Serum Biomarkers of Inflammation and Turnover of Joint Cartilage Can Help Differentiate Psoriatic Arthritis (PsA) Patients from Osteoarthritis (OA) Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Assessment OA Group
2.2. Clinical Assessment PsA Group
2.3. Determination of the Biomarker Levels of Inflammation and Cartilage Turnover in Serum
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | osteoarthritis |
| PsA | psoriatic arthritis |
| HC | healthy control group |
| IL-18 | interleukin-18 |
| IL-20 | interleukin-20 |
| IL-6 | interleukin-6 |
| MMP-1 | metalloproteinase-1 |
| MMP-3 | metalloproteinase-3 |
| COMP | cartilage oligomeric matrix protein |
| PG-AG | aggrecan |
| YKL-40 | human cartilage glycoprotein |
| DIP | distal interphalangeal joint |
| PIP | proximal interphalangeal joint |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
| Lequesne Index | Index of Severity for Osteoarthritis of the Knee |
| BSA | Body Surface Area |
| PASI | Psoriasis Area and Severity Index |
| DLQI | Dermatology Life Quality Index |
| TJC | the number of tender joints |
| SJC | the number of swollen joints |
| MCP-1 | monocyte chemoattractant protein-1 |
| NGF | nerve growth factor |
| ROC | receiver operating characteristic curve |
References
- Monibi, F.; Roller, B.L.; Stoker, A.; Garner, B.C.; Bal, S.; Cook, J.L. Identification of Synovial Fluid Biomarkers for Knee Osteoarthritis and Correlation with Radiographic Assessment. J. Knee Surg. 2015, 29, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best Pr. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64, ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Krajewska-Włodarczyk, M.; Bechtold, A.; Żuber, Z.; Wojtkiewicz, M.; Wojtkiewicz, J. Role of Microparticles in the Pathogenesis of Inflammatory Joint Diseases. Int. J. Mol. Sci. 2019, 20, 5453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, H.; Ritchlin, C.T. Altered Bone Biology in Psoriatic Arthritis. Curr. Rheumatol. Rep. 2012, 14, 349–357. [Google Scholar] [CrossRef] [Green Version]
