Abstract
Gallbladder (GB) diseases represent various lesions including gallstones, cholesterol polyps, adenomyomatosis, and GB carcinoma. This review aims to summarize the role of endoscopic ultrasound (EUS) in the diagnosis of GB lesions. EUS provides high-resolution images that can improve the diagnosis of GB polypoid lesions, GB wall thickness, and GB carcinoma staging. Contrast-enhancing agents may be useful for the differential diagnosis of GB lesions, but the evidence of their effectiveness is still limited. Thus, further studies are required in this area to establish its usefulness. EUS combined with fine-needle aspiration has played an increasing role in providing a histological diagnosis of GB tumors in addition to GB wall thickness.
1. Introduction
Gallbladder (GB) diseases are relatively common and represent a variety of lesions including gallstones, cholesterol polyps, adenomyomatosis (ADM), and GB carcinoma. The most common disease is gallstones, affecting 10–15% of the adult population in the USA [1]. GB polyps have an estimated prevalence of approximately 5% in the global population [2,3,4], while GB carcinoma has an incidence of approximately 0.4 and 27 per 100,000 and in 100,000 people, respectively [5]. Northern India, Korea, Japan, and Central/Eastern Europe including Slovakia, Czech Republic, and Slovenia have also reported a higher prevalence than the worldwide average [6]. In contrast, GB carcinoma is rare in the western world (USA, UK, Canada, Australia, and New Zealand) [7].
Endoscopic ultrasound (EUS) has a high spatial resolution that can improve the diagnosis of GB polypoid lesions, GB wall thickness, and GB carcinoma staging. Vascularity can be evaluated using contrast-enhancing agents. Therefore, contrast-enhanced EUS may be useful for the differential diagnosis of GB lesions. However, the evidence of their effectiveness is still limited, and further studies are required in this area to establish its usefulness. EUS combined with fine-needle aspiration has played an increasing role in providing a histological diagnosis of GB tumors in addition to GB wall thickness. The role of EUS in the diagnosis of GB lesions has been demonstrated in many studies, and this review aims to summarize the role of EUS in the diagnosis of GB pathologies.
2. Literature Research
We conducted our literature research in the PubMed and Scopus electronic databases using combinations of the following key words: endoscopic ultrasound, gallbladder diseases, gallbladder neoplasm, and gallbladder carcinoma/cancer. We narrowed the research timeline to the period from 1991 to 2021. After initial selection based on exclusion criteria such as languages other than English, lack of access to a paper, and after having removed duplicates, we reviewed papers for further evaluation. From these papers, we mainly referred to our own papers and adopted 65 papers for this review. This review is written in the style of narrative review.
3. EUS Compared with Transabdominal Ultrasound to Detect GB Lesions
Transabdominal ultrasound (TUS) is used as a primary screening modality for gallbladder lesions because it is a relatively inexpensive, minimally invasive, and simple examination. However, EUS is considered superior to TUS for biliary system imaging because of the proximity of the duodenum to GB and higher resolution images by using higher ultrasound frequencies than TUS (5–12 versus 2–5 MHz) [8,9].
TUS has a sensitivity of 98% for gallstone detection [10]. However, TUS still has particularly some difficulty in identifying microlithiasis [10]. EUS can sometimes detect microlithiasis in the gallbladder in patients with grossly noncalculous biliary colic and normal TAUS findings. EUS demonstrated 92.6–100% and 55.6–91% of sensitivity and specificity, respectively, for the diagnosis of GB microlithiasis that had a negative result on TUS [11,12,13]. The benefit of using EUS over TUS has been best demonstrated in the diagnosis of microlithiasis.
The sensitivity of TUS in the detection of polypoid lesions of the gallbladder ranges from 36% to 99% depending on the presence of gallstones [14,15]. The current study found no studies for the detection of gallbladder polypoid lesions on EUS. The accuracy of TUS for the diagnosis of polypoid gallbladder lesions was reported to be 70–90% [16]. However, its diagnostic accuracy is strongly affected by the TUS technology and the ability of sonographers [17].
4. Differential Diagnosis of GB Lesions
GB lesions are broadly divided into protuberant lesions and wall-thickening lesions [18,19]. Protuberant lesions are defined as a focal elevation or a protrusion that can be distinguished from the surrounding mucosa [15,20]. Protuberant lesions are a comprehensive category that includes various diseases (Table 1). Protuberant lesions are first divided into two types (i.e., neoplastic and non-neoplastic protuberant lesions). Neoplastic protuberant lesions include adenomas and malignant lesions (e.g., GB carcinomas). However, non-neoplastic protuberant lesions include cholesterol polyps, inflammatory polyps, localized ADM, and hyperplasia. Neoplastic polypoid lesions should be treated by surgical resection, while non-neoplastic polypoid lesions can be observed serially.

Table 1.
Classification of GB protuberant lesions.
Moreover, wall-thickening lesions denote lesions in which the GB wall is diffusely thickened. GB wall-thickening is defined as the GB wall measuring >4 mm and can be the result of various processes (Table 2) [21].

Table 2.
Classification of GB wall-thickening lesions.
4.1. Differential Diagnosis of GB Protuberant Lesions
Several studies have evaluated EUS in the differential diagnosis of GB protuberant lesions [22,23,24,25,26,27,28,29]. The differential diagnosis for neoplastic and non-neoplastic lesions is based on size, number, morphology, surface contour, internal echotexture, and internal structure (Table 3). Among these findings, classifying them into pedunculated or sessile (broad-based) types is very important. Most pedunculated lesions are benign, and cholesterol polyps are the most common. Multiple polyps measuring ≤10 mm are highly likely to be cholesterol polyps [30]. However, malignant tumors are included in rare cases. These lesions are frequently incidental findings during abdominal examinations, and precisely distinguishing benign lesions from malignancies is important. EUS can visualize the layered structure of the GB and provide high-resolution images using high ultrasound frequencies. The characteristic findings of cholesterol polyps on EUS are a deeply notched granular surface and morular morphology. The internal echo is rough or granular, and highly echogenic punctiform foci reflecting cholesterolosis are visible (Figure 1) [25]. Peduncles are thin. Thus, they are often unobserved even on EUS [26]. Akatsu et al. [27] described the presence of hyperechoic spots, and multiple microcysts were important indicators of non-neoplastic lesions. Kimura et al. [24] described a granular contour, and a spotty internal echo pattern in the pedunculated polypoid lesions indicated benign pathology.

Table 3.
EUS features of major GB protuberant lesions.
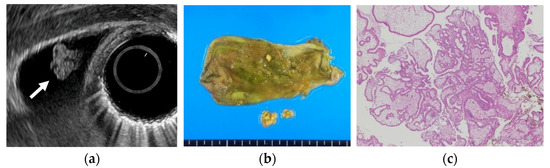
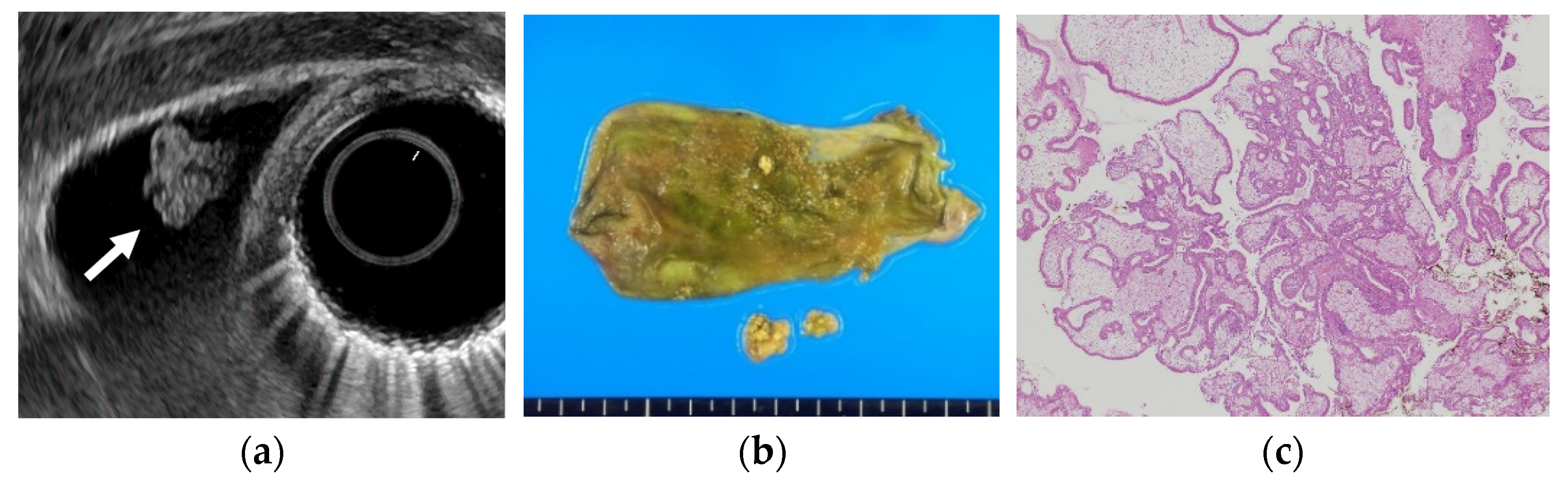
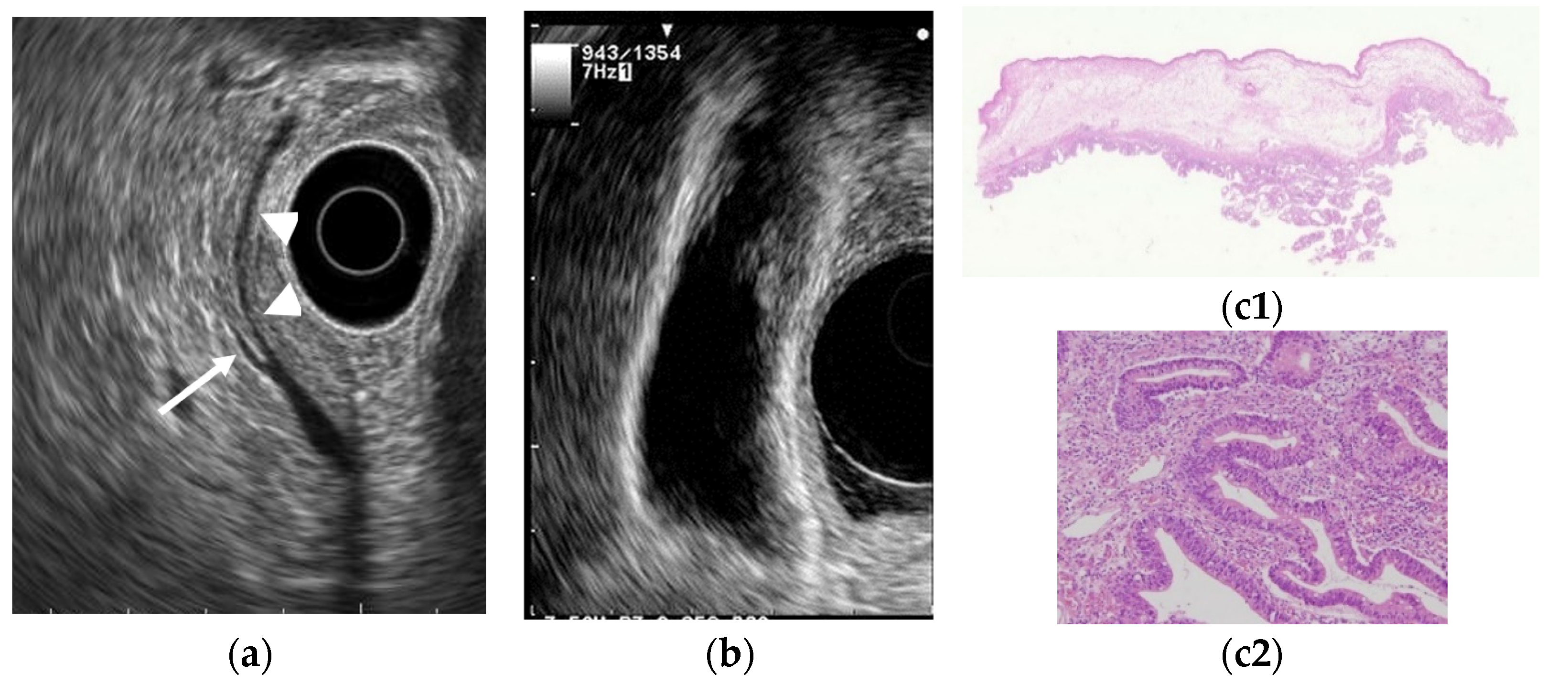
Figure 1.
Cholesterol polyp. (a) EUS shows a cholesterol polyp as a granular-surfaced pedunculated lesion. The internal echo is heterogeneous with a hyperechoic spot (arrow). (b) Photograph of the gross pathologic specimen after cholecystectomy shows a yellowish polyp. (c) H-E stain of the specimen demonstrates an aggregation of foamy cells under the epithelium.
EUS shows an adenoma as a homogeneously isoechoic pedunculated or subpedunculated mass with a nodular or relatively smooth surface and an adenocarcinoma (pedunculated type) as a heterogeneously echogenic pedunculated mass with a nodular or smooth surface (Figure 2) [27,31]. Differentiation between adenomas and adenocarcinomas based on imaging is considered difficult. Thus, Cho et al. focused on relatively hypoechoic areas at the cores of the polyps, reporting the presence of such hypoechoic cores on EUS to be a strong predictive factor for neoplastic polyps. The overall accuracy of EUS in differentiating neoplastic from non-neoplastic lesions is 86.5–97% [22,25]. The accuracy of EUS in differentiating neoplastic from non-neoplastic polypoid lesions <10 mm was reported to be low [23]. Moreover, EUS is considered useful for guiding the treatment of larger pedunculated polyps [24].
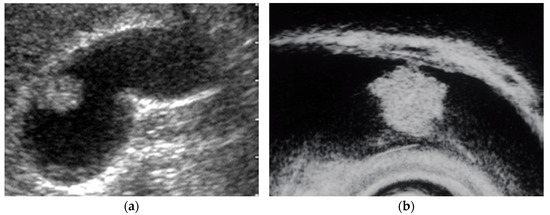
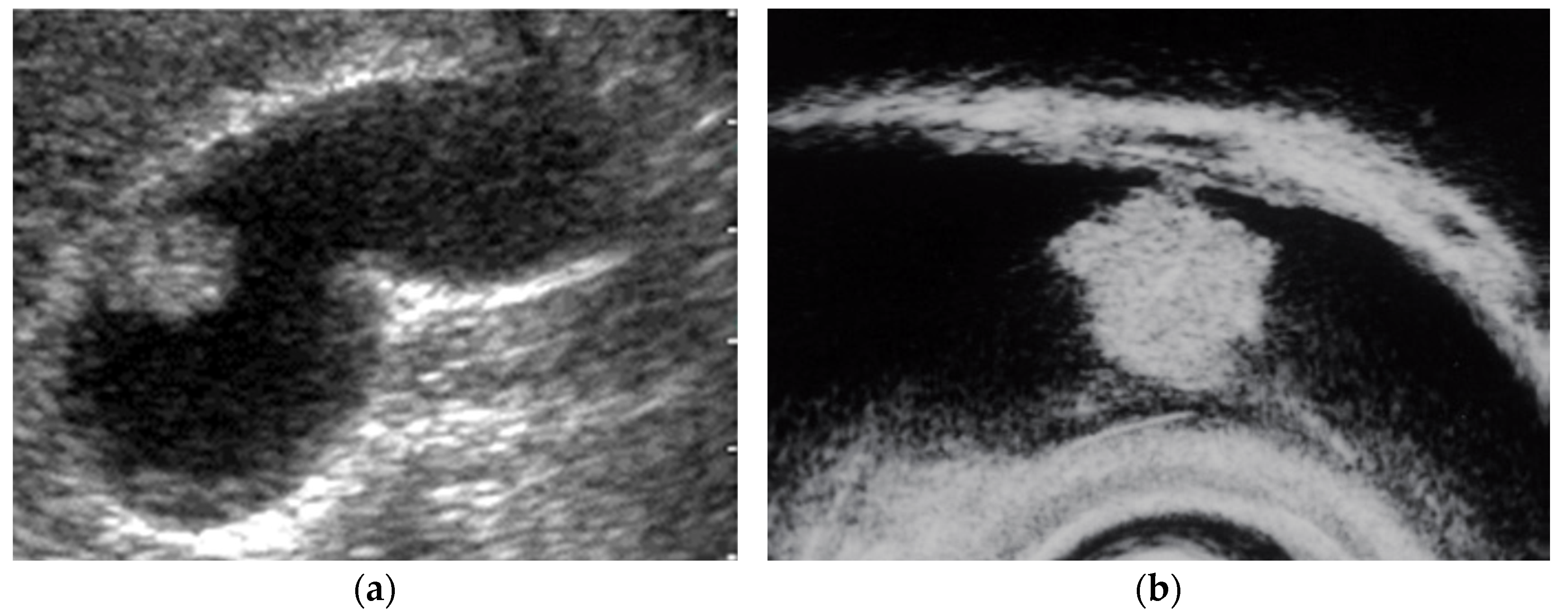
Figure 2.
Pedunculated GB carcinoma. (a) TUS image shows a relatively smooth surface, solid internal echogenicity polyp, but TUS does not depict the nature of the base of the lesion. (b) EUS image shows a pedunculated lesion. This lesion was GB adenocarcinoma with invasion depth pT1a (M) as a result of surgery.
EUS scoring systems have been proposed to differentiate between non-neoplastic and neoplastic GB protuberant lesions to aid diagnosis. Sadamoto et al. [32] reported the usefulness of an EUS score based on a coefficient of multivariate analysis: score = (maximum diameter in millimeter) + (internal echo pattern score; heterogeneous = 4, homogeneous = 0) + (hyperechoic spot score; present = −5, absence = 0). The sensitivity and specificity were 77.8% and 82.7%, respectively, in the differential diagnosis of neoplastic and non-neoplastic polyps using a cutoff score of >12. Choi et al. [33] have proposed another EUS scoring system for differential diagnosis of GB lesions between 5 and 15 mm based on layer structure, echo patterns, polyp margin, presence of a stalk, and the number of polyps. Moreover, the sensitivity and specificity were 81% and 86%, respectively, using a cutoff score of six.
Moreover, sessile lesions include localized ADM, carcinomas, and debris. Sessile GB carcinomas present with irregular internal echoes that are equal to or slightly hypoechoic to the liver parenchyma by EUS. Early GB carcinomas may be often accompanied by thickening of the inner hypoechoic layer around the main protuberant lesion [17,34].
EUS can visualize localized ADM as a sessile polypoid lesion with small cystic areas corresponding to the proliferation of Rokitansky–Aschoff sinuses (RAS; Figure 3) [27]. Comet tail artifacts are also occasionally observed owing to multipath reflection from RAS or intramural calculi. Several cases of GB carcinoma concomitant with ADM have been recently reported [35,36,37,38,39]. Therefore, the possibility of concomitant GB carcinoma with ADM in sessile lesions with multiple microcysts should be kept in mind.
