Differences in Hemodynamic Alteration between Atherosclerotic Occlusive Lesions and Moyamoya Disease: A Quantitative 15O-PET Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PET Study
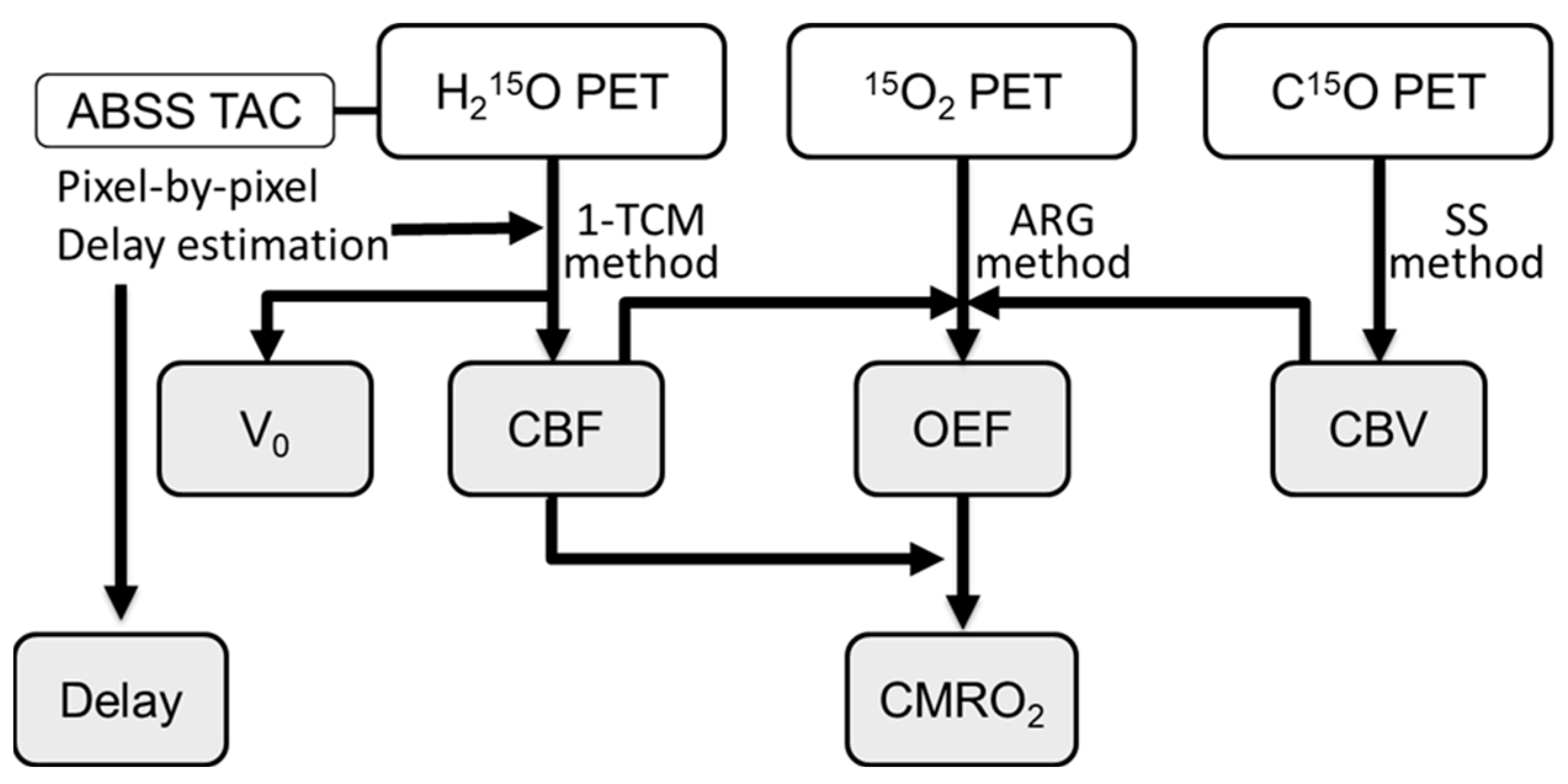
2.3. Calculation of Hemodynamic PET Images
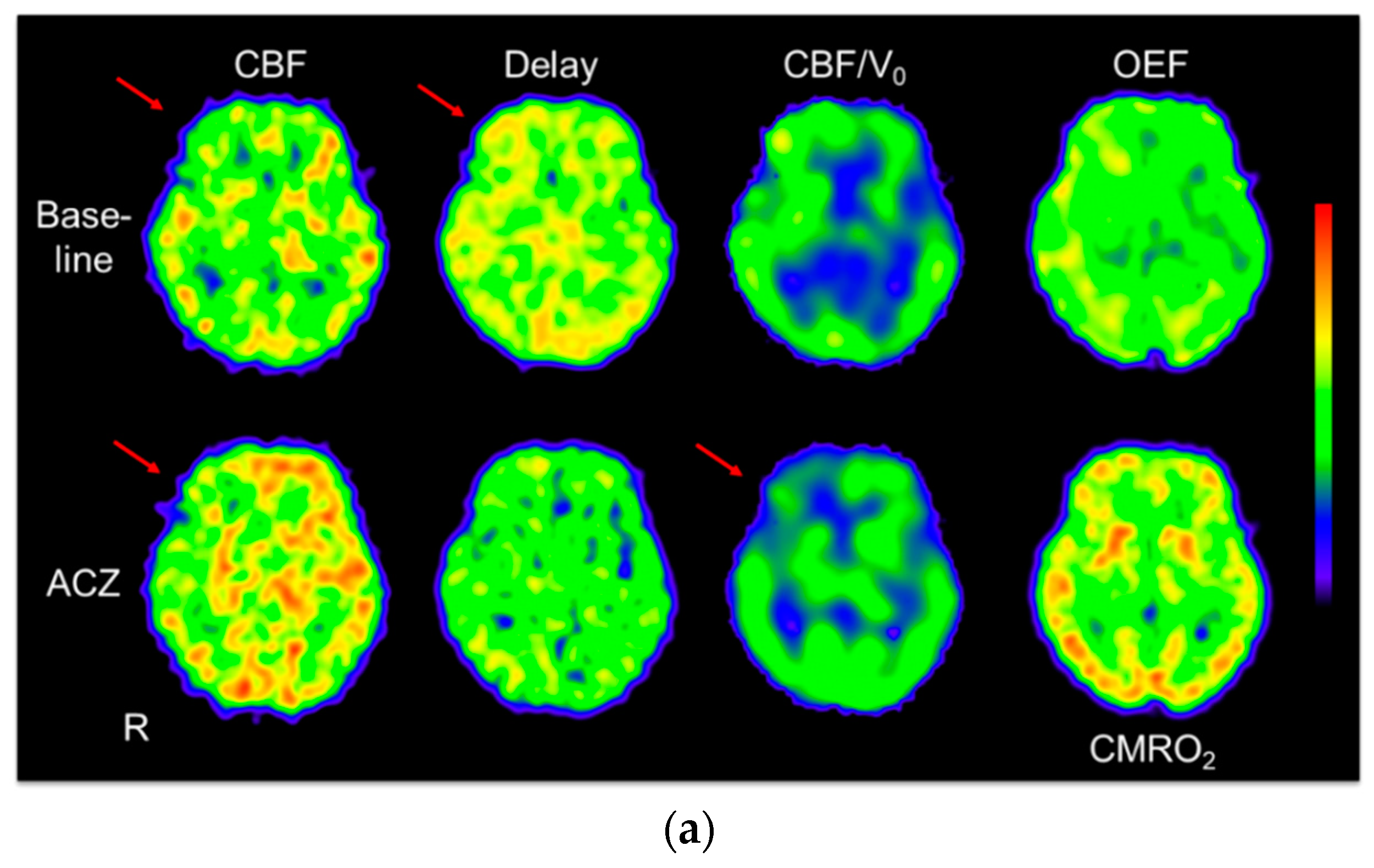
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powers, W.J. Atherosclerotic carotid artery occlusion. Curr. Treat Options Cardiovasc. Med. 2003, 5, 501–509. [Google Scholar] [CrossRef]
- Flaherty, M.L.; Flemming, K.D.; McClelland, R.; Jorgensen, N.W.; Brown, R.D., Jr. Population-based study of symptomatic internalcarotid artery occlusion: Incidence and long-term follow-up. Stroke 2004, 35, e349–e352. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.D.; Kramer, C.L.; Mandrekar, J.; Lanzino, G.; Rabinstein, A.A. Asymptomatic caritid stenosis: Risk of progression and development of symptoms. Cerebrovasc. Dis. 2015, 40, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Trombatore, P.; Lozupone, E.; Gaudino, S.; Milonia, L.; Alexandre, A.; Valente, I.; D’Argento, F.; Frassanito, P.; Pedicelli, A. A Rare Case of Postinfectious Moyamoya Syndrome: Case Report and Review of the Literature. World Neurosurg. 