- McGonagle, D.; Khan, M.A.; Marzo-Ortega, H.; Oʼconnor, P.; Gibbon, W.; Emery, P. Enthesitis in spondyloarthropathy. Curr. Opin. Rheumatol. 1999, 11, 244–250. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Mease, P.J.; Jackson, J.M.; Eisen, D.; Xia, H.A.; Asare, C.; Stevens, S.R. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists’ offices. J. Dermatol. Treat. 2006, 17, 279–287. [Google Scholar] [CrossRef]
- Tan, A.L.; Grainger, A.J.; Tanner, S.F.; Emery, P.; McGonagle, D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: Are they the same? Arthritis Rheum. 2006, 54, 1328–1333. [Google Scholar] [CrossRef]
- McGonagle, D.; Lories, R.J.U.; Tan, A.L.; Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007, 56, 2482–2491. [Google Scholar] [CrossRef]
- Benjamin, M.; McGonagle, D. Histopathologic changes at “synovio-entheseal complexes” suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 2007, 56, 3601–3609. [Google Scholar] [CrossRef] [PubMed]
- Plato, C.C.; Norris, A.H. Osteoarthritis of the hand: Age-specific joint-digit prevalence rates1. Am. J. Epidemiol. 1979, 109, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, T.; Bruges-Armas, J.; Evison, G.; Cohen, M.; Lovell, C.; McHugh, N.J. The cervical spine in psoriatic arthritis: A clinical and radiological study. Rheumatology 1994, 33, 255–259. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Hermann, K.-G.A.; Tan, A.L. Differentiation between osteoarthritis and psoriatic arthritis: Implications for pathogenesis and treatment in the biologic therapy era. Rheumatology 2015, 54, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waszczykowski, M.; Bednarski, I.; Narbutt, J.; Waszczykowska, E.; Lesiak, A.; Fabiś, J. Interleukin-18, interleukin-20, and matrix metalloproteinases (MMP-1, MMP-3) as markers of psoriatic arthritis disease severity and their correlations with biomarkers of inflammation and turnover of joint cartilage. Adv. Dermatol. Allergol. 2020, 37. [Google Scholar] [CrossRef]
- Waszczykowski, M.; Fabiś-Strobin, A.; Bednarski, I.; Narbutt, J.; Fabiś, J. Serum and synovial fluid concentrations of interleukin-18 and interleukin-20 in patients with osteoarthritis of the knee and their correlation with other markers of inflammation and turnover of joint cartilage. Arch. Med. Sci. 2020, 16. [Google Scholar] [CrossRef]
- Waszczykowski, M.; Bednarski, I.; Lesiak, A.; Waszczykowska, E.; Narbutt, J.; Fabiś, J. The influence of tumour necrosis factor α inhibitors treatment—Etanercept on serum concentration of biomarkers of inflammation and cartilage turnover in psoriatic arthritis patients. Adv. Dermatol. Allergol. 2020, 37. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef] [Green Version]
- De Ceuninck, F.; Sabatini, M.; Pastoureau, P. Recent progress toward biomarker identification in osteoarthritis. Drug Discov. Today 2011, 16, 443–449. [Google Scholar] [CrossRef]
- Cretu, D.; Prassas, I.; Saraon, P.; Batruch, I.; Gandhi, R.; Diamandis, E.P.; Chandran, V. Identification of psoriatic arthritis mediators in synovial fluid by quantitative mass spectrometry. Clin. Proteom. 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przepiera-Będzak, H.; Fischer, K.; Brzosko, M. Extra-Articular Symptoms in Constellation with Selected Serum Cytokines and Disease Activity in Spondyloarthritis. Mediat. Inflamm. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeQuesne, M.G. The algofunctional indices for hip and knee osteoarthritis. J. Rheumatol. 1997, 24, 779–781. [Google Scholar] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Barr, S.; Bellamy, N.; Buchanan, W.W.; Chalmers, A.; Ford, P.M.; Kean, W.F.; Kraag, G.R.; Gerecz-Simon, E.; Campbell, J. A comparative study of signal versus aggregate methods of outcome measurement based on the WOMAC Osteoarthritis Index. Western Ontario and McMaster Universities Osteoarthritis Index. J. Rheumatol. 1994, 21, 2106–2112. [Google Scholar]
- Wolfe, F.; Kong, S.X. Rasch analysis of the Western Ontario MacMaster Questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann. Rheum. Dis. 1999, 58, 563–568. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 2008, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Mease, P.J. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesit. Arthritis Rheum. 2011, 63, S64–S85. [Google Scholar] [CrossRef]
- Long, C.C.; Finlay, A.Y. The finger-tip unit—a new practical measure. Clin. Exp. Dermatol. 1991, 16, 444–447. [Google Scholar] [CrossRef]
- Ashcroft, D.M.; Po, A.L.W.; Williams, H.C.; Griffiths, C.E. Clinical measures of disease severity and outcome in psoriasis: A critical appraisal of their quality. Br. J. Dermatol. 1999, 141, 185–191. [Google Scholar] [CrossRef]
- Finlay, A.; Khan, G. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Mease, P.J.; Strand, V.; Healy, P.; Helliwell, P.; Fitzgerald, O.; Gottlieb, A.B.; Krueger, G.G.; Nash, P.; Ritchlin, C.T.; et al. Consensus on a core set of domains for psoriatic arthritis. J. Rheumatol. 2007, 34, 1167–1170. [Google Scholar] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Wang, H.; Peng, J.; Wang, B.; Lu, X.; Zheng, J.Z.; Wang, K.; Tu, X.M.; Feng, C. Inconsistency Between Univariate and Multiple Logistic Regressions. Shanghai Arch. Psychiatry. 2017, 29, 124–128. [Google Scholar] [PubMed]
- Bursac, Z.; Gauss, C.H.; Williams, K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Chandran, V.; Abji, F.; Perruccio, A.V.; Gandhi, R.; Li, S.; Cook, R.J.; Gladman, D.D. Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis. Ann. Rheum. Dis. 2019, 78, 796–801. [Google Scholar] [CrossRef]
- Blagojevic-Bucknall, M.; Jinks, C.; Jeffery, A.; Jordan, K. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Eder, L.; Abji, F.; Rosen, C.F.; Chandran, V.; Gladman, D.D. The Association Between Obesity and Clinical Features of Psoriatic Arthritis: A Case-control Study. J. Rheumatol. 2017, 44, 437–443. [Google Scholar] [CrossRef]
- Cañete, J.D.; Mease, P. The link between obesity and psoriatic arthritis. Ann. Rheum. Dis. 2012, 71, 1265–1266. [Google Scholar] [CrossRef] [Green Version]
- Oliveria, S.A.; Felson, D.T.; Reed, J.I.; Cirillo, P.A.; Walker, A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; Scarpa, R.; Padula, A.; D’Angelo, S. Role of Trauma in Psoriatic Arthritis. J. Rheumatol. 2008, 35, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- El-Arman, M.M.; El-Fayoumi, G.; El-Shal, E.; El-Boghdady, I.; El-Ghaweet, A. Aggrecan and Cartilage Oligomeric Matrix Protein in Serum and Synovial Fluid of Patients with Knee Osteoarthritis. HSS J. 2010, 6, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, V.; Cook, R.J.; Edwin, J.; Shen, H.; Pellett, F.J.; Shanmugarajah, S.; Rosen, C.F.; Gladman, D.D. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology 2010, 49, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Chandran, V. Soluble Biomarkers May Differentiate Psoriasis from Psoriatic Arthritis. J. Rheumatol. Suppl. 2012, 89, 65–66. [Google Scholar] [CrossRef]
- Otterness, I.; Swindell, A.; Zimmerer, R.; Poole, A.; Ionescu, M.; Weiner, E. An analysis of 14 molecular markers for monitoring osteoarthritis: Segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthr. Cartil. 2000, 8, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.J.; Hetland, M.L.; Sørensen, I.J.; Østergaard, M.; Nielsen, H.J.; Johansen, J.S. Circulating levels of interleukin-6, vascular endothelial growth factor, YKL-40, matrix metalloproteinase-3, and total aggrecan in spondyloarthritis patients during 3 years of treatment with TNFα inhibitors. Clin. Rheumatol. 2010, 29, 1301–1309. [Google Scholar] [CrossRef]
- Pedersen, S.J.; Sørensen, I.J.; Lambert, R.G.; Hermann, K.-G.; Garnero, P.; Johansen, J.S.; Madsen, O.R.; Hansen, A.; Hansen, M.S.; Thamsborg, G.; et al. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor α inhibitors: A study of radiographic progression, inflammation on magnetic resonance imaging, and c. Arthritis Rheum. 2011, 63, 3789–3800. [Google Scholar] [CrossRef]
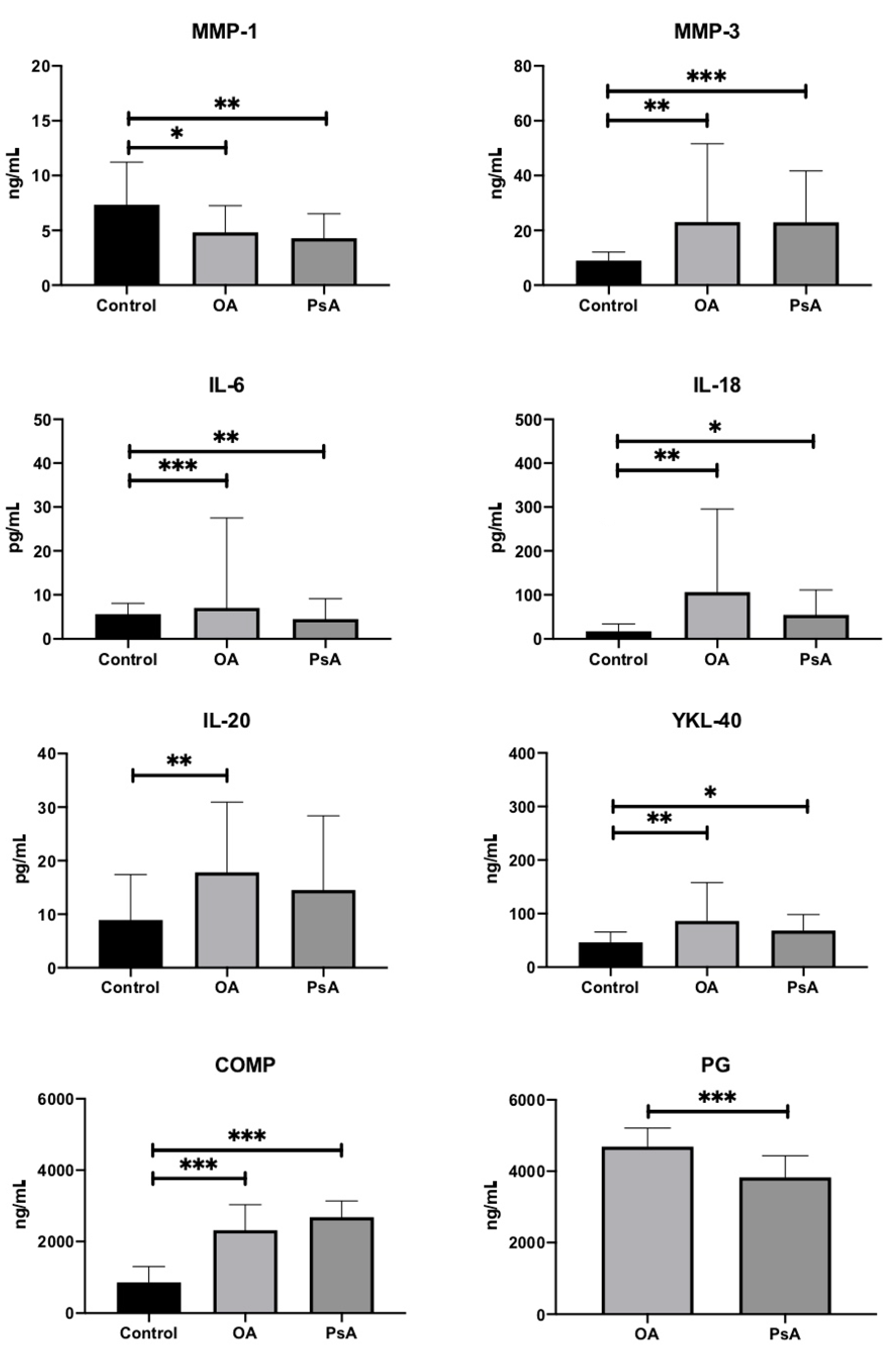
| Variable | OA (n = 22) | PsA (n = 22) | HC (n = 23) | p-Value * |
|---|---|---|---|---|
| Age (years) | 62.23 ± 12.16 | 52.82 ± 6.02 | 54.71 ± 7.40 | 0.108 |
| Gender (female/male) | 11/11 | 12/10 | 12/11 | 0.9554 |
| Disease duration (yeas) | 12.9 ± 6.4 | 14.2 ± 7.2 | N/A | - |
| Kellgren-Lawrence Grading (0–4) | 3 (2–4) | N/A | N/A | - |
| WOMAC score (0–100) | 46.42 ± 8.93 | N/A | N/A | - |
| Lequesne index (0–24) | 10.31 ± 2.29 | N/A | N/A | - |
| PASI (%) | N/A | 16.53 ± 3.93 | N/A | - |
| DLQI | N/A | 13.87 ± 2.13 | N/A | - |
| BSA (%) | N/A | 26.47 ± 8.41 | N/A | - |
| CRP (mg/L) | N/A | 9.48 ± 8.54 | N/A | - |
| TJC + SJC | N/A | 10.56 ± 2.53 | N/A | - |
| MMP-1 (ng/mL) | 4.83 ± 2.43 | 4.29 ± 2.23 | 7.33 ± 3.89 | 0.003 |
| MMP-3 (ng/mL) | 23.06 ± 28.55 | 22.95 ± 18.72 | 8.99 ± 3.08 | <0.001 |
| PG-AG (ng/mL) | 4689.32 ± 518.47 | 3828.7 ± 603.01 | N/A | <0.001 |
| IL-6 (pg/mL) | 7.01 ± 20.52 | 4.48 ± 4.61 | 5.61 ± 2.45 | <0.001 |
| IL-18 (pg/mL) | 106 ± 189.76 | 54.34 ± 56.64 | 16.73 ± 17 | 0.001 |
| YKL-40 (ng/mL) | 86.23 ± 71.58 | 68.03 ± 30.21 | 46.06 ± 19.41 | 0.003 |
| IL-20 (pg/mL) | 17.8 ± 13.13 | 14.51 ± 13.87 | 8.9 ± 8.5 | 0.011 |
| COMP (ng/mL) | 2315.61 ± 715.4 | 2683.91 ± 453.07 | 862.58 ± 441.31 | <0.001 |
| Biomarker | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| MMP-1 | 0.925 (0.702–1.218) | 0.579 | - | - |
| MMP-3 | 0.999 (0.973–1.025) | 0.915 | - | - |
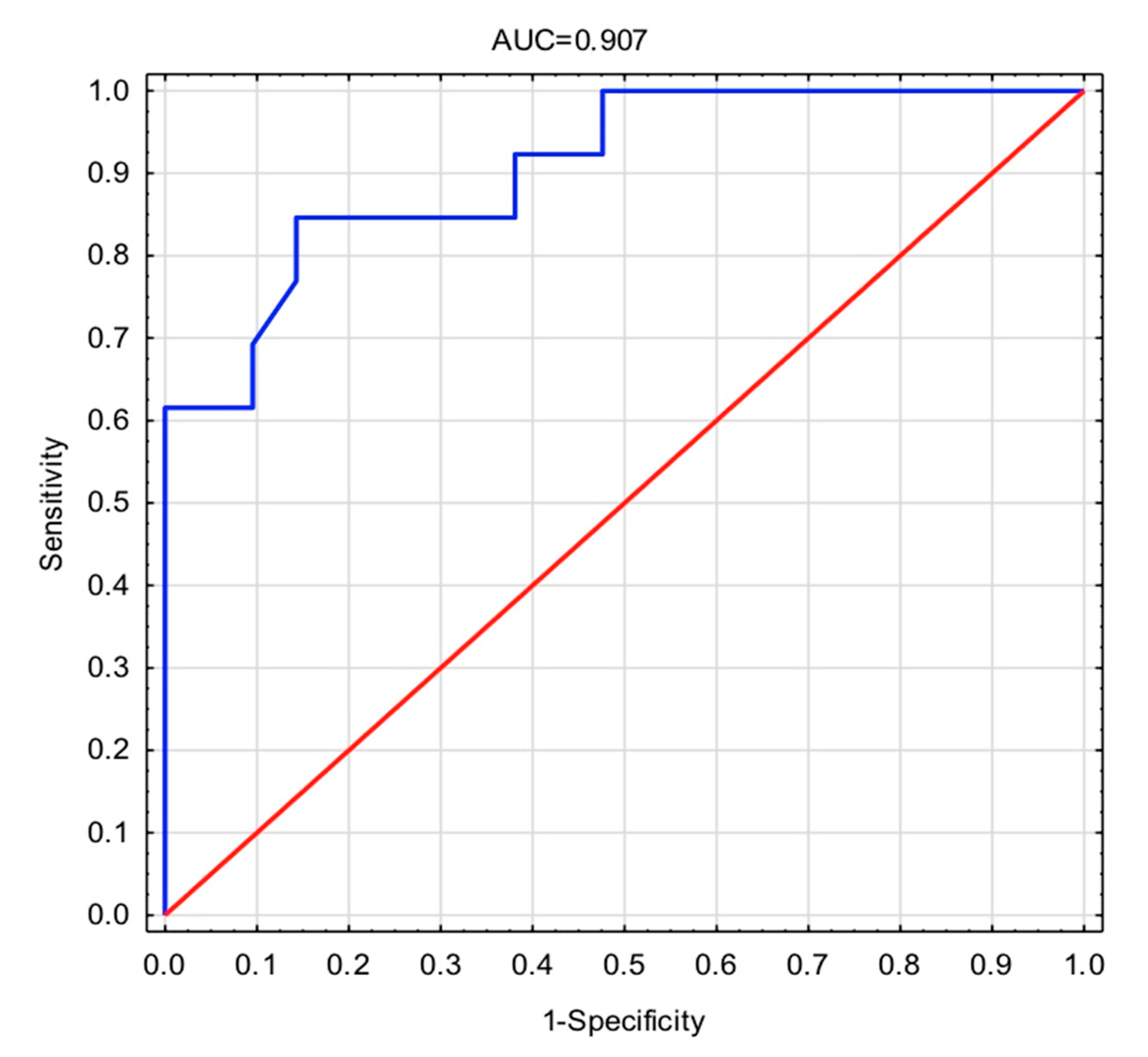
| PG-AG | 0.997 (0.995–0.999) | 0.007 | 0.995 (0.991–0.999) | 0.023 |
| IL-6 | 0.982 (0.925–1.043) | 0.555 | - | - |
| IL-18 | 0.997 (0.990–1.004) | 0.419 | - | - |
| YKL-40 | 0.996 (0.984–1.009) | 0.561 | - | - |
| IL-20 | 0.967 (0.911–1.027) | 0.277 | - | - |
| COMP | 1.001 (1.000–1.002) | 0.124 | 1.003 (1.000–1.005) | 0.026 |
| Statistic | Value |
|---|---|
| −2 Log(Likelihood) | −11.511 |
| Cox-Snell’s R2 | 0.480 |
| Nagelkerke’s R2 | 0.652 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszczykowski, M.; Fabiś-Strobin, A.; Bednarski, I.; Lesiak, A.; Narbutt, J.; Fabiś, J. Serum Biomarkers of Inflammation and Turnover of Joint Cartilage Can Help Differentiate Psoriatic Arthritis (PsA) Patients from Osteoarthritis (OA) Patients. Diagnostics 2021, 11, 52. https://doi.org/10.3390/diagnostics11010052
Waszczykowski M, Fabiś-Strobin A, Bednarski I, Lesiak A, Narbutt J, Fabiś J. Serum Biomarkers of Inflammation and Turnover of Joint Cartilage Can Help Differentiate Psoriatic Arthritis (PsA) Patients from Osteoarthritis (OA) Patients. Diagnostics. 2021; 11(1):52. https://doi.org/10.3390/diagnostics11010052
Chicago/Turabian StyleWaszczykowski, Michał, Anna Fabiś-Strobin, Igor Bednarski, Aleksandra Lesiak, Joanna Narbutt, and Jarosław Fabiś. 2021. "Serum Biomarkers of Inflammation and Turnover of Joint Cartilage Can Help Differentiate Psoriatic Arthritis (PsA) Patients from Osteoarthritis (OA) Patients" Diagnostics 11, no. 1: 52. https://doi.org/10.3390/diagnostics11010052





