The Current Approach to the Diagnosis and Classification of Mirizzi Syndrome
Abstract
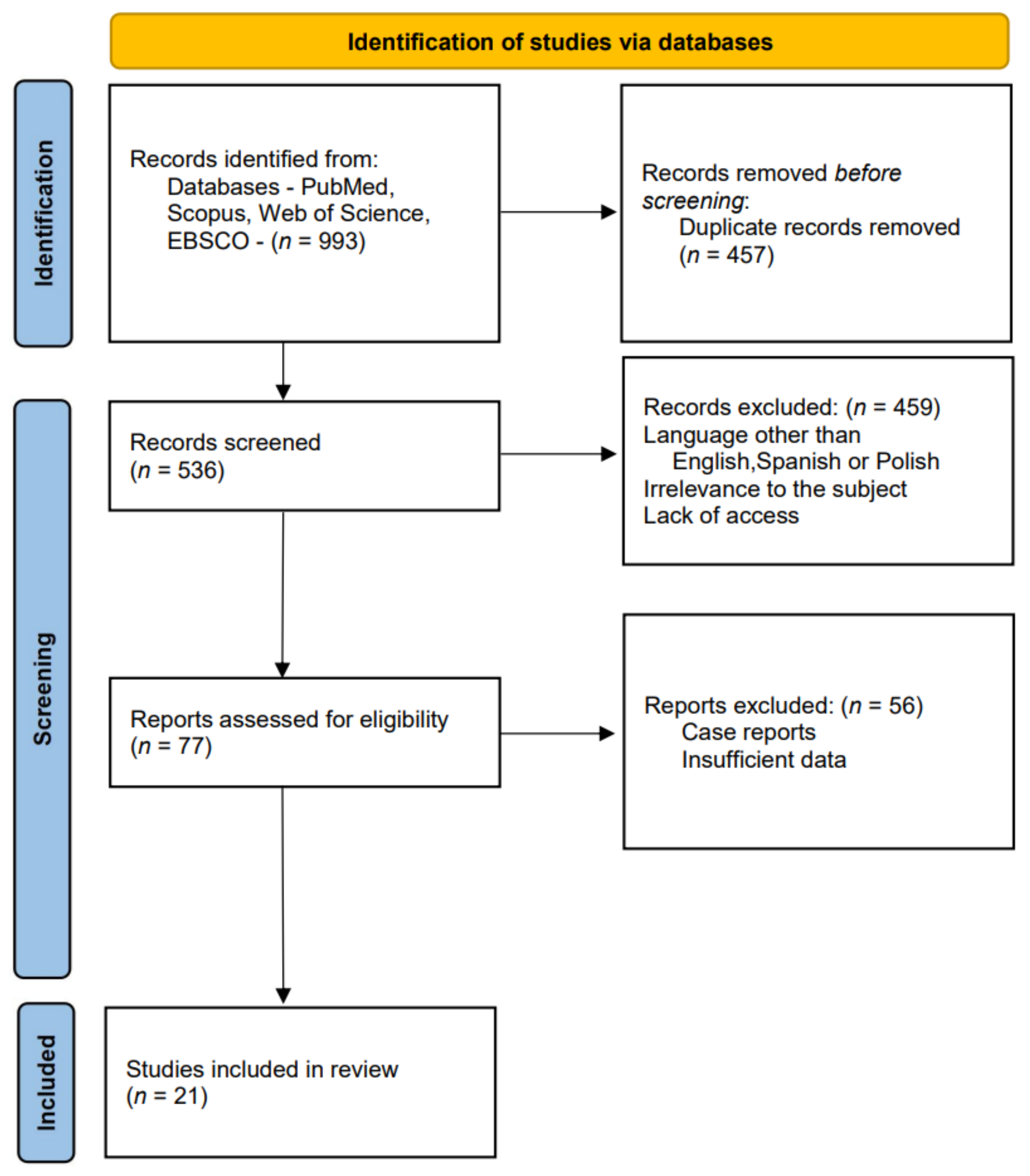
:1. Introduction
1.1. Pathophysiology
- Atrophic gallbladder with thick or thin walls;
- Obliterated cystic duct;
- Cystic duct—long, parallel to the common bile duct and with low insertion;
- Cystic duct—short cystic duct with another anatomical variation;
- Bile duct—partially obstructed due to the external compression or a gallstone eroding from the gallbladder;
- Distal bile duct with normal thin walls and no distended lumen;
- Proximal bile duct—dilated with inflamed walls;
- Abnormal communication between the bile duct and the gallbladder;
- Fistula between the gallbladder and stomach, duodenum, colon or other structures.
