0) | 3944 (16.0) | |
| 65–69 | 712 (11.6) | 2848 (11.6) | |
| 70–74 | 388 (6.3) | 1552 (6.3) | |
| 75–79 | 170 (2.8) | 680 (2.8) | |
| 80–84 | 50 (0.8) | 200 (0.8) | |
| 85+ | 11 (0.2) | 44 (0.2) | |
| Sex, n (%) | 1.000 | ||
| Male | 3786 (61.4) | 15,144 (61.4) | |
| Female | 2377 (38.6) | 9508 (38.6) | |
| Income group, n (%) | 1.000 | ||
| 1 (lowest) | 756 (12.3) | 3024 (12.3) | |
| 2 | 727 (11.8) | 2908 (11.8) | |
| 3 | 932 (15.1) | 3728 (15.1) | |
| 4 | 1349 (21.9) | 5396 (21.9) | |
| 5 (highest) | 2399 (38.9) | 9596 (38.9) | |
| Residence, n (%) | 1.000 | ||
| Urban | 2856 (46.3) | 11,424 (46.3) | |
| Rural | 3307 (53.7) | 13,228 (53.7) | |
| Obesity, n (%) | 0.003 1 | ||
| Underweight | 104 (1.7) | 508 (2.1) | |
| Normal | 2003 (32.5) | 8456 (34.3) | |
| Overweight | 1829 (29.7) | 6815 (27.6) | |
| Obese I | 2052 (33.3) | 8144 (33.0) | |
| Obese II | 175 (2.8) | 729 (3.0) | |
| Smoking status, n (%) | <0.001 1 | ||
| Nonsmoker | 4047 (65.7) | 15,960 (64.7) | |
| Past smoker | 908 (14.7) | 3226 (13.1) | |
| Current smoker | 1208 (19.6) | 5466 (22.2) | |
| Alcohol drinking, n (%) | 0.198 | ||
| <1 time a week | 4034 (65.5) | 15,920 (64.6) | |
| ≥1 time a week | 2129 (34.5) | 8732 (35.4) | |
| CCI score, n (%) | <0.001 1 | ||
| 0 | 3983 (64.6) | 17,809 (72.2) | |
| 1 | 1008 (16.4) | 3124 (12.7) | |
| 2 | 557 (9.0) | 1722 (7.0) | |
| 3 | 273 (4.4) | 808 (3.3) | |
| ≥4 | 342 (5.6) | 1189 (4.8) | |
| Dyslipidemia, n (%) | 1608 (26.1) | 5067 (20.6) | <0.001 1 |
| Days of statin use (day), mean (SD) | 57.8 (168.3) | 44.3 (148.5) | <0.001 2 |
| Total cholesterol (mg/dL) mean (SD) | 197.1 (37.2) | 199.0 (37.9) | 0.001 2 |
| Characteristics | Odds Ratios for Dyslipidemia | |||||
|---|---|---|---|---|---|---|
| Crude 2 | p-Value | Model 1 2,3 | p -Value | Model 2 2,4 | p -Value | |
| CRS total (n = 6163) | 1.39 (1.30–1.48) | <0.001 1 | 1.37 (1.27–1.48) | <0.001 1 | 1.36 (1.26–1.47) | <0.001 1 |
| Control (n = 24,652) | 1.00 | 1.00 | 1.00 | |||
| CRScNP (n = 2958) | 1.28 (1.15–1.41) | <0.001 1 | 1.33 (1.18–1.49) | <0.001 1 | 1.31 (1.17–1.47) | <0.001 1 |
| Control (n = 11,832) | 1.00 | 1.00 | 1.00 | |||
| CRSsNP (n = 3205) | 1.48 (1.36–1.62) | <0.001 1 | 1.42 (1.28–1.57) | <0.001 1 | 1.42 (1.28–1.57) | <0.001 1 |
| Control (n = 12,820) | 1.00 | 1.00 | 1.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, J.H.; Min, C.; Park, M.W.; Byun, S.H.; Lee, H.-J.; Song, C.M.; Park, B.; Choi, H.G. Association between Dyslipidemia and Chronic Rhinosinusitis in a Korean Population. Diagnostics 2021, 11, 26. https://doi.org/10.3390/diagnostics11010026
Wee JH, Min C, Park MW, Byun SH, Lee H-J, Song CM, Park B, Choi HG. Association between Dyslipidemia and Chronic Rhinosinusitis in a Korean Population. Diagnostics. 2021; 11(1):26. https://doi.org/10.3390/diagnostics11010026
Chicago/Turabian StyleWee, Jee Hye, Chanyang Min, Min Woo Park, Soo Hwan Byun, Hyo-Jeong Lee, Chang Myeon Song, Bumjung Park, and Hyo Geun Choi. 2021. "Association between Dyslipidemia and Chronic Rhinosinusitis in a Korean Population" Diagnostics 11, no. 1: 26. https://doi.org/10.3390/diagnostics11010026