2020, 140, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Hashimoto, N.; Yoshimoto, T.; Iwasaki, Y.; Research Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: Results of multicenter survey in Japan. Stroke 2007, 38, 1430–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, M.R.; Derdeyn, C.P.; Robert, L.G., Jr.; Grubb, L.R., Jr.; Powers, W.J.; Zipfel, G.J.M. Extracranial-intracranial bypass for ischemic cerebrovascular disease: What have we learned from the Carotid Occlusion Surgery Study? Neurosurg. Focus 2014, 36, E9. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Press, G.A.; Grubb, R.L., Jr.; Gado, M.; Raichle, M.E. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann. Intern. Med. 1987, 106, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Baron, J.C.; Bousser, M.G.; Rey, A.; Guillard, A.; Comar, D.; Castaigne, P. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke 1981, 12, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Zaharchuk, G.; Guzman, R.; Achrol, A.; Bell-Stephens, T.; Steinberg, G.K. Quantitative hemodynamic studies in moyamoya disease: A review. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef]
- Powers, W.J.; Zazulia, A.R. PET in Cerebrovascular disease PET. PET Clin. 2010, 5, 83–106. [Google Scholar] [CrossRef] [Green Version]
- Okazawa, H.; Kudo, T. Clinical impact of hemodynamic parameter measurement for cerebrovascular disease using positron emission tomography and 15O-labeled tracers. Ann. Nucl. Med. 2009, 23, 217–227. [Google Scholar] [CrossRef]
- Ohta, S.; Meyer, E.; Fujita, H.; Reutens, D.C.; Evans, A.; Gjedde, A. Cerebral [15O]water clearance in humans determined by PET: I. Theory and normal values. J. Cereb. Blood Flow Metab. 1996, 16, 765–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.M.; Tsujikawa, T.; Mori, T.; Kiyono, Y.; Okazawa, H. Pixel-by-pixel precise delay correction for mesuament of cerebral hemodynamic parameters in H215O PET study. Ann. Nucl. Med. 2017, 31, 283–294. [Google Scholar] [CrossRef] [Green Version]
- De Grado, T.R.; Turkington, T.G.; Williams, J.J.; Stearns, C.W.; Hoffman, J.M.; Coleman, R.E. Performance characteristics of a whole-body PET scanner. J. Nucl. Med. 1994, 35, 1398. [Google Scholar]
- Okazawa, H.; Yamauchi, H.; Sugimoto, K.; Takahashi, M.; Toyoda, H.; Kishibe, Y. Quantitative comparison of the bolus and steady-state methods for measurement of cerebral perfusion and oxygen metabolism: Positron emission tomography study using 15O-gas and water. J. Cereb. Blood Flow Metab. 2001, 21, 793–803. [Google Scholar] [CrossRef]
