Psychometric Evaluation of the Krogh-Poulsen Test for the Diagnosis of the Temporomandibular Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size
2.4. Measurements
2.5. Procedure
2.6. Data Analysis
3. Results
3.1. Factorial Analysis
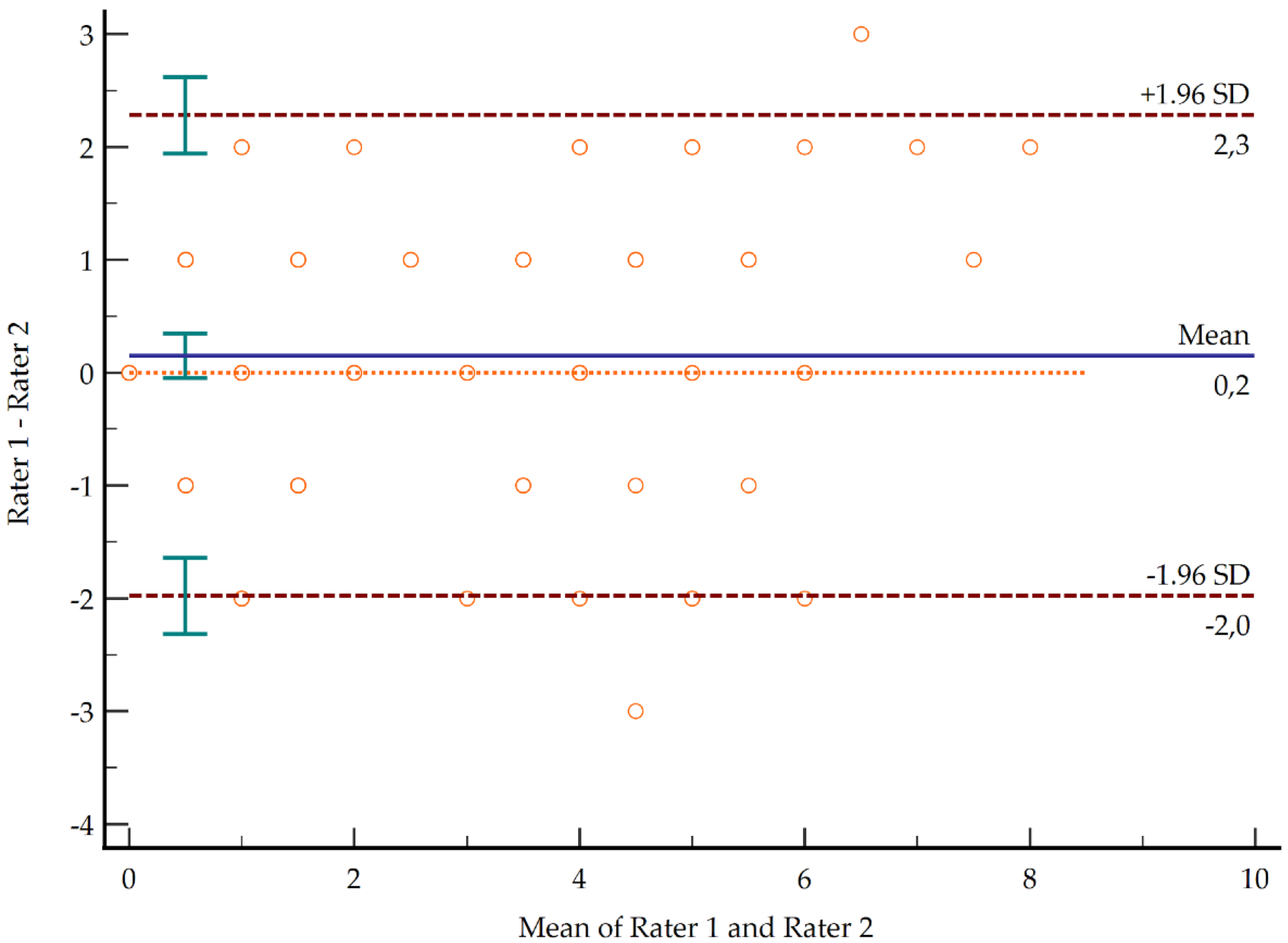
3.2. Reliability and Inter-Rater Agreement
3.3. Concurrent Validity
3.4. Discriminant Validity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gauer, R.L.; Semidey, M.J. Diagnosis and Treatment of Temporomandibular Disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar] [PubMed]
- Herb, K.; Cho, S.; Stiles, M.A. Temporomandibular joint pain and dysfunction. Curr. Pain Headache Rep. 2006, 10, 408–414. [Google Scholar] [CrossRef] [PubMed]
- International Association of the Study of Pain. Orofacial Pain [Internet]. 2013. Available online: https://www.iasp-pain.org/GlobalYear/OrofacialPain2013 (accessed on 3 May 2021).