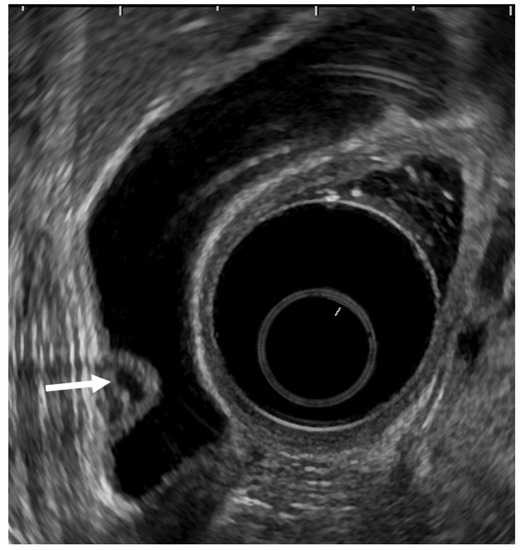
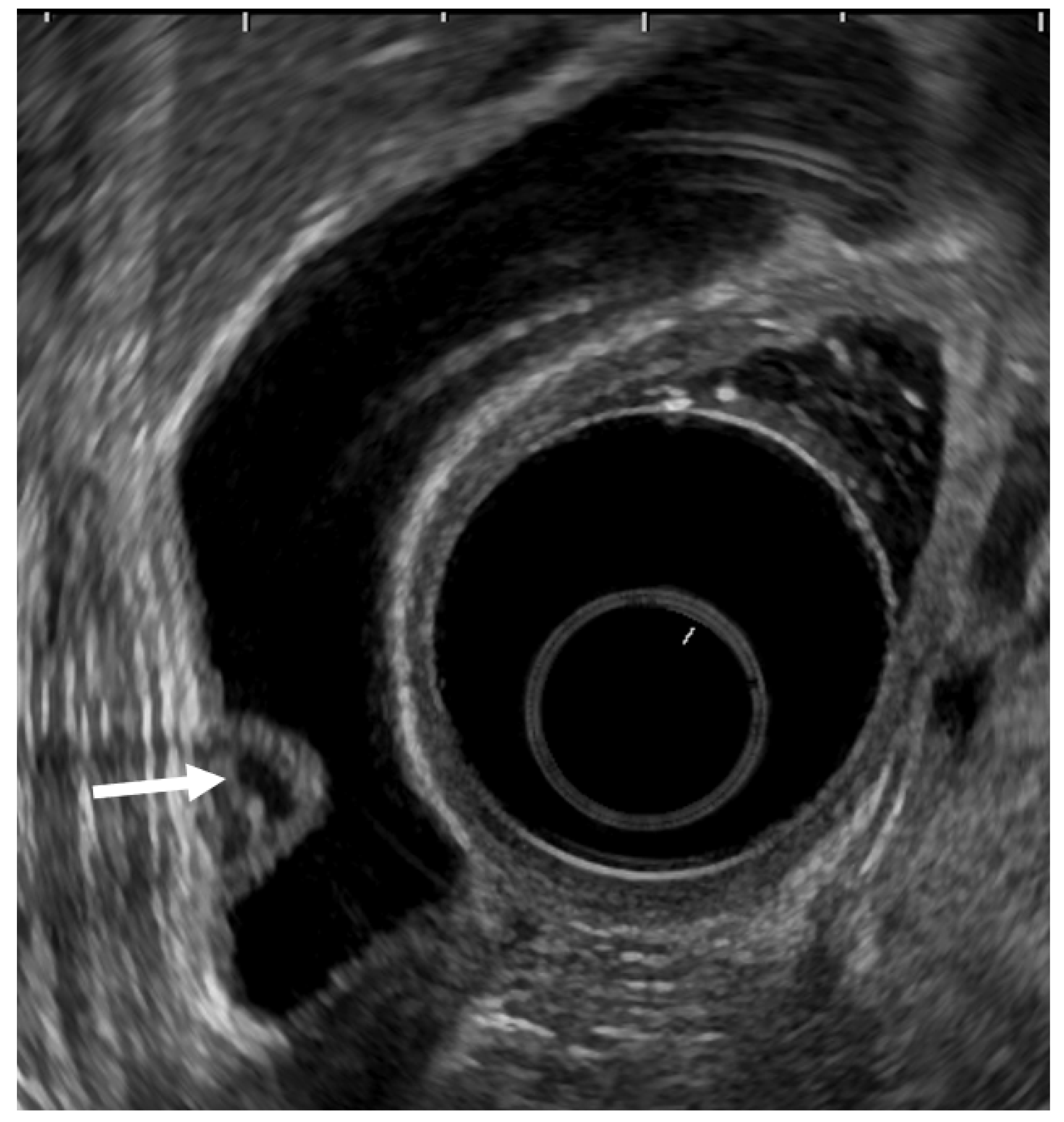
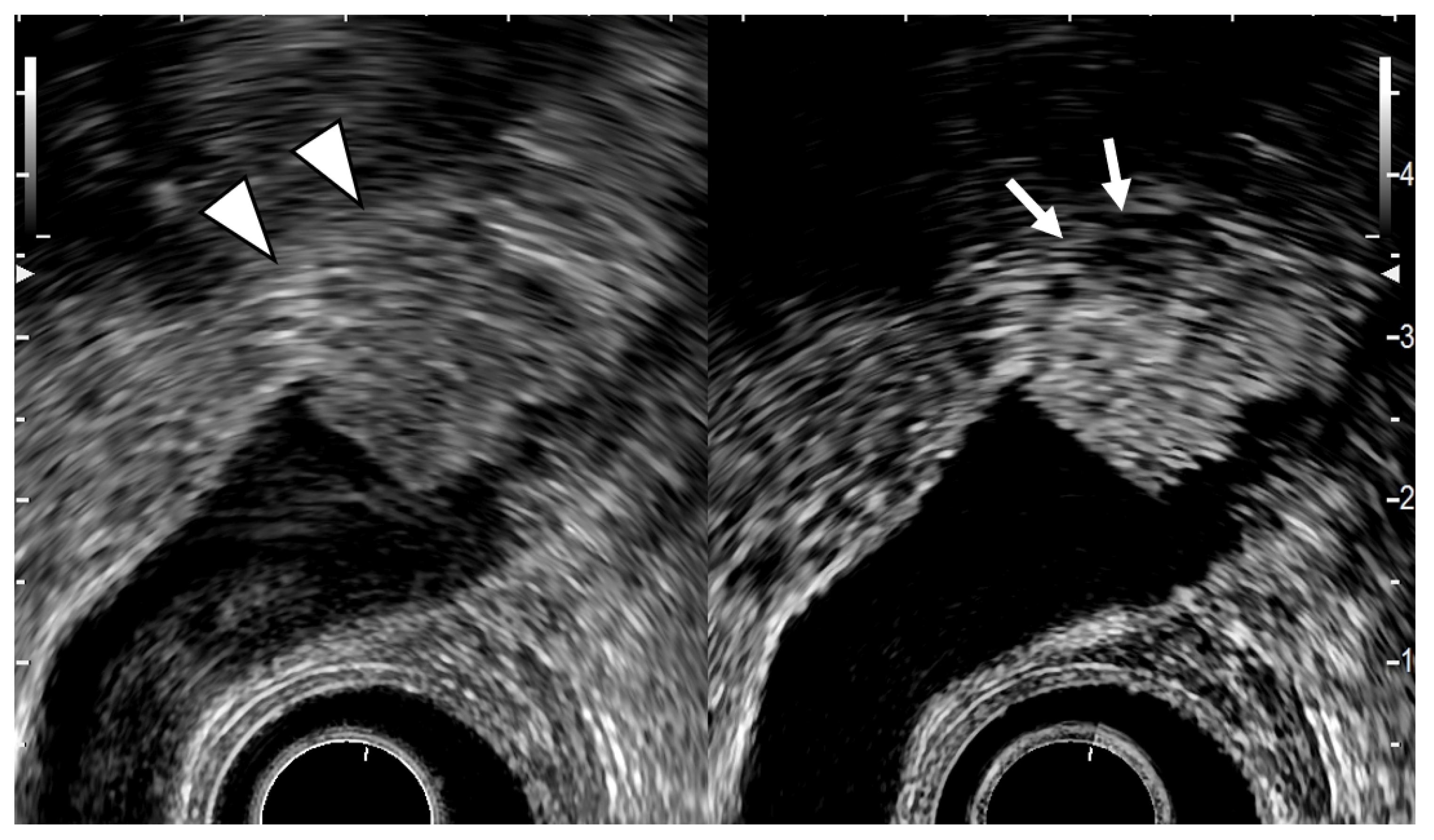
Figure 3.
EUS image ADM (localized type). EUS shows localized ADM as a sessile polypoid lesion with anechoic areas (arrow) corresponding to RAS proliferation. The surface is relatively smooth.
4.2. Differential Diagnosis of GB Wall-Thickening Lesions
GB wall-thickening can be associated with a myriad of disorders. Therefore, GB wall-thickening poses difficulty in differentiating between benign processes (e.g., inflammation and malignant tumor). Thus, distinguishing early-stage cancer from benign wall-thickening of GB is important [40]. The contour of the lesion, patterns of wall thickness, intramural cystic space, and patterns of GB wall enhancement are used as differential points (Table 4). EUS can better define the characteristics of GB wall-thickening.

Table 4.
EUS features of major GB wall-thickening lesions.
ADM can sometimes mimic GB carcinoma. The layers of a thickened GB wall are usually preserved in ADM, but there are microcysts with bright echoes arising from the cystic spaces. The thickened wall has a smooth surface but occasionally exhibits surface irregularity, reflecting hyperplastic changes. A key point in its diagnosis is the confirmation of the presence of cystic anechoic spots reflecting RAS inside the thickened wall (Figure 4) [26].
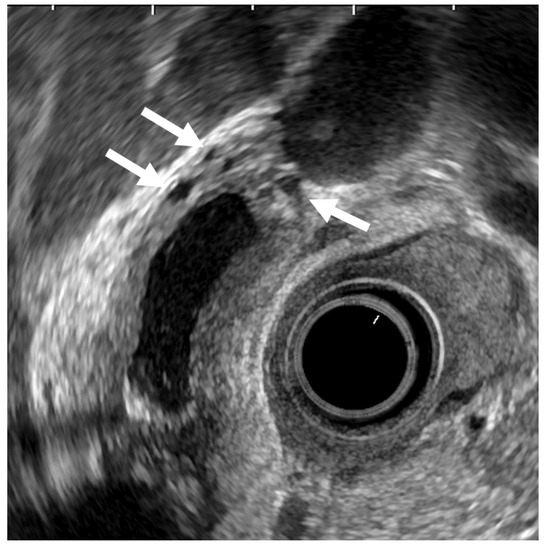
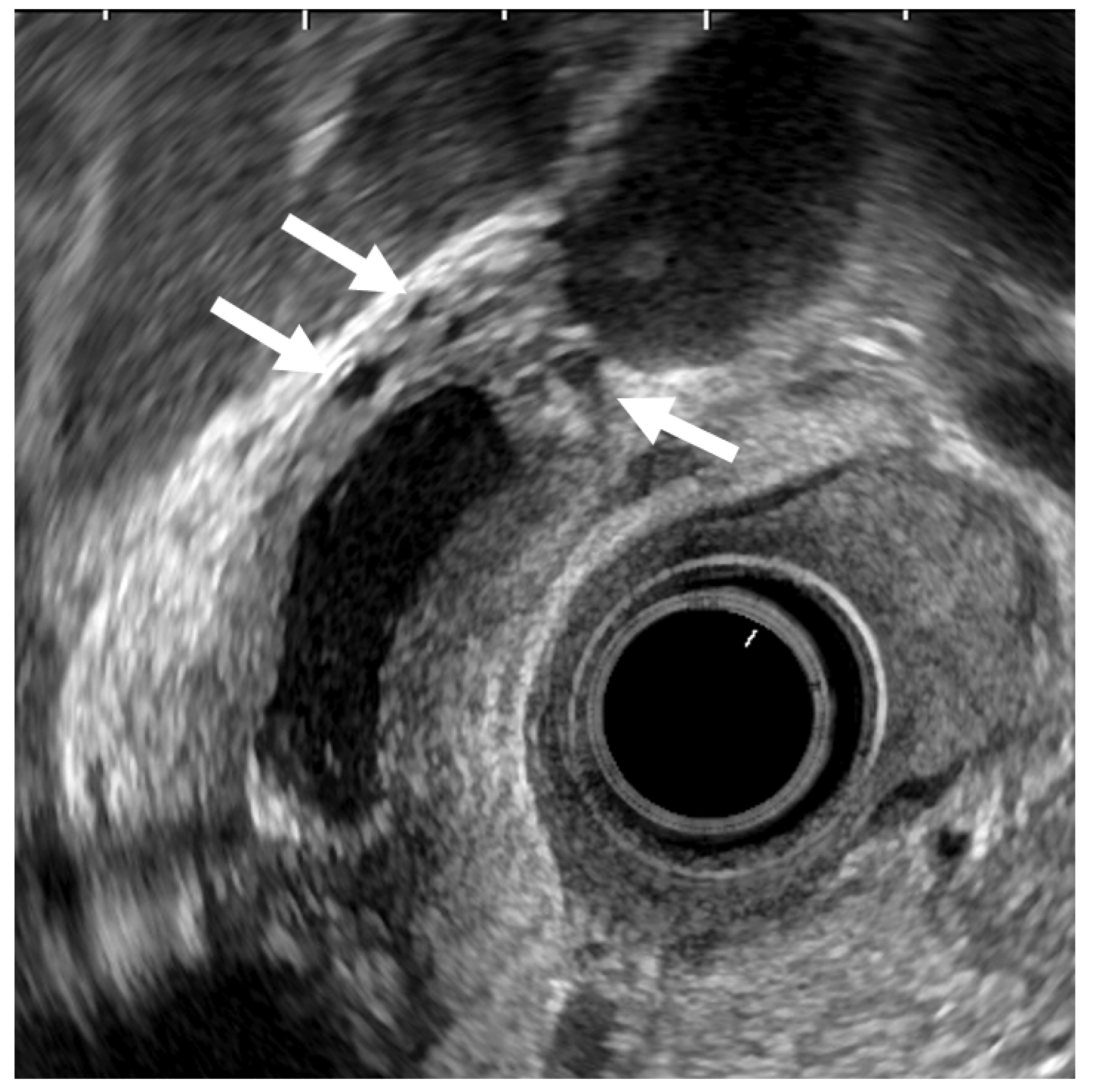
Figure 4.
EUS image of ADM (diffuse type). The GB wall is diffusely thickened, and the layers of a thickened GB wall are preserved. Some anechoic areas (arrows) are visualized in the GB thickened wall.
Xanthogranulomatous cholecystitis (XGC) is an uncommon disease involving chronic GB inflammation. Its clinical presentation is like that of cholecystitis, making it very difficult to distinguish from GB carcinoma because of marked tissue-destructive changes. In addition, imaging findings resemble those of GB carcinomas. EUS can sometimes visualize hyperechoic nodules in the GB wall, probably representing XGC. However, differentiation between benign and malignant types based on EUS alone is frequently difficult [41]. A report also exists that EUS-FNA was useful for preoperative differential diagnosis between GB carcinoma and XGC [42].
Epithelial height is increased, cellular proliferative activity is accelerated, and a mechanism from hyperplasia to dysplasia and carcinoma is speculated in hyperplasia of the gallbladder mucous membrane accompanying anomalous pancreaticobiliary junction because anomalous pancreaticobiliary junction permits reflux of pancreatic juice into the bile duct. EUS can delineate abnormal connections between pancreatobiliary ducts as clearly as endoscopic retrograde cholangiopancreatography [43]. GB lesions should be suspected to be malignant when EUS shows abnormal connections between pancreatobiliary ducts (Figure 5).
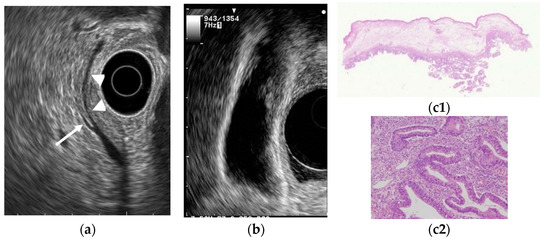
Figure 5.
GB carcinoma is associated with pancreaticobiliary maljunction without biliary dilatation. (a) EUS shows the bile duct (arrowhead) and main pancreatic duct (arrow) communicated inside the pancreas. (b) EUS shows the irregular GB wall-thickening on fundus without wall layer structure disruption. (c1,c2) H–E stain of the specimen demonstrates adenocarcinoma with tumor in situ stage.
Differentiation from GB ADM and chronic cholecystitis is problematic in wall-thickening-type GB carcinoma. However, the mucous membrane is irregular or papillated, thickened areas do not have a uniform thickness, and the layered structure is ill-defined in GB carcinoma. Furthermore, microcysts and comet tail artifacts reflecting RAS are usually not observed. Mizuguchi et al. [44] reported that the loss of multiple layer patterns of the GB wall demonstrated by EUS was the most specific finding in diagnosing GB carcinoma. Kim et al. [45] noted that EUS findings of GB wall thickness exceeding 10 mm and hypoechoic internal echogenicity as independent predictive factors for neoplasm. However, differentiating malignant lesions from benign GB wall-thickening remains difficult.
5. GB Carcinoma Staging
EUS has been used in GB carcinoma staging because it can demonstrate the multilayer GB wall structure. In 1990, Mitake et al. [46] reported the effectiveness of EUS in determining tumor-invasion extent. The differentiation between early and advanced-stage tumors was 79.5% accurate, and the overall accuracy for depth of tumor invasion was 76.9%. No detailed examination was made in this study on whether each layer structure of the GB wall delineated by EUS corresponds to the mucosa, muscularis propria, subserosal layer, or serosa. In 1995, Fujita et al. [47] analyzed the layer structure of the GB wall delineated by EUS in detail using the pinning method for excised specimens. Consequently, the inner hypoechoic layer in a two-layer structure was clarified, and the hypoechoic middle layer in a three-layer structure includes not only the muscularis propria but also the fibrous layer of the subserosa. The outermost hyperechoic layer represents the adipose layer of the subserosa and the serosa. In 1998, Watanabe et al. [48] also analyzed the layer structure of the GB wall delineated by using intraductal ultrasonography, which has a higher frequency than EUS. The GB wall was composed of three layers: the innermost, middle, and outermost layers. Little fibrous tissue was seen and the second layer on sonograms was approximately identical to the muscularis propria in cases in which the thickness of the second layer was <500 µm. However, the second layer included not only the muscularis propria but also the fibrous layer of the subserosa in cases in which the thickness of the second layer was >500 µm. A consensus almost exists if GB wall-thickening is present, the inner hypoechoic layer includes not only the muscularis propria but also the fibrous layer of the subserosa, and the outer hyperechoic layer includes the adipose layer of the subserosa and serosa.
Fujita et al. [49] classified EUS images into four categories: type A, a pedunculated mass with a finely nodular surface and without abnormality of the neighboring gallbladder wall; type B, a broad-based mass with an irregular surface and no disruption of the outer hyperechoic layer of the gallbladder wall; type C, irregularity of the outer hyperechoic layer due to mass echo; and type D, disruption of the outer hyperechoic layer by mass echo (Figure 6). They then assigned the image types EUS to T stages for GB carcinomas. Type A would be a tumor in situ (Tis); type B, T1 or possibly T2; type C, T2; and type D, T3 or higher. Each of the four EUS image categories correlated well with the histologic invasion depth (Table 5).
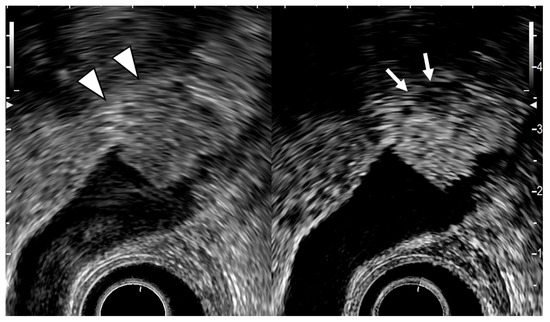
Figure 6.