- Mintun, M.A.; Raichle, M.E.; Martin, W.R.W.; Herscovitch, P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J. Nucl. Med. 1984, 25, 177–187. [Google Scholar] [PubMed]
- Okazawa, H.; Tsuchida, T.; Kobayashi, M.; Arai, Y.; Pagani, M.; Isozaki, M.; Yonekura, Y. Can the detection of misery perfusion in chronic cerebro- vascular disease be based on reductions in baseline CBF and vasoreactivity? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 121–129. [Google Scholar] [CrossRef]
- Vafaee, M.; Murase, K.; Gjedde, A.; Meyer, E. Dispersion correction for automatic sampling of O-15-labeled H2O and red blood cells. In Quantification of Brain Function Using PET; Myers, R., Cunningham, V., Bailey, D., Jones, T., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 72–75. [Google Scholar]
- Okazawa, H.; Yamauchi, H.; Sugumoto, K.; Toyoda, H.; Kishibe, Y.; Takahashi, M. Effects of acetazolamide on cerebral blood flow, blood volume and oxygen metabolism: A PET study with healthy volunteers. J. Cereb. Blood Flow Metab. 2001, 21, 1472–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, E. Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by the H215O autoradiographic method and dynamic PET. J. Nucl. Med. 1989, 30, 1069–1078. [Google Scholar]