1.2. Aim of the Review
2. Literature Research
3. Classification
4. Symptoms and Laboratory Results
5. Imaging
6. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senra, F.; Navaratne, L.; Acosta, A.; Martínez-Isla, A. Laparoscopic Management of Type II Mirizzi Syndrome. Surg. Endosc. 2020, 34, 2303–2312. [Google Scholar] [CrossRef] [Green Version]
- Al-Akeely, M.H.A.; Alam, M.K.; Bismar, H.A.; Khalid, K.; Al-Teimi, I.; Al-Dossary, N.F. Mirizzi Syndrome: Ten Years Experience from a Teaching Hospital in Riyadh. World J. Surg. 2005, 29, 1687–1692. [Google Scholar] [CrossRef]
- Chawla, A.; Bosco, J.I.; Lim, T.C.; Srinivasan, S.; Teh, H.S.; Shenoy, J.N. Imaging of Acute Cholecystitis and Cholecystitis-Associated Complications in the Emergency Setting. Singap. Med. J. 2015, 56, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Mithani, R.; Schwesinger, W.H.; Bingener, J.; Sirinek, K.R.; Gross, G.W.W. The Mirizzi Syndrome: Multidisciplinary Management Promotes Optimal Outcomes. J. Gastrointest. Surg. 2008, 12, 1022–1028. [Google Scholar] [CrossRef]
- Chen, H.; Siwo, E.A.; Khu, M.; Tian, Y. Current Trends in the Management of Mirizzi Syndrome: A review of literature. Medicine 2018, 97, e9691. [Google Scholar] [CrossRef]
- Shirah, B.H.; Shirah, H.A.; Albeladi, K.B. Mirizzi Syndrome: Necessity for Safe Approach in Dealing with Diagnostic and Treatment Challenges. Ann. Hepato-Biliary-Pancreat Surg. 2017, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Senthil, G.; Prakash, A.; Behari, A.; Singh, R.K.; Kapoor, V.K.; Saxena, R. Mirizzi’s Syndrome: Lessons Learnt from 169 Patients at a Single Center. Korean J. Hepato-Biliary-Pancreatic Surg. 2016, 20, 17. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Li, Z.; Zhao, E.; Zhang, H.; Cui, N. Appraisal of Diagnosis and Surgical Approach for Mirizzi Syndrome. ANZ J. Surg. 2012, 82, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.; Khan, N.; Shah, A.; Khan, A. Post-Cholecystectomy Mirizzi’s Syndrome: Magnetic Resonance Cholangiopancreatography Demonstration. Saudi J. Gastroenterol. 2010, 16, 295–298. [Google Scholar] [CrossRef]
- Nassar, A.H.M.; Nassar, M.K.; Gil, I.C.; Ng, H.J.; Yehia, A.M. One-Session Laparoscopic Management of Mirizzi Syndrome: Feasible and Safe in Specialist Units. Surg. Endosc. 2020, 35, 3295–3296. [Google Scholar] [CrossRef] [PubMed]
- Ibrarullah, M.; Mishra, T.; Das, A.P. Mirizzi Syndrome. Indian J. Surg. 2008, 70, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Beorlequi, J.; Cabezali Sánchez, R.; Monsalve Laguna, E.; Soriano Gil-Albarellos, P.; Moreno de Marcos, N. Nuevas Posibilidades Diagnósticas y Terapéuticas En El Síndrome de Mirizzi. Anales de Medicina Interna 2007, 24, 281–284. [Google Scholar] [CrossRef]
- Pablo, A.; Mariano, P.; Luis, B.; Rafael García, F.T. Mirizzi Syndrome: Prevalence, Diagnosis and Treatment. Acta Gastroenterol. Latinoam. 2014, 44, 323–328. [Google Scholar]
- Tung, K.L.M.; Tang, C.N.; Lai, E.C.H.; Yang, G.P.C.; Chan, O.C.Y.; Li, M.K.W. Robot-Assisted Laparoscopic Approach of Management for Mirizzi Syndrome. Surg. Laparosc. Endosc. Percutaneous Tech. 2013, 23, e17–e21. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Urquijo, M.; Gil-Galindo, G.; Rodarte-Shade, M. Mirizzi Syndrome from Type i to Vb: A Single Center Experience. Turk. J. Surg. 2020, 36, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Singh, K.K. Mirizzi Syndrome: Laparoscopic Management by Subtotal Cholecystectomy. Surg. Endosc. Other Interv. Tech. 2006, 20, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, M.A. Mirizzi Syndrome: History, Current Knowledge and Proposal of a Simplified Classification. World J. Gastroenterol. 2012, 18, 4639–4650. [Google Scholar] [CrossRef]
- Ji, Y.F.; Gao, Y.; Xie, M. The Use of Different Pathology Classification Systems in Preoperative Imaging of Mirizzi Syndrome. Arch. Med. Sci. 2019, 15, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Payá-Llorente, C.; Vázquez-Tarragón, A.; Alberola-Soler, A.; Martínez-Pérez, A.; Martínez-López, E.; Santarrufina-Martínez, S.; Ortiz-Tarín, I.; Armañanzas-Villena, E. Mirizzi Syndrome: A New Insight Provided by a Novel Classification. Ann. Hepato-Biliary-Pancreatic Surg. 2017, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Kamalesh, N.; Prakash, K.; Pramil, K.; George, T.; Sylesh, A.; Shaji, P. Laparoscopic Approach Is Safe and Effective in the Management of Mirizzi Syndrome. J. Minim. Access Surg. 2015, 11, 246. [Google Scholar] [CrossRef]
- Davlatov, S.; Rakhmanov, K.; Qurbonov, N.; Vafayeva, I.; Abduraxmanov, D. Current State of the Problem Treatment of Mirizzi Syndrome (Literature Review). Int. J. Pharm. Res. 2020, 12, 1931–1939. [Google Scholar] [CrossRef]
- Corlette, M.B.; Bismuth, H. Biliobiliary Fistula: A Trap in the Surgery of Cholelithiasis. Arch. Surg. 1975, 110, 377–383. [Google Scholar] [CrossRef]
- McSherry, C.K.; Ferstenberg, H.; Virshup, H. The Mirizzi Syndrome: Suggested Classification and Surgical Therapy. Surg Gastroenterol. 1982, 1, 219–225. [Google Scholar]
- Csendes, A.; Diaz, J.C.; Burdiles, P.; Maluenda, F.; Nava, O. Mirizzi Syndrome and Cholecystobiliary Fistula: A Unifying Classification. Br. J. Surg. 1989, 76, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Beltran, M.A.; Csendes, A.; Cruces, K.S. The Relationship of Mirizzi Syndrome and Cholecystoenteric Fistula: Validation of a Modified Classification. World J. Surg. 2008, 32, 2237–2243. [Google Scholar] [CrossRef]
- Nagakawa, T.; Ohta, T.; Kayahara, M.; Ueno, K.; Konishi, I.; Sanada, H.; Miyazaki, I. A New Classification of Mirizzi Syndrome from Diagnostic and Therapeutic Viewpoints. Hepatogastroenterology 1997, 44, 63–67. [Google Scholar] [PubMed]
- Solis-Caxaj, C.A. Mirizzi Syndrome: Diagnosis, Treatment and a Plea for a Simplified Classification. World J. Surg. 2009, 33, 1783–1784. [Google Scholar] [CrossRef]
- Erben, Y.; Benavente-Chenhalls, L.A.; Donohue, J.M.; Que, F.G.; Kendrick, M.L.; Reid-Lombardo, K.M.; Farnell, M.B.; Nagorney, D.M. Diagnosis and Treatment of Mirizzi Syndrome: 23-Year Mayo Clinic Experience. J. Am. Coll. Surg. 2011, 213, 114–119. [Google Scholar] [CrossRef]
- Waisberg, J.; Corona, A.; De Abreu, I.W.; Farah, J.F.D.M.; Lupinacci, R.A.; Goffi, F.S. Benign Obstruction of the Common Hepatic Duct (Mirizzi Syndrome): Diagnosis and Operative Management. Arq. Gastroenterol. 2005, 42, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.; Rahman, S.H.; Toogood, G.J.; Prasad, K.R.; Lodge, J.P.A.; Guillou, P.J.; Menon, K.V. Mirizzi’s Syndrome—Results from a Large Western Experience. HBP 2006, 8, 474–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lledó, J.B.; Barber, S.M.; Ibañez, J.C.; Torregrosa, A.G.; Lopez-Andujar, R. Update on the Diagnosis and Treatment of Mirizzi Syndrome in Laparoscopic Era: Our Experience in 7 Years. Surg. Laparosc. Endosc. Percutaneous Tech. 2014, 24, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Seah, W.M.; Koh, Y.X.; Cheow, P.C.; Chow, P.K.H.; Chan, C.Y.; Lee, S.Y.; Ooi, L.L.P.J.; Chung, A.Y.F.; Goh, B.K.P. A Retrospective Review of the Diagnostic and Management Challenges of Mirizzi Syndrome at the Singapore General Hospital. Dig. Surg. 2018, 35, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.L.V.D.; Kumar, A.; Sikora, S.S.; Saxena, R.; Kapoor, V.K. Mirizzi Syndrome and Gallbladder Cancer. J. Hepatobiliary Pancreat. Surg. 2006, 13, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hong, T.; Li, B.; Liu, W.; He, X.; Zheng, C. Mirizzi Syndrome: Our Experience with 27 Cases in PUMC Hospital. Chin. Med. Sci. J. 2013, 28, 172–177. [Google Scholar] [CrossRef]
- Ahlawat, S.K.; Singhania, R.; Al-Kawas, F.H. Mirizzi Syndrome. Curr. Treat. Options Gastroenterol. 2007, 10, 102–110. [Google Scholar] [CrossRef]
- Wehrmann, T.; Riphaus, A.; Martchencko, K.; Kokabpick, S.; Pauka, H.; Stergiou, N.; Frenz, M.B. Intraductal Ultrasonography in the Diagnosis of Mirizzi Syndrome. Endoscopy 2006, 38, 717–722. [Google Scholar] [CrossRef]
- Kwon, A.H.; Inui, H. Preoperative Diagnosis and Efficacy of Laparoscopic Procedures in the Treatment of Mirizzi Syndrome. J. Am. Coll. Surg. 2007, 204, 409–415. [Google Scholar] [CrossRef]
- Testini, M.; Sgaramella, L.I.; De Luca, G.M.; Pasculli, A.; Gurrado, A.; Biondi, A.; Piccinni, G. Management of Mirizzi Syndrome in Emergency. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 28–32. [Google Scholar] [CrossRef]
- Mar, W.A.; Shon, A.M.; Lu, Y.; Yu, J.H.; Berggruen, S.M.; Guzman, G.; Ray, C.E.; Miller, F. Imaging Spectrum of Cholangiocarcinoma: Role in Diagnosis, Staging, and Posttreatment Evaluation. Abdom. Radiol. 2016, 41, 553–567. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Delhaye, M.; Charette, N.; Farina, A. Challenging Biliary Strictures: Pathophysiological Features, Differential Diagnosis, Diagnostic Algorithms, and New Clinically Relevant Biomarkers—Part 1. Therap. Adv. Gastroenterol. 2020, 13, 175628482092729. [Google Scholar] [CrossRef]
- Tataria, R.D.; Salgaonkar, H.P.; Maheshwari, G.; Halder, P.J. Mirizzi’s Syndrome: A Scoring System for Preoperative Diagnosis. Saudi J. Gastroenterol. 2018, 24, 274–281. [Google Scholar] [CrossRef]
- Yun, E.J.; Choi, C.S.; Yoon, D.Y.; Seo, Y.L.; Chang, S.K.; Kim, J.S.; Woo, J.Y. Combination of Magnetic Resonance Cholangiopancreatography and Computed Tomography for Preoperative Diagnosis of the Mirizzi Syndrome. J. Comput. Assist. Tomogr. 2009, 33, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fan, Y.; Wu, S. Safety and Feasibility of Laparoscopic Approaches for the Management of Mirizzi Syndrome: A Systematic Review. Surg. Endosc. 2020, 34, 4717–4726. [Google Scholar] [CrossRef] [PubMed]
- Nag, H.H.; Nekarakanti, P.K. Laparoscopic versus Open Surgical Management of Patients with Mirizzi’s Syndrome: A Comparative Study. J. Minim. Access Surg. 2020, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Gong, J.-P. Mirizzi Syndrome: Experience in Diagnosis and Treatment of 25 Cases. Am. Surg. 2012, 78, 61–65. [Google Scholar] [CrossRef]
- Salgado-Garza, G.; Hernandez-Arriaga, P.; Gonzalez-Urquijo, M.; Díaz-Elizondo, J.A.; Flores-Villalba, E.; Rojas-Méndez, J.; Rodarte-Shade, M. Single-Operator Cholangioscopy and Electrohydraulic Lithotripsy for the Treatment of Mirizzi Syndrome. Ann. Med. Surg. 2021, 62, 274–277. [Google Scholar] [CrossRef]
- Tazuma, S.; Unno, M.; Igarashi, Y.; Inui, K.; Uchiyama, K.; Kai, M.; Tsuyuguchi, T.; Maguchi, H.; Mori, T.; Yamaguchi, K.; et al. Evidence-Based Clinical Practice Guidelines for Cholelithiasis 2016. J. Gastroenterol. 2017, 53, 276–300. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Chen, Y.L. Diagnosis and Treatment of Xanthogranulomatous Cholecystitis: A Report of 39 Cases. Cell Biochem. Biophys. 2012, 64, 131–135. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Hotta, M.; Sher, L.; Selby, R.R.; Parekh, D.; Buxbaum, J.; Stapfer, M. Complicated Gallstone Disease: Diagnosis and Management of Mirizzi Syndrome. Surg. Endosc. 2017, 31, 2215–2222. [Google Scholar] [CrossRef]

| Authors | McSherry et al.-1982 | Csendes et al.-1989 and Complemented in 2008 | Beltrán et al.-2012 | Payá-Llorente et al.-2017 | Nagakawa et al.-1997 |
|---|---|---|---|---|---|
| Classification | Type I—external compression of the bile duct | Type I—external compression of the bile duct | Type I—external compression of the bile duct | Type 1—external compression of the bile duct | Type I—external compression of the bile duct |
| Type II—cholecystobiliary fistula | Type II—cholecystobiliary fistula—up to 1/3 of the bile duct wall erosion | Type IIa—cholecystobiliary fistula involving <50% of the bile duct diameter | Type 2—cholecystobiliary fistula involving <50% of the bile duct diameter | Type II—cholecystobiliary fistula | |
| Type III—cholecystobiliary fistula—up to 2/3 of the bile duct wall erosion | |||||
| Type IV—cholecystobiliary fistula—complete destruction of the bile duct wall and fusion with gallbladder | Type IIb—cholecystobiliary fistula involving >50% of the bile duct diameter | Type 3—cholecystobiliary fistula involving >50% of the bile duct diameter | |||
| Type Va—cholecystoenteric fistula | Type IIIa—cholecystoenteric fistula | Subtypes describing cholecystoenteric fistula: A-no fistula/B-fistula without gallstone ileus/C-fistula with gallstone ileus | Type III—gallstones in the cystic duct and common hepatic duct confluence | ||
| Type Vb—cholecystoenteric fistula with gallstone ileus | Type IIIb—cholecystoenteric fistula with gallstone ileus | Type IV—stricture without stones (e.g., due to cholecystitis) |
| Authors and the Year of Publication | Period of Studied Patients | Analysed Patients | The Patients with MS | Men/Women | Classification | Patients Diagnosed Preoperatively | Patients Diagnosed with: | Patients Diagnosed Intraoperatively or Postoperatively | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US | CT | MRCP | ERCP | Other | |||||||||
| 1 | Seah et al., 2018 [32] | November 2001–June 2012 | 10,176 | 64 | 27/37 | Csendes | 48 (75%) | 4/35 (11.4%) | 16/40 (40%) | 24/27 (88.9%) | 29/44 (65.9%) | - | 16 (25%) |
| 2 | Acquafresca et al., 2014 [13] | July 2007–June 2013 | 2160 | 14 | 6/8 | Csendes | 3 (21.43%) | 1/14 (7.14%) | - | 2/14 (14.3%) | - | - | 11 (78.57%) |
| 3 | Xu et al., 2013 [34] | January 1988–November 2011 | 8697 | 27 | 8/19 | Csendes | - | 12/27 (44.4%) | 4/8 (50%) | 7/9 (77.8%) | 17/17 (100%) | - | - |
| 4 | Erben et al., 2011 [28] | January 1987–December 2009 | 21,450 | 36 | 19/17 | McSherry | - | 13/27 (48%) | 10/24 (42%) | - | 20/32 (63%) | - | - |
| 5 | Wehrmann et al., 2006 [36] | January 2002–December 2004 | 2089 | 30 | - | - | - | - | 1 (3%) | 12/19 (63%) | - | IDUS 29/30 (97%); EUS 11/15 (73%) | - |
| 6 | Kamalesh et al., 2015 [20] | January 2006–August 2013 | 1530 | 20 | 11/9 | Csendes | 12 (60%) | - (40%) | - (33%) | - (100%) | 13/18 (72%) | EUS - (63%) | 8 (40%) |
| 7 | Kwon et al., 2007 [37] | April 1992–December 2005 | 2012 | 24 | 15/9 | McSherry | 20 (83%) | - | - | - | 9/13 (69%) | IVC-SCT 11/11 (100%) | 4 (17%) |
| 8 | Cui et al., 2012 [8] | January 2004–January 2010 | 29,875 | 198 | 88/110 | Csendes | - | Suggested in 154/198 (77.8%) | Performed in 89 to differentiate from malignant disease | Suggested in 163 | - | - | - |
| 9 | Kumar et al., 2016 [7] | 1989–2011 | 169 | 169 | 62/107 | Csendes | 54 (32%) | 17 (10%) | suspected in 5 | 5 | 27 | - | 115 (68%) |
| 10 | Payá-Llorente et al., 2017 [19] | January 2000–October 2015 | 4853 | 28 | 7/21 | Csendes | 19 | 14 (50%) | 4 | 2 | - | - | - |
| 11 | Testini et al., 2016 [38] | March 2006–February 2016 | 919 | 18 | 6/12 | Csendes | 5 (27.8%) | - | - | 5/5 (100%) | - | - | 13 (72.2%) |
| 12 | Nassar et al., 2020 [10] | February 1992–February 2020 | 5740 | 58 | 20/38 | Csendes | 7 (12%) | 1/34 (2.9%) | 0/11 | 3/12 (25%) | 3/10 (30%) | - | 51 (88%) |
| 13 | Gomez et al., 2006 [30] | August 1994–August 2005 | 33 | 33 | 15/18 | McSherry | 28 (84.4%) | 4 | 3 | 10 | 11 | - | 5 (15.2%) |
| 14 | Shirah et al., 2017 [6] | January 2003–December 2012 | - | 64 | 15/49 | Csendes | 30 (46.9%) | 13/64 (20.3%) | 14 | - | 17 | - | 34 (53.1%) |
| 15 | Prasad et al., 2006 [33] | 1989–2003 | 4800 | 133 | 42/91 | Csendes | - (32%) | - | - | - | - | - | - (68%) |
| 16 | Lledó et al., 2014 [31] | January 2006–November 2012 | 1168 | 35 | - | Csendes | 24 (68.5%) | - (51%) | - | - (76%) | - | - | 11 (31.5%) |
| 17 | Waisberg et al., 2005 [29] | November 1997–June 2003 | 8 | 8 | 4/4 | Csendes | 1 (12.5%) | One patient diagnosed by combining US, CT and ERCP | 7 (87.5%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klekowski, J.; Piekarska, A.; Góral, M.; Kozula, M.; Chabowski, M. The Current Approach to the Diagnosis and Classification of Mirizzi Syndrome. Diagnostics 2021, 11, 1660. https://doi.org/10.3390/diagnostics11091660
Klekowski J, Piekarska A, Góral M, Kozula M, Chabowski M. The Current Approach to the Diagnosis and Classification of Mirizzi Syndrome. Diagnostics. 2021; 11(9):1660. https://doi.org/10.3390/diagnostics11091660
Chicago/Turabian StyleKlekowski, Jakub, Aleksandra Piekarska, Marta Góral, Marta Kozula, and Mariusz Chabowski. 2021. "The Current Approach to the Diagnosis and Classification of Mirizzi Syndrome" Diagnostics 11, no. 9: 1660. https://doi.org/10.3390/diagnostics11091660
APA StyleKlekowski, J., Piekarska, A., Góral, M., Kozula, M., & Chabowski, M. (2021). The Current Approach to the Diagnosis and Classification of Mirizzi Syndrome. Diagnostics, 11(9), 1660. https://doi.org/10.3390/diagnostics11091660