- Fernandes, G.; Student, G.; Lúcia Franco, A.; Aparecida de Godoi Gonçalves, D.; Geraldo Speciali, J.; Eduardo Bigal, M.; Maria Camparis, C. Temporomandibular Disorders, Sleep Bruxism, and Primary Headaches Are Mutually Associated. J. OROFAC PAIN 2013, 27, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama Magalhães, B.; de Melo Freitas, J.L.; da Sillva Barbosa, A.C.; Scheidegger Neves Gueiros, M.C.; Faria Gomes, S.G.; Rosenblatt, A.; de Caldas, A.F., Jr. Temporomandibular disorder: Otologic implications and its relationship to sleep bruxism. Braz. J. Otorhinolaryngol. 2018, 84, 614–619. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Dental and Craniofacial Research. Prevalence of TMJD and Its Sings and Symptoms. Available online: https://www.nidcr.nih.gov/research/data-statistics/facial-pain/prevalence (accessed on 3 May 2021).
- Plesh, O.; Adams, S.H.; Gansky, S.A. Racial/Ethnic and gender prevalences in reported common pains in a national sample. J. Orofac. Pain 2011, 25, 25–31. [Google Scholar] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral. Fac. Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Poulsen, W.; Olsson, A. Occlusal disharmonies and dysfunction of the stomatognathic system. Dent. Clin. N. Am. 1966, 627–635. [Google Scholar]
- Farfán-Verástegui, L.G.; Aguirre-Aguilar, A.A. Asociación Entre Sintomatología Clínica de Trastornos Temporomandibulares y Posición Condilar Según el Índice de Krogh Poulsen en Escolares de 15 a 17 Años de Edad en el Distrito de Moche, 2014; Universidad Nacional de Trujillo: Trujillo, Perú, 2014. [Google Scholar]
- Helkimo, M. Studies on function and dysfunction of the masticatory system: IV. Age and sex distribution of symptoms of dysfunction of the masticatory system in lapps in the north of Finland. Acta Odontol. Scand. 1974, 32, 255–267. [Google Scholar] [CrossRef]
- Alonso-Royo, R.; Sánchez-Torrelo, C.M.; Ibáñez-Vera, A.J.; Zagalaz-Anula, N.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Rodríguez-Almagro, D.; Lomas-Vega, R. Validity and Reliability of the Helkimo Clinical Dysfunction Index for the Diagnosis of Temporomandibular Disorders. Diagnostics 2021, 11, 472. [Google Scholar] [CrossRef]
- Salazar, J.L.C. Sensibilidad y especificiad del índice de Krogh Poulsen en el Diagnóstico de los Transtornos Temporomandibulares. Odontol. Sanmarquina 1999, 1, 16–20. [Google Scholar]
- Hobart, J.C.; Cano, S.J.; Warner, T.T.; Thompson, A.J. What sample sizes for reliability and validity studies in neurology? J. Neurol. 2012, 259, 2681–2694. [Google Scholar] [CrossRef]
- Meng, X.; Brunet, A.; Turecki, G.; Liu, A.; D’Arcy, C.; Caron, J. Risk factor modifications and depression incidence: A 4-year longitudinal Canadian cohort of the Montreal Catchment Area Study. BMJ Open 2017, 7, 15156. [Google Scholar] [CrossRef] [Green Version]
- Henríquez Sánchez, P.; Doreste Alonso, J. Smoking. Prevalence and Attitudes Among Health Science Students. Aten Primaria 1996, 18, 436–441. [Google Scholar] [CrossRef]
- World Health Organization. 2013–2020 Global Action Plan for the Prevention and Control of Noncommunicable Diseases; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Da Cunha, S.C.; Nogueira Bessa, R.V.; Pinto Duarte, Â.; Do Egito Vasconcelos, B.C.; de Cavalcanti Almeida, R.A. Analysis of helkimo and craniomandibular indexes for temporomandibular disorder diagnosis on rheumatoid arthritis patients. Braz. J. Otorhinolaryngol. 2007, 73, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Bigaton, D.; de Castro, E.M.; Pires, P.F. Factor and Rasch analysis of the Fonseca anamnestic index for the diagnosis of myogenous temporomandibular disorder. Braz. J. Phys. Ther. 2017, 21, 120–126. [Google Scholar] [CrossRef]
- Sánchez-Torrelo, C.; Zagalaz-Anula, N.; Alonso-Royo, R.; Ibáñez-Vera, A.; López-Collantes, J.; Rodríguez-Almagro, D.; Obrero-Gaitán, E.; Lomas-Vega, R. Transcultural Adaptation and Validation of the Fonseca Anamnestic Index in a Spanish Population with Temporomandibular Disorders. J. Clin. Med. 2020, 9, 3230. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.F.; de Castro, E.M.; Pelai, E.B.; de Arruda, A.B.C.; Rodrigues-Bigaton, D. Analysis of the accuracy and reliability of the Short-Form Fonseca Anamnestic Index in the diagnosis of myogenous temporomandibular disorder in women. Braz. J. Phys. Ther. 2018, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Castarlenas, E.; Roy, R.; Tomé Pires, C.; Racine, M.; Pathak, A.; Miró, J. The Utility and Construct Validity of Four Measures of Pain Intensity: Results from a University-Based Study in Spain. Pain Med. 2019, 20, 2411–2420. [Google Scholar] [CrossRef]