- Iida, H.; Kanno, I.; Miura, S.; Murakami, M.; Takahashi, K.; Uemura, K. Error analysis of a quantitative cerebral blood flow measurement using H2O autoradiography and positron emission tomography, with respect to the dispersion of the input function. J. Cereb. Blood Flow Metab. 1986, 6, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, H.; Okazawa, H.; Kishibe, Y.; Sugimoto, K.; Takahashi, M. Oxygen extraction fraction and acetazolamide reactivity in symptomatic carotid artery disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 33–37. [Google Scholar]
- Okazawa, H.; Yamauchi, H.; Toyoda, H.; Sugimoto, K.; Fujibayashi, Y.; Yonekura, Y. Relationship between vasodilatation and cerebral blood flow increase in impaired hemodynamics: A PET study with the acetazolamide test in cerebrovascular disease. J. Nucl. Med. 2003, 44, 1875–1883. [Google Scholar]
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef] [Green Version]
- Okazawa, H.; Yamauchi, H.; Sugimoto, K.; Takahashi, M. Differences of vasodilatory capacity and changes in cerebral blood flow induced by acetazolamide in patients with cerebrovascular disease. J. Nucl. Med. 2003, 44, 1371–1378. [Google Scholar] [PubMed]
- Hokari, M.; Kuroda, S.; Shiga, T.; Nakayama, N.; Tamaki, N.; Iwasaki, Y. Combination of a mean transit time measurement with an acetazolamide test increases predictive power to identify elevated oxygen extraction fraction in occlusive carotid artery diseases. J. Nucl. Med. 2008, 49, 1922–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, H.; Higashi, T.; Kagawa, S.; Nishii, R.; Kudo, T.; Sugimoto, K.; Okazawa, H.; Fukuyama, H. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012, 135, 2515–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Subject | SOL | MMD |
|---|---|---|
| Number | 24 | 18 |
| Age [years, mean ± SD] (range) | 66.6 ± 11.3 (37–84) | 33.1 ± 16.2 † (12–70) |
| Sex | ||
| Male | 20 | 7 |
| Female | 4 | 11 |
| Stroke | 11 | 10 |
| Lesion side | ||
| Right | 10 | 6 |
| Left | 14 | 6 |
| Bilateral | 0 | 6 |
| Steno-occlusion | ||
| ICA (stenosis/occlusion) | 17 (9/8) | |
| MCA (stenosis/occlusion) | 7 (3/4) |
| SOL (n = 24) | MMD (n = 18) | ||||
|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral (n = 12) | Contralateral (n = 12) | Bilateral (n = 6 × 2) | |
| BP (mmHg) | |||||
| Baseline | 91.9 ± 9.8 | 91.9 ± 11.8 | |||
| Post-ACZ | 91.8 ± 11.3 | 94.1 ± 13.3 | |||
| PaCO2 (mmHg) | |||||
| Baseline | 40.5 ± 4.4 | 39.1 ± 3.6 | |||
| Post-ACZ | 39.5 ± 4.1 | 38.0 ± 3.5 | |||
| CBF (mL/min/100 g) | |||||
| Baseline | 47.9 ± 7.4 c,* | 53.0 ± 6.0 | 51.8 ± 8.5 | 55.4 ± 7.9 * | 47.8 ± 8.9 |
| Post-ACZ | 55.3 ± 8.6 a,c,* | 66.3 ± 6.1 a,*,† | 57.6 ± 10.0 b,* | 65.1 ± 8.6 a,* | 54.0 ± 12.5 b,g,† |
| CVR (% Change) | 16.2 ± 12.4 c | 25.7 ± 8.7 | 11.5 ± 11.9 | 18.1 ± 11.0 | 13.6 ± 12.7 |
| V0 (mL/100 g) | |||||
| Baseline | 1.26 ± 0.48 d | 1.59 ± 0.56 * | 1.21 ± 0.46 | 1.36 ± 0.43 | 1.10 ± 0.37 g,* |
| Post-ACZ | 1.30 ± 0.37 c | 1.93 ± 0.57 † | 1.50 ± 0.74 | 1.75 ± 0.84 | 1.14 ± 0.15 g,† |
| CBV (mL/100 g) | 3.91 ± 0.72 e,† | 3.62 ± 0.63 †,* | 5.23 ± 1.67 g,† | 4.77 ± 1.07 †,* | 5.79 ± 1.71 g,† |
| CMRO2 (mL/min/100 g) | 2.86 ± 0.35 d,† | 3.00 ± 0.27 * | 3.32 ± 0.37 † | 3.40 ± 0.28 †,* | 2.96 ± 0.49 f |
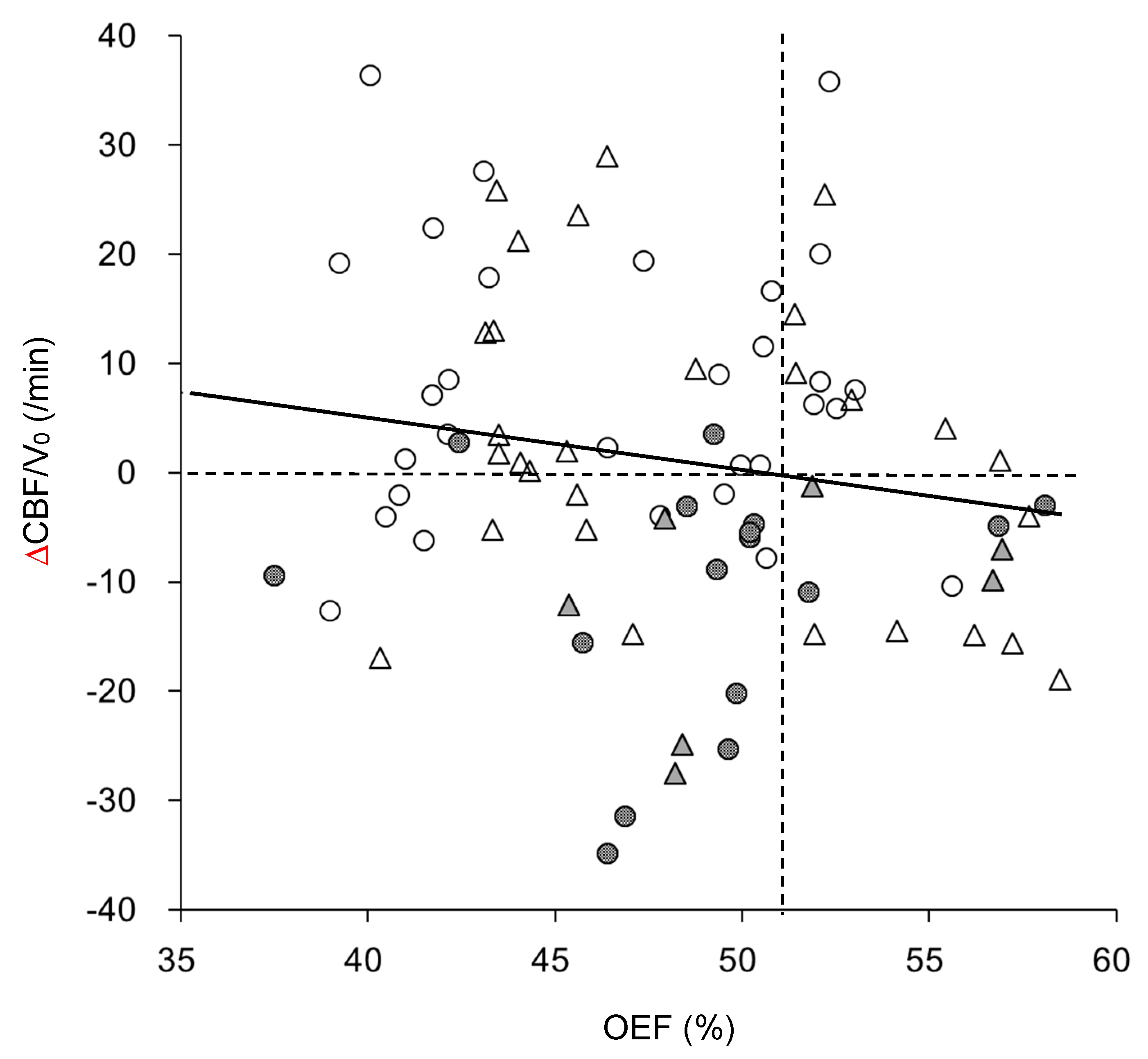
| OEF (%) | 49.0 ± 16.0 d | 45.2 ± 5.8 † | 48.7 ± 5.9 | 46.2 ± 2.7 | 52.5 ± 5.2 g,† |
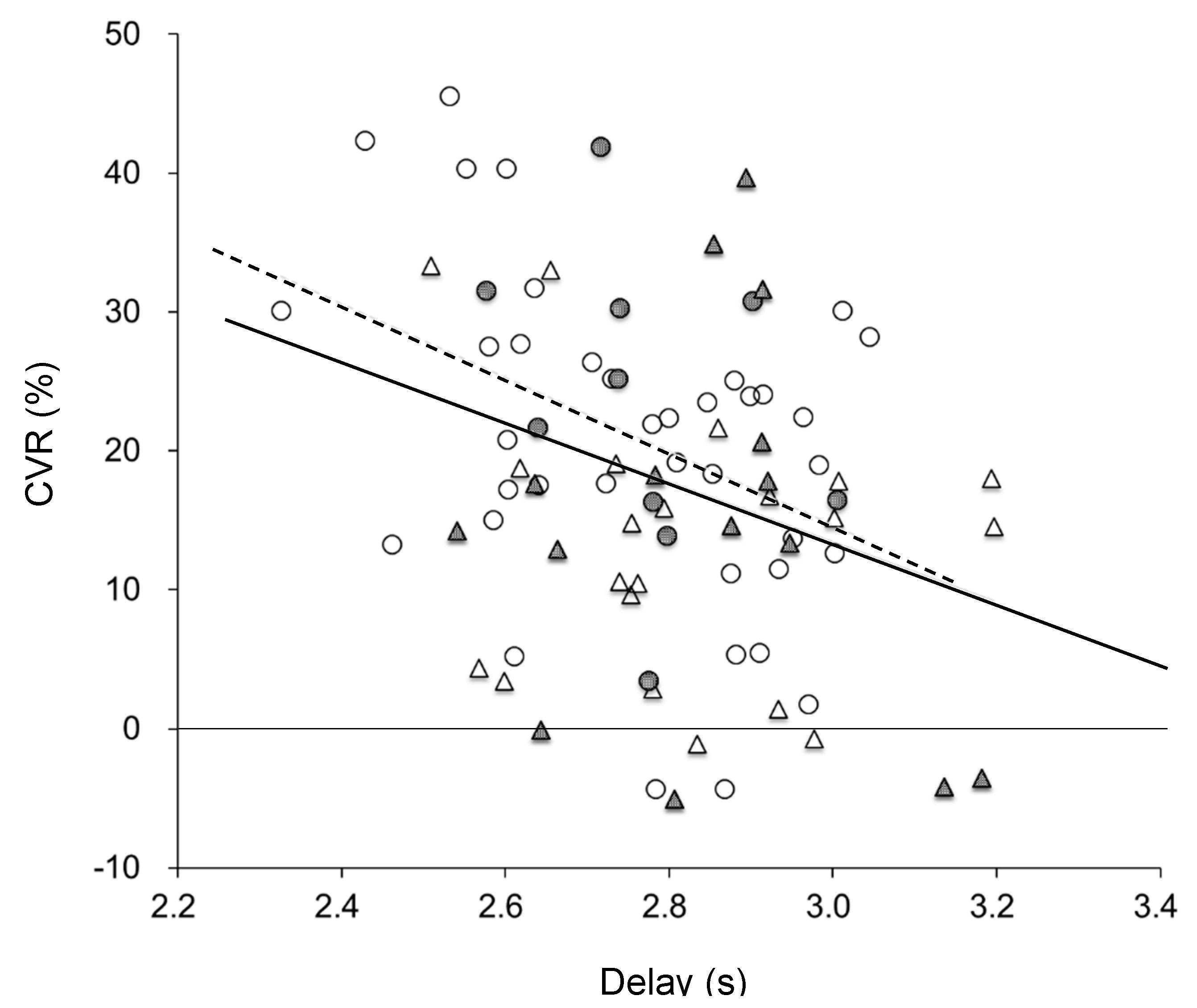
| Delay (s) | 2.82 ± 0.15 c | 2.68 ± 0.18 † | 2.78 ± 0.14 | 2.77 ± 0.14 | 2.96 ± 0.21 f,† |
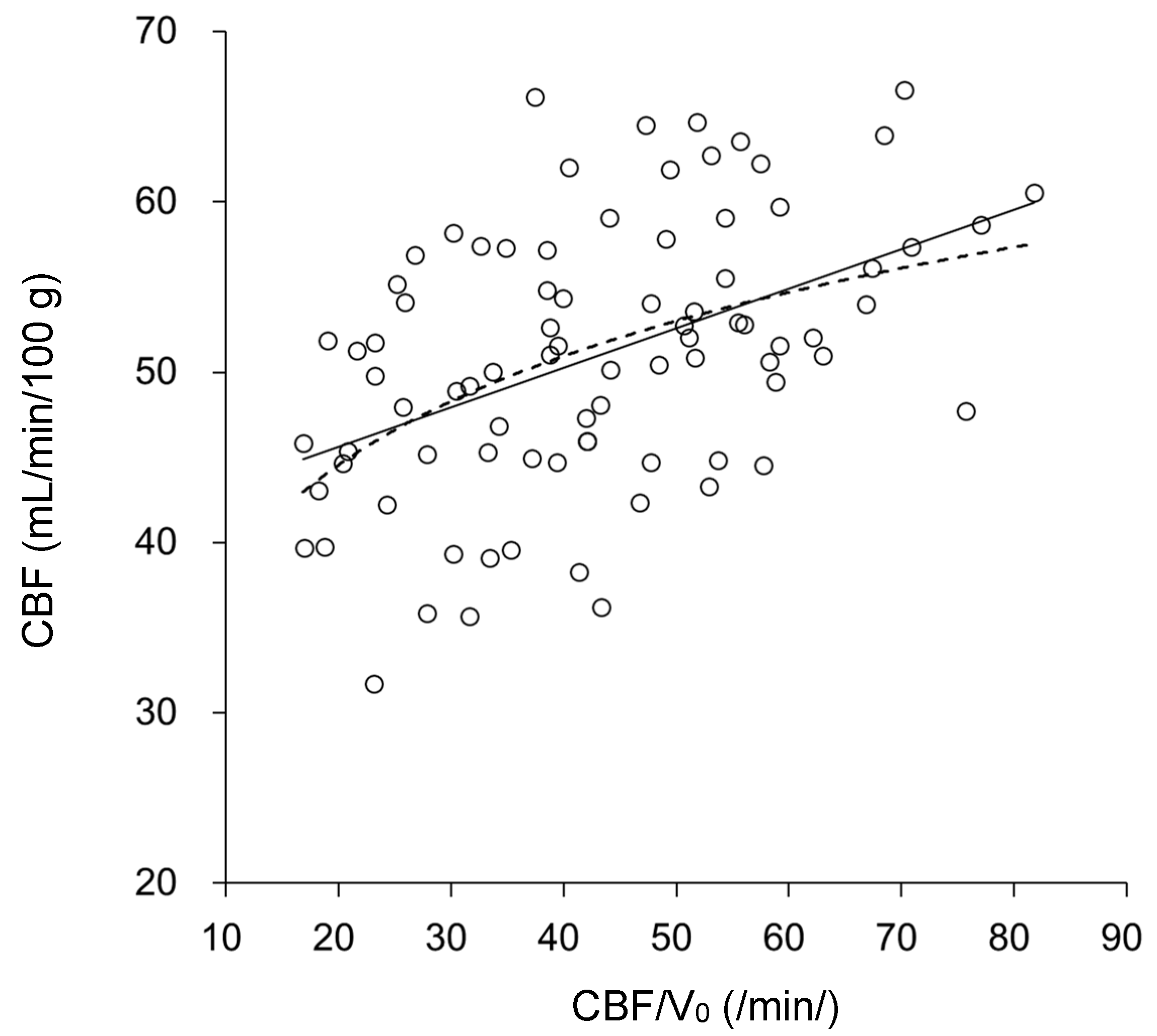
| CBF/V0 (/min) | 41.8 ± 12.3 | 39.7 ± 10.3 | 42.1 ± 13.3 | 42.2 ± 13.5 | 45.0 ± 13.0 |
| ΔCBF/V0(/min) | −0.63 ± 12.3 | 0.28 ± 15.2 | −1.45 ± 16.2 | 0.07 ± 0.14 | −1.48 ± 11.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igarashi, C.; Okazawa, H.; Islam, M.M.; Tsujikawa, T.; Higashino, T.; Isozaki, M.; Kikuta, K.-i. Differences in Hemodynamic Alteration between Atherosclerotic Occlusive Lesions and Moyamoya Disease: A Quantitative 15O-PET Study. Diagnostics 2021, 11, 1820. https://doi.org/10.3390/diagnostics11101820
Igarashi C, Okazawa H, Islam MM, Tsujikawa T, Higashino T, Isozaki M, Kikuta K-i. Differences in Hemodynamic Alteration between Atherosclerotic Occlusive Lesions and Moyamoya Disease: A Quantitative 15O-PET Study. Diagnostics. 2021; 11(10):1820. https://doi.org/10.3390/diagnostics11101820
Chicago/Turabian StyleIgarashi, Chiaki, Hidehiko Okazawa, Muhammad M. Islam, Tetsuya Tsujikawa, Toshifumi Higashino, Makoto Isozaki, and Ken-ichiro Kikuta. 2021. "Differences in Hemodynamic Alteration between Atherosclerotic Occlusive Lesions and Moyamoya Disease: A Quantitative 15O-PET Study" Diagnostics 11, no. 10: 1820. https://doi.org/10.3390/diagnostics11101820
APA StyleIgarashi, C., Okazawa, H., Islam, M. M., Tsujikawa, T., Higashino, T., Isozaki, M., & Kikuta, K.-i. (2021). Differences in Hemodynamic Alteration between Atherosclerotic Occlusive Lesions and Moyamoya Disease: A Quantitative 15O-PET Study. Diagnostics, 11(10), 1820. https://doi.org/10.3390/diagnostics11101820