EUS image of GB carcinoma. The conventional EUS (left) shows that a broad-based elevated lesion is found at the fundus of the GB with hypoechoic (arrowhead) in the deep part of the lesion and rupture of the lateral hyperechoic layer. In the contrast-enhanced EUS (right), the contrast effect of most of the lesion is good, but the deep part of the lesion is poorly contrasted (arrow). It can be diagnosed from these findings as GB carcinoma with invasion depth T3a (SE).

Table 5.
EUS classification of GB carcinoma and correlation with T staging (adapted from [49]).
The following is a summary of the diagnosis of the depth of invasion by EUS.
- Those whose lesions can be diagnosed as pedunculated GB carcinomas can be diagnosed as Tis or T1a (M) depth of invasion.
- Sessile gallbladder carcinomas with thinning or irregularity of the outer hyperechoic layer can be diagnosed as gallbladder carcinoma with T2 (SS) invasion depth.
- In cases where the outer hyperechoic layer is retained, the depth of GB carcinoma invasion may extend to T1a(M), T1b(MP), or T2(SS), depending on the case, and differentiation is impossible even by EUS.
6. Contrast-Enhanced EUS
Contrast-enhanced EUS has been used to improve diagnostic accuracy based on the different levels of vascularity and blood flow that are found across different pathologic processes. Limited studies of contrast-enhanced EUS exist in the differential diagnosis of GB polyps or GB wall-thickening while many studies using contrast-enhanced EUS imaging have focused on pancreatic lesions. Hirooka et al. [50] reported that enhancement was observed in GB adenocarcinomas by contrast-enhanced endoscopic ultrasonography using sonicated albumin, but not in adenosquamous carcinomas and cholesterol polyps. They also reported that the depth of tumor invasion was assessed accurately in 11 of 14 cases (78.6%) in noncontrast EUS, while the assessment was accurate in 13 of 14 cases (92.9%) using contrast-enhanced EUS (Table 6). Latter studies were based on the second-generation contrast agents (e.g., SonoVue® and Sonazoid®). The perfusion patterns were classified as diffuse enhancement, perfusion defect, and without enhancement in a contrast-enhanced harmonic EUS study by Choi et al. [51]. The vessels were categorized as regular spotty, irregular, or no vessels. This study reported that the presence of irregular vessel pattern and the perfusion defect on contrast-enhanced EUS can diagnose GB carcinomas in GB polyps measuring at least 10 mm with a sensitivity and specificity of 93.5% and 93.2%, respectively, versus 90.0% and 91.1% for conventional EUS. Kamata et al. [52] also reported that GB carcinoma was characterized by irregular vessels in the vascular image and heterogeneous enhancement in the perfusion image (Figure 7). The sensitivity, specificity, and accuracy for the diagnosis of carcinoma on contrast-enhanced harmonic EUS was 90%, 98%, and 96%, respectively, in this study.

Table 6.
The sensitivity and specificity on CH-EUS for the diagnosis of GB malignancy.
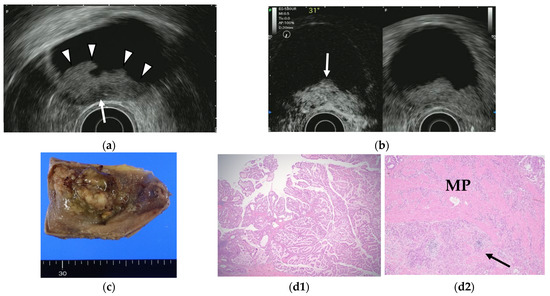
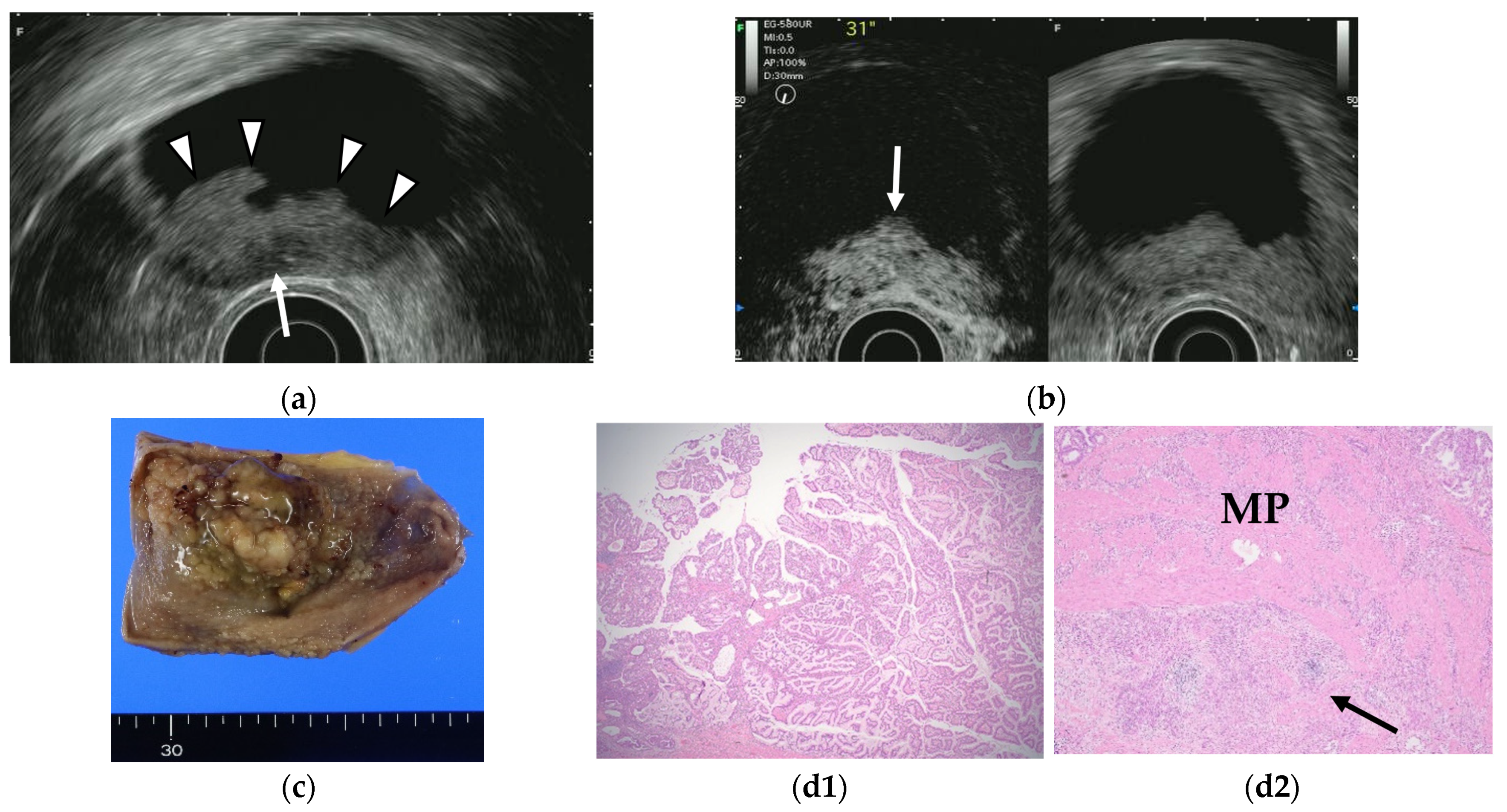
Figure 7.
GB carcinoma. (a) Conventional EUS shows elevated lesions with conspicuous surface irregularities (arrowheads) observed in the gallbladder body. A hypoechoic region is observed in the deep part of the lesion (arrow), and the outer hyperechoic layer is also irregular, suggesting infiltration into the subserosal layer. (b) The contrast-enhanced harmonic EUS image after the injection of Sonazoid® shows that lesions in the gallbladder body (arrow) have a strong heterogeneous staining effect from the early stage of contrast enhancement. (Left contrast-enhanced harmonic mode, right B-mode) (c) Photograph of the gross pathologic specimen after cholecystectomy shows that the papillary neoplasm with a maximum diameter of 55 mm is found from the body to the bottom of the gallbladder. (d1,d2): H-E stain of the specimen demonstrates atypical epithelial cells grow papillary. Infiltration into the subserosal layer is observed in a part of the deep part of the tumor with infiltration and hyperplasia of poorly differentiated adenocarcinoma. (MP muscularis propria).
Another study by Imazu et al. [53] using contrast-enhanced EUS in the differential diagnosis of GB wall-thickening demonstrated inhomogeneous enhancement as a strong predictive factor of malignant GB wall-thickening (Figure 8). The same study reported that overall sensitivity, specificity, and accuracy for diagnosing malignant GB wall-thickening for EUS and contrast-enhanced EUS, respectively, were 83.3% versus 89.6%, 65% versus 98% (p < 0.001), and 73.1% versus 94.4% (p < 0.001).
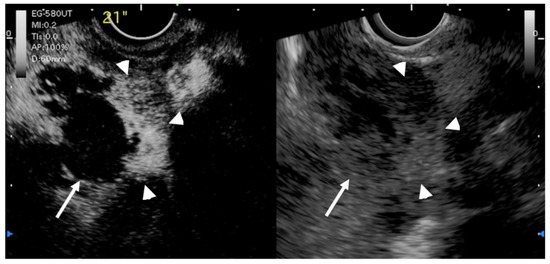
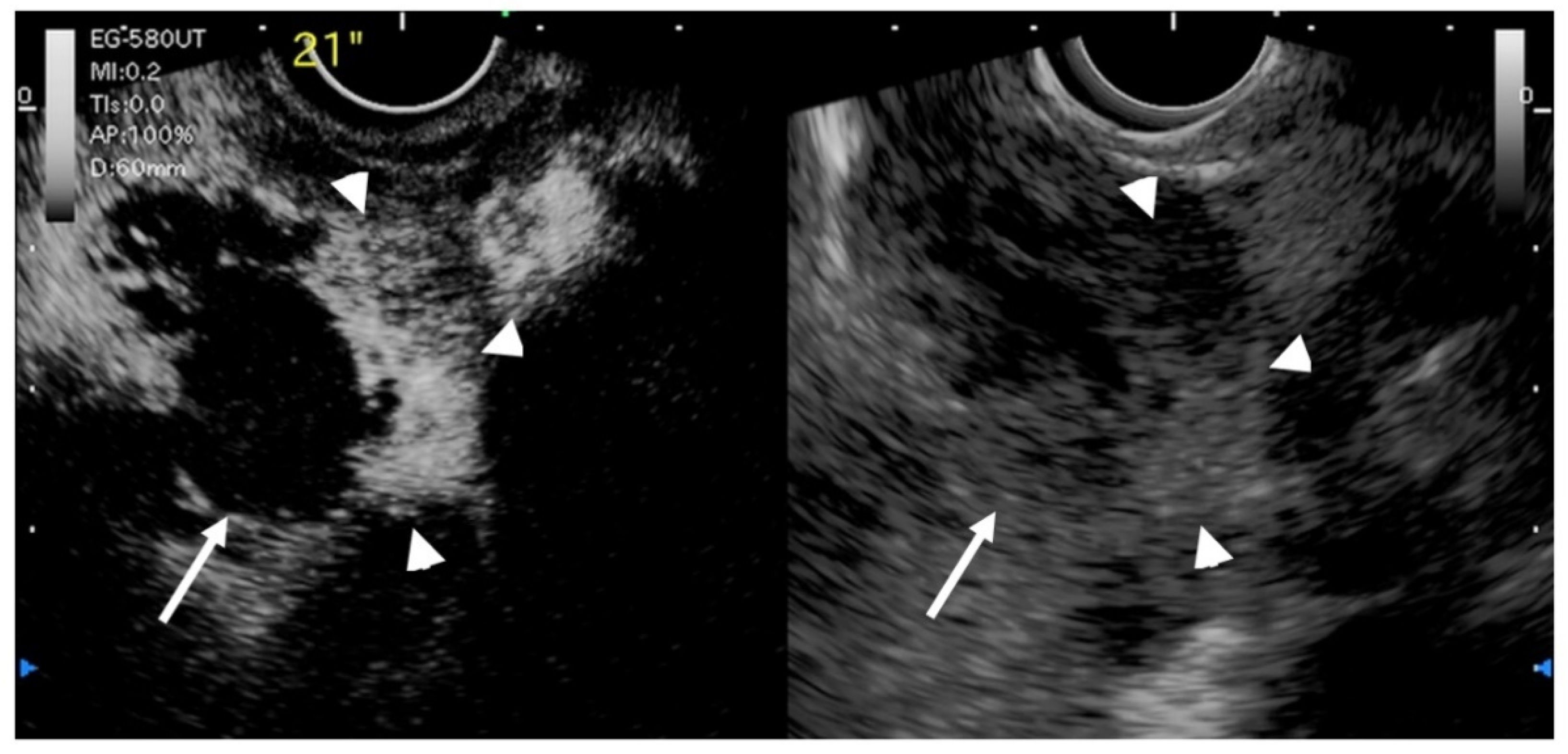
Figure 8.
EUS image of GB carcinoma. Irregular wall-thickening of the GB (arrowhead) is observed. In the conventional EUS image (right), a structure is found inside the GB and the lumen is unknown. The contrast-enhanced harmonic image 21 s after the injection of Sonazoid® (left) shows heterogeneous enhancement in the thickened wall (arrowhead). The structure inside the GB is not enhanced and can be diagnosed as biliary sludge (arrow) rather than a neoplasm.
Xue Liang and Xiang Jing [56] reported a meta-analysis of contrast-enhanced ultrasound and contrast-enhanced harmonic EUS (CH-EUS) for the diagnosis of GB malignancy. The pooled sensitivities of CH-EUS and specificities were 0.92 and 0.89 (Table 6), respectively, in this meta-analysis. On CH-EUS, the heterogeneous enhancement could be indicative of malignant lesions with a sensitivity and specificity of 0.94 and 0.92, respectively.
However, further accumulation of knowledge is desired because no large-scale study on contrast-enhanced harmonic EUS in GB diseases to date.
7. EUS-FNA for GB Lesions
The EUS-FNA role in tissue sampling for pancreatic and gastrointestinal lesions has been established. However, its role in the diagnosis of GB lesions has not been elucidated. The pathological diagnosis of GB lesions to rely on cytological examination of the bile obtained by endoscopic transpapillary gallbladder drainage tube is often necessary [57]. However, cases are sometimes experienced where a definitive diagnosis cannot be made pathologically even with this method. In addition, cytology using endoscopic naso-GB drainage has problems (e.g., perforation of the cystic duct when using a guidewire). In such cases, the pathological search by EUS-FNA is useful if the tumor can be visualized by EUS. Hijioka et al. [58] have reported that FNA can be performed in GB lesions without compromising diagnostic performance or safety. Moreover, the diagnostic performance of EUS-FNA in GB lesions is high with a sensitivity, specificity, and diagnostic accuracy of 80–100%, 100%, and 83–100%, respectively (Table 7) [58,59,60,61,62,63], which was superior to endoscopic transpapillary GB aspiration with cytology [62] or endoscopic retrograde cholangiography-guided sampling [58]. No report exists of bile peritonitis or tumor seeding in EUS-FNA in GB disease.

Table 7.
The results on EUS-FNA for the diagnosis of GB malignancy.
EUS-FNA are not recommended for resectable GB carcinoma, because this procedure may induce biliary peritonitis and would also cause peritoneal dissemination, like transabdominal US-guided FNA cytology [65]. The strategy for obtaining tissue from gallbladder tumors is first to try to obtain tissue from gallbladder tumors by endoscopic retro grade cholangiography (ERC) biopsy, then from liver or lymph node metastases by EUS-FNA, and finally from gallbladder tumors by EUS-FNA [66]. Indications of EUS-FNA of gallbladder mass lesions should include the following:
- GB large lesions; only if the lesion can be punctured without the needle passing through the GB lumen (Figure 9) or lesions with the very thickened GB wall [67].
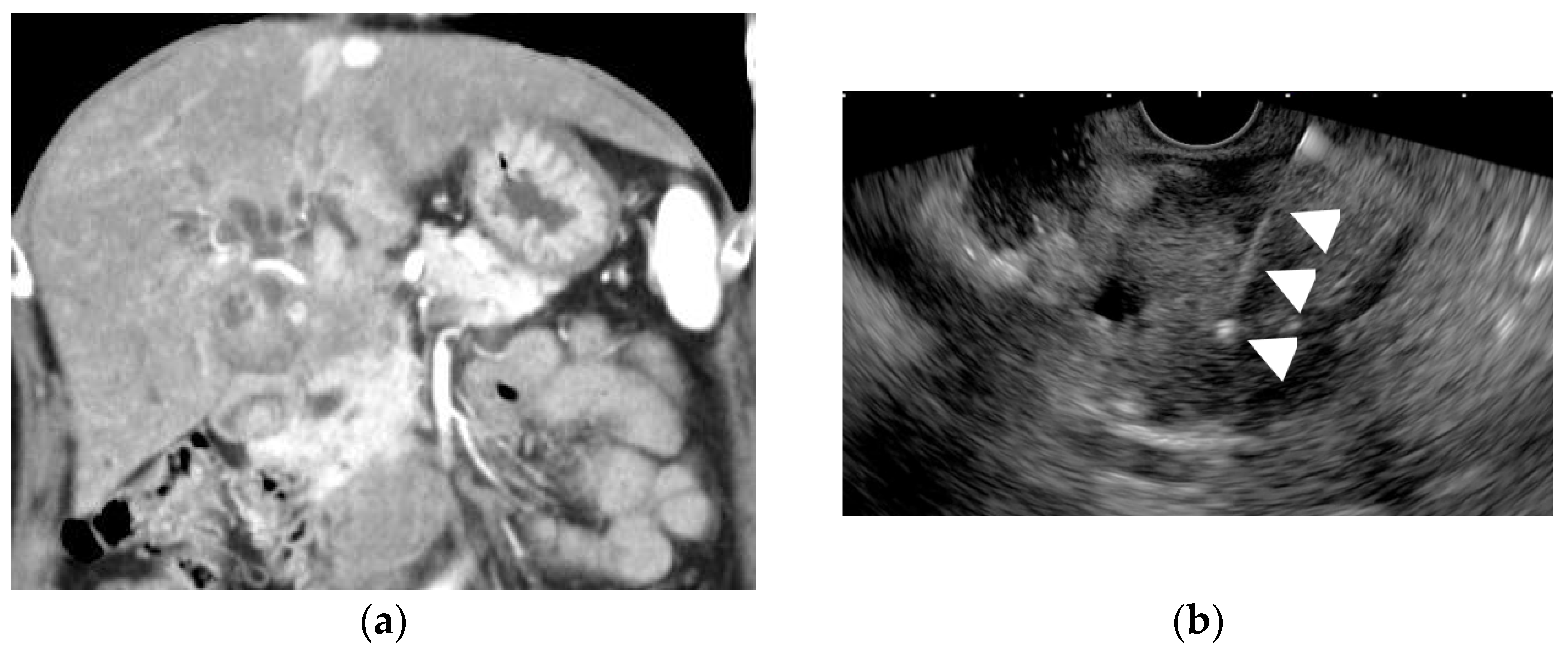
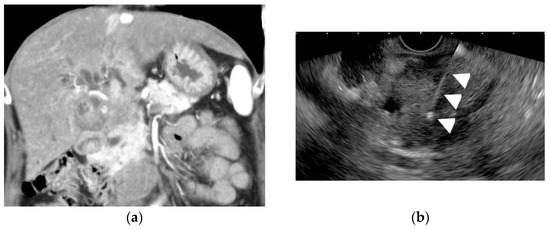 Figure 9. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for a GB lesion. (a) Enhanced CT scan shows a GB lesion. (b) EUS-FNA of a GB mass lesion. The arrowhead shows the FNA needle inside the lesion. The pathology result showed adenocarcinoma.
Figure 9. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for a GB lesion. (a) Enhanced CT scan shows a GB lesion. (b) EUS-FNA of a GB mass lesion. The arrowhead shows the FNA needle inside the lesion. The pathology result showed adenocarcinoma. - In patients with gallbladder tumors accompanied by liver and/or lymph node metastasis, the liver and/or lymph node metastasis should be punctured before the gallbladder tumor is punctured.
- When it is difficult to categorize a lesion as benign or malignant, or when the surgery is extremely invasive, EUS-FNA should beconsidered.
When taking a sample from the gallbladder, it is important that the needle does not pass through the lumen of the gallbladder [68]. When directly puncturing the GB wall, it takes care to gain stroke distance by tangentially puncturing the gallbladder wall. However, the wall may move if the gallbladder lumen remains, and puncturing is often difficult. Thus, it is best to puncture the neck side of GB. In cases where lesions have invaded the liver, it is recommended to puncture either the liver parenchyma as the invasion site or the gallbladder wall that is in contact with the liver parenchyma. Regional lymphadenopathy is often noted in unresectable advanced GB carcinoma. EUS-FNA from the regional lymph nodes is preferable considering the risks (e.g., invasive biliary fistula and peritoneal dissemination).
8. Conclusions
EUS provides higher resolution images of GB lesions using the higher frequency and closer proximity to GB when compared to conventional TUS. The EUS is highly useful for the detection of GB lesions, the differential diagnosis of GB polypoid lesions and GB wall thickness, and the evaluation of staging of GB carcinomas. In the future, the importance of EUS in this field is expected to grow further with the use and establishment of contrast-enhanced EUS and EUS-FNA for GB lesions.
Author Contributions
S.H., R.M. searched and reviewed previous papers under the supervision of K.N., T.K., N.K., T.S. and Y.H.; N.K., K.F. drafted the manuscript and Y.H., M.N., Y.N. edited it. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the review of previously published articles except for the sample pictures of nine cases.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data supporting reported results can be found in the references at the end of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stinton, L.M.; Shaffer, E.A. Epidemiology of gallbladder disease: Cholelithiasis and cancer. Gut Liver 2012, 6, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Segawa, K.; Arisawa, T.; Niwa, Y.; Suzuki, T.; Tsukamoto, Y.; Goto, H.; Hamajima, E.; Shimodaira, M.; Ohmiya, N. Prevalence of gallbladder polyps among apparently healthy Japanese: Ultrasonographic study. Am. J. Gastroenterol. 1992, 87, 630–633. [Google Scholar]
- Okamoto, M.; Okamoto, H.; Kitahara, F.; Kobayashi, K.; Karikome, K.; Miura, K.; Matsumoto, Y.; Fujino, M.A. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am. J. Gastroenterol. 1999, 94, 446–450. [Google Scholar] [CrossRef]
- Lin, W.R.; Lin, D.Y.; Tai, D.I.; Hsieh, S.Y.; Lin, C.Y.; Sheen, I.S.; Chiu, C.T. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: Analysis of 34 669 cases. J. Gastroenterol. Hepatol. 2008, 23, 965–969. [Google Scholar] [CrossRef]
- Kanthan, R.; Senger, J.L.; Ahmed, S.; Kanthan, S.C. Gallbladder Cancer in the 21st Century. J. Oncol. 2015, 2015, 967472. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.H.; Lau, W.Y. Gallbladder cancer—A comprehensive review. Surgeon 2008, 6, 101–110. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Vijayakumar, A.; Patil, V.; Mallikarjuna, M.N.; Shivaswamy, B.S. Early diagnosis of gallbladder carcinoma: An algorithm approach. ISRN Radiol. 2013, 2013, 239424. [Google Scholar] [CrossRef] [PubMed]
- Wennmacker, S.Z.; Lamberts, M.P.; Di Martino, M.; Drenth, J.P.; Gurusamy, K.S.; van Laarhoven, C.J. Transabdominal ultrasound and endoscopic ultrasound for diagnosis of gallbladder polyps. Cochrane Database Syst. Rev. 2018, 8, Cd012233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijioka, S.; Okusaka, T. Enormous Potential of Endoscopic Ultrasound-guided Liver Biopsies. Intern. Med. 2021, 60, 1655–1656. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, P.L.; Burhenne, H.J. Real-time ultrasonography. Diagnostic technique of choice in calculous gallbladder disease. N. Engl. J. Med. 1980, 302, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
- Dahan, P.; Andant, C.; Lévy, P.; Amouyal, P.; Amouyal, G.; Dumont, M.; Erlinger, S.; Sauvanet, A.; Belghiti, J.; Zins, M.; et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut 1996, 38, 277–281. [Google Scholar] [CrossRef]
- Thorbøll, J.; Vilmann, P.; Jacobsen, B.; Hassan, H. Endoscopic ultrasonography in detection of cholelithiasis in patients with biliary pain and negative transabdominal ultrasonography. Scand. J. Gastroenterol. 2004, 39, 267–269. [Google Scholar] [CrossRef]
- Ardengh, J.C.; Malheiros, C.A.; Rahal, F.; Pereira, V.; Ganc, A.J. Microlithiasis of the gallbladder: Role of endoscopic ultrasonography in patients with idiopathic acute pancreatitis. Rev. Assoc. Med. Bras. 2010, 56, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Sun, Y.G.; Wang, Z. Polypoid lesions of the gallbladder: Diagnosis and indications for surgery. Br. J. Surg. 1992, 79, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Terzi, C.; Sökmen, S.; Seçkin, S.; Albayrak, L.; Uğurlu, M. Polypoid lesions of the gallbladder: Report of 100 cases with special reference to operative indications. Surgery 2000, 127, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Yoshino, J.; Miyoshi, H. Diagnosis of gallbladder tumors. Intern. Med. 2011, 50, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Okaniwa, S. Role of conventional ultrasonography in the diagnosis of gallbladder polypoid lesions. J. Med. Ultrason. 2021, 48, 149–157. [Google Scholar] [CrossRef]
- Okaniwa, S. Everything you need to know about ultrasound for diagnosis of gallbladder diseases. J. Med. Ultrason. 2021, 48, 145–147. [Google Scholar] [CrossRef]
- Okaniwa, S. How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound? Diagnostics 2021, 11, 784. [Google Scholar] [CrossRef]
- Mellnick, V.M.; Menias, C.O.; Sandrasegaran, K.; Hara, A.K.; Kielar, A.Z.; Brunt, E.M.; Doyle, M.B.; Dahiya, N.; Elsayes, K.M. Polypoid lesions of the gallbladder: Disease spectrum with pathologic correlation. Radiographics 2015, 35, 387–399. [Google Scholar] [CrossRef]
- van Breda Vriesman, A.C.; Engelbrecht, M.R.; Smithuis, R.H.; Puylaert, J.B. Diffuse gallbladder wall thickening: Differential diagnosis. AJR Am. J. Roentgenol. 2007, 188, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Atomi, Y.; Yamato, T. Endoscopic ultrasonography for differential diagnosis of polypoid gall bladder lesions: Analysis in surgical and follow up series. Gut 2000, 46, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, Y.K.; Cho, W.Y.; Lee, T.H.; Cho, Y.D.; Moon, J.H.; Lee, J.S.; Shim, C.S. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J. Gastroenterol. 2009, 15, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K. Differential diagnosis of large-sized pedunculated polypoid lesions of the gallbladder by endoscopic ultrasonography: A prospective study. J. Gastroenterol. 2001, 36, 619–622. [Google Scholar] [CrossRef]
- Azuma, T.; Yoshikawa, T.; Araida, T.; Takasaki, K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am. J. Surg. 2001, 181, 65–70. [Google Scholar] [CrossRef]
- Tanaka, K.; Katanuma, A.; Hayashi, T.; Kin, T.; Takahashi, K. Role of endoscopic ultrasound for gallbladder disease. J. Med. Ultrason. 2021, 48, 187–198. [Google Scholar] [CrossRef]
- Akatsu, T.; Aiura, K.; Shimazu, M.; Ueda, M.; Wakabayashi, G.; Tanabe, M.; Kawachi, S.; Kitajima, M. Can endoscopic ultrasonography differentiate nonneoplastic from neoplastic gallbladder polyps? Dig. Dis. Sci. 2006, 51, 416–421. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, J.Y.; Kim, Y.J.; Kim, H.M.; Kim, H.J.; Hong, S.P.; Park, S.W.; Chung, J.B.; Song, S.Y.; Bang, S. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest. Endosc. 2009, 69, 1244–1250. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Kim, Y.J.; Ryu, J.K.; Kim, Y.T.; Lee, J.Y.; Han, J.K. Diagnostic accuracy of transabdominal high-resolution US for staging gallbladder cancer and differential diagnosis of neoplastic polyps compared with EUS. Eur. Radiol. 2017, 27. [Google Scholar] [CrossRef]
- Christensen, A.H.; Ishak, K.G. Benign tumors and pseudotumors of the gallbladder. Report of 180 cases. Arch. Pathol. 1970, 90, 423–432. [Google Scholar]
- Cocco, G.; Basilico, R.; Delli Pizzi, A.; Cocco, N.; Boccatonda, A.; D’Ardes, D.; Fabiani, S.; Anzoletti, N.; D’Alessandro, P.; Vallone, G.; et al. Gallbladder polyps ultrasound: What the sonographer needs to know. J. Ultrasound. 2021, 24, 131–142. [Google Scholar] [CrossRef]
- Sadamoto, Y.; Oda, S.; Tanaka, M.; Harada, N.; Kubo, H.; Eguchi, T.; Nawata, H. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy 2002, 34, 959–965. [Google Scholar] [CrossRef]
- Choi, W.B.; Lee, S.K.; Kim, M.H.; Seo, D.W.; Kim, H.J.; Kim, D.I.; Park, E.T.; Yoo, K.S.; Lim, B.C.; Myung, S.J.; et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest. Endosc. 2000, 52, 372–379. [Google Scholar] [CrossRef]
- Kimura, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Horaguchi, J.; Takasawa, O. A case of pedunculated polypoid cancer associated with flat-type cancer of the gallbladdeR. Dig. Endosc. 2005, 17, 93–96. [Google Scholar] [CrossRef]
- Kawarada, Y.; Sanda, M.; Mizumoto, R.; Yatani, R. Early carcinoma of the gallbladder, noninvasive carcinoma originating in the Rokitansky-Aschoff sinus: A case report. Am. J. Gastroenterol. 1986, 81, 61–66. [Google Scholar] [PubMed]
- Kurihara, K.; Mizuseki, K.; Ninomiya, T.; Shoji, I.; Kajiwara, S. Carcinoma of the gall-bladder arising in adenomyomatosis. Acta Pathol. Jpn. 1993, 43, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Nakai, T.; Hayashi, S.; Satake, T. Noninvasive carcinoma of the gallbladder arising in localized type adenomyomatosis. Am. J. Gastroenterol. 1988, 83, 670–674. [Google Scholar]
- Nabatame, N.; Shirai, Y.; Nishimura, A.; Yokoyama, N.; Wakai, T.; Hatakeyama, K. High risk of gallbladder carcinoma in elderly patients with segmental adenomyomatosis of the gallbladder. J. Exp. Clin. Cancer Res. 2004, 23, 593–598. [Google Scholar]
- Kai, K.; Ide, T.; Masuda, M.; Kitahara, K.; Miyoshi, A.; Miyazaki, K.; Noshiro, H.; Tokunaga, O. Clinicopathologic features of advanced gallbladder cancer associated with adenomyomatosis. Virchows Arch. 2011, 459, 573–580. [Google Scholar] [CrossRef]
- Miyoshi, H.; Inui, K.; Katano, Y.; Tachi, Y.; Yamamoto, S. B-mode ultrasonographic diagnosis in gallbladder wall thickening. J. Med. Ultrason. 2021, 48, 175–186. [Google Scholar] [CrossRef]
- Muguruma, N.; Okamura, S.; Okahisa, T.; Shibata, H.; Ito, S.; Yagi, K. Endoscopic sonography in the diagnosis of xanthogranulomatous cholecystitis. J. Clin. Ultrasound. 1999, 27, 347–350. [Google Scholar] [CrossRef]
- Hijioka, S.; Mekky, M.A.; Bhatia, V.; Sawaki, A.; Mizuno, N.; Hara, K.; Hosoda, W.; Shimizu, Y.; Tamada, K.; Niwa, Y.; et al. Can EUS-guided FNA distinguish between gallbladder cancer and xanthogranulomatous cholecystitis? Gastrointest. Endosc. 2010, 72, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Mitake, M.; Nakazawa, S.; Naitoh, Y.; Kimoto, E.; Tsukamoto, Y.; Yamao, K.; Inui, K. Value of endoscopic ultrasonography in the detection of anomalous connections of the pancreatobiliary duct. Endoscopy 1991, 23, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, M.; Kudo, S.; Fukahori, T.; Matsuo, Y.; Miyazaki, K.; Tokunaga, O.; Koyama, T.; Fujimoto, K. Endoscopic ultrasonography for demonstrating loss of multiple-layer pattern of the thickened gallbladder wall in the preoperative diagnosis of gallbladder cancer. Eur. Radiol. 1997, 7, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.H.; Park, D.I.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Choi, S.H. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig. Dis. Sci. 2012, 57, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Mitake, M.; Nakazawa, S.; Naitoh, Y.; Kimoto, E.; Tsukamoto, Y.; Asai, T.; Yamao, K.; Inui, K.; Morita, K.; Hayashi, Y. Endoscopic ultrasonography in diagnosis of the extent of gallbladder carcinoma. Gastrointest. Endosc. 1990, 36, 562–566. [Google Scholar] [CrossRef]
- Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Yago, A.; Mochizuki, F. Analysis of the Layer Structure of the Gallbladder Wall Delineated by Endoscopic Ultrasound Using the Pinning Method. Dig. Endosc. 1995, 7, 353–356. [Google Scholar] [CrossRef]
- Watanabe, Y.; Goto, H.; Naitoh, Y.; Hirooka, Y.; Itoh, A.; Taki, T.; Hayakawa, S.; Hayakawa, T. Usefulness of intraductal ultrasonography in gallbladder disease. J. Ultrasound Med. 1998, 17, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Yago, A. Diagnosis of the depth of invasion of gallbladder carcinoma by EUS. Gastrointest. Endosc. 1999, 50, 659–663. [Google Scholar] [CrossRef]
- Hirooka, Y.; Naitoh, Y.; Goto, H.; Ito, A.; Hayakawa, S.; Watanabe, Y.; Ishiguro, Y.; Kojima, S.; Hashimoto, S.; Hayakawa, T. Contrast-enhanced endoscopic ultrasonography in gallbladder diseases. Gastrointest. Endosc. 1998, 48, 406–410. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, D.W.; Choi, J.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest. Endosc. 2013, 78, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Nishida, N.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig. Endosc. 2018, 30, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Imazu, H.; Mori, N.; Kanazawa, K.; Chiba, M.; Toyoizumi, H.; Torisu, Y.; Koyama, S.; Hino, S.; Ang, T.L.; Tajiri, H. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig. Dis. Sci. 2014, 59, 1909–1916. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Konno, N.; Suzuki, R.; Asama, H.; Hikichi, T.; Watanabe, K.; Waragai, Y.; Kikuchi, H.; Takasumi, M.; et al. The efficacy of contrast-enhanced harmonic endoscopic ultrasonography in diagnosing gallbladder cancer. Sci. Rep. 2016, 6, 25848. [Google Scholar] [CrossRef]
- Leem, G.; Chung, M.J.; Park, J.Y.; Bang, S.; Song, S.Y.; Chung, J.B.; Park, S.W. Clinical Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography in the Differential Diagnosis of Pancreatic and Gallbladder Masses. Clin. Endosc. 2018, 51, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Jing, X. Meta-analysis of contrast-enhanced ultrasound and contrast-enhanced harmonic endoscopic ultrasound for the diagnosis of gallbladder malignancy. BMC Med. Inform. Decis. Mak. 2020, 20, 235. [Google Scholar] [CrossRef]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Moriyasu, F.; Yamagishi, T.; Serizawa, H. Preoperative diagnosis and management of thick-walled gallbladder based on bile cytology obtained by endoscopic transpapillary gallbladder drainage tube. Gastrointest. Endosc. 2006, 64, 512–519. [Google Scholar] [CrossRef]
- Hijioka, S.; Hara, K.; Mizuno, N.; Imaoka, H.; Ogura, T.; Haba, S.; Mekky, M.A.; Bhatia, V.; Hosoda, W.; Yatabe, Y.; et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J. Hepatobiliary Pancreat Sci. 2012, 19, 650–655. [Google Scholar] [CrossRef]
- Jacobson, B.C.; Pitman, M.B.; Brugge, W.R. EUS-guided FNA for the diagnosis of gallbladder masses. Gastrointest. Endosc. 2003, 57, 251–254. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Eloubeidi, M.A. Endoscopic ultrasound-guided fine-needle aspiration in the evaluation of gallbladder masses. Endoscopy 2005, 37, 751–754. [Google Scholar] [CrossRef]
- Meara, R.S.; Jhala, D.; Eloubeidi, M.A.; Eltoum, I.; Chhieng, D.C.; Crowe, D.R.; Varadarajulu, S.; Jhala, N. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: Analysis of 53 cases. Cytopathology 2006, 17, 42–49. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.K.; Jang, J.W.; Kim, T.G.; Ryu, C.H.; Park, D.H.; Lee, S.S.; Seo, D.W.; Kim, M.H. Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions. Hepatogastroenterology 2012, 59, 1691–1695. [Google Scholar] [CrossRef]
- Ogura, T.; Kurisu, Y.; Masuda, D.; Imoto, A.; Onda, S.; Kamiyama, R.; Hayashi, M.; Mohamed, M.; Uchiyama, K.; Higuchi, K. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for thick-walled gallbladders? Dig. Dis. Sci. 2014, 59, 1917–1924. [Google Scholar] [CrossRef]
- Singla, V.; Agarwal, R.; Anikhindi, S.A.; Puri, P.; Kumar, M.; Ranjan, P.; Kumar, A.; Sharma, P.; Bansal, N.; Bakshi, P.; et al. Role of EUS-FNA for gallbladder mass lesions with biliary obstruction: A large single-center experience. Endosc. Int. Open 2019, 7, E1403–E1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepatobiliary Pancreat Sci. 2021, 28, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yamashita, Y.; Itonaga, M.; Ashida, R.; Kitano, M. Usefulness of EUS-FNA with contrast-enhanced harmonic imaging for diagnosis of gallbladder tumor. Endosc. Ultrasound. 2021, 10, 224–226. [Google Scholar] [CrossRef]
- Chantarojanasiri, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Kongkam, P.; Goto, H. The role of endoscopic ultrasound in the diagnosis of gallbladder diseases. J. Med. Ultrason. 2017, 44, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Nagashio, Y.; Ohba, A.; Maruki, Y.; Okusaka, T. The Role of EUS and EUS-FNA in Differentiating Benign and Malignant Gallbladder Lesions. Diagnostics 2021, 11, 1586. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).