- Andrade Ortega, J.A.; Delgado Martínez, A.D.; Almécija Ruiz, R. Validation of a Spanish version of the Neck Disability Index. Spine 2010, 35, 85–89. [Google Scholar] [CrossRef]
- Martin, M.; Blaisdell, B.; Kwong, J.W.; Bjorner, J.B. The Short-Form Headache Impact Test (HIT-6) was psychometrically equivalent in nine languages. J. Clin. Epidemiol. 2004, 57, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.; Garmendia, I.; Martín, E.; García-Tapia, R. Cultural adaptation of 2 questionnaires for health measurement in patients with vertigo. Acta Otorrinolaringol. Esp. 2000, 51, 572–580. [Google Scholar]
- Jacobson, G.P.; Newman, C.W. The Development of the Dizziness Handicap Inventory lected to ensure that the scale had both content and face validity. An example of a probe question for functional aspects of dizziness would be: “Because of your prob¬ lem, do you restrict your travel fo. Arch. Otolaryngol. Head. Neck Surg. 1990, 116, 425–427. [Google Scholar]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretation of SF-36 and SF-12 questionnaires in Spain: Physical and mental components. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, P.; Lorenzo-Seva, U. Assessing the quality and appropiateness of factor solutions and factor score estimates in exploratory item factor analysis. Educ. Psychol. Meas. 2018, 78, 762–780. [Google Scholar] [CrossRef]
- Kvålseth, T.O. An Alternative Interpretation of the Linearly Weighted Kappa Coefficients for Ordinal Data. Psychometrika 2018, 83, 618–627. [Google Scholar] [CrossRef]
- Landis, J.; Koch, G.G. The Measurement of the Observer Agreement for Categorial Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciencies, 2nd ed.; Hillsdale, N.J., Ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1998; ISBN 0805802835. [Google Scholar]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, S.; Pawah, S.; Gola, S.; Bakshi, M. Analysis of Helkimo index for temporomandibular disorder diagnosis in the dental students of Faridabad city: A cross-sectional study. J. Indian Prosthodont Soc. 2016, 17, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Bergholz, P. Examiner agreement with the Krogh-Poulsen method of clinical functional analysis. Dtsch. Zahnarztl. Z. 1985, 40, 182–185. [Google Scholar]
- Pangidis, C. Teaching of functional occlusion according to Krogh-Poulsen. Zahnarztebl Bad. Wurttemb 1974, 2, 189–193. [Google Scholar]
- Solberg, W.K.; Woo, M.W.; Houston, J.B. Prevalence of mandibular dysfunction in young adults. J. Am. Dent. Assoc. 1979, 98, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaum, T.; Voss, R. Statistical considerations on the evaluation of the Krogh-Poulsen clinical functional analysis. Dtsch. Zahnarztl. Z. 1978, 33, 439–445. [Google Scholar] [PubMed]
- Von Kummer, R.; Bourquain, H.; Bastianello, S.; Bozzao, L.; Manelfe, C.; Meier, D.; Hacke, W. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 2001, 219, 95–100. [Google Scholar] [CrossRef]

| TMD | n = 63 | Healthy | n = 56 | All | n = 119 | ||
|---|---|---|---|---|---|---|---|
| Gender | Female | 59 | 93.7% | 32 | 57.14% | 91 | 76.5% |
| Male | 4 | 6.3% | 24 | 42.86% | 28 | 23.5% | |
| Job | Active | 12 | 19.0% | 14 | 25.00% | 26 | 21.8% |
| Not active | 51 | 81.0% | 42 | 75.00% | 93 | 78.2% | |
| Study Level | Primary | 7 | 11.1% | 14 | 25.00% | 21 | 17.6% |
| Secondary | 28 | 44.4% | 30 | 53.57% | 58 | 48.7% | |
| University | 28 | 44.4% | 12 | 21.43% | 40 | 33.6% | |
| Physical Activity | No | 27 | 42.9% | 20 | 35.71% | 47 | 39.5% |
| Yes | 36 | 57.1% | 36 | 64.29% | 72 | 60.5% | |
| Income | <20,000 | 35 | 55.6% | 36 | 64.29% | 71 | 59.7% |
| >20,000 | 28 | 44.4% | 20 | 35.71% | 48 | 40.3% | |
| Smoker | Non-smoker | 43 | 68.3% | 34 | 60.71% | 77 | 64.7% |
| Smoker | 8 | 12.7% | 7 | 12.50% | 15 | 12.6% | |
| Occasional smoker | 6 | 9.5% | 7 | 12.50% | 13 | 10.9% | |
| Ex-smoker | 6 | 9.5% | 8 | 14.29% | 14 | 11.8% | |
| Drinker | Non-drinker | 21 | 33.3% | 21 | 37.50% | 42 | 35.3% |
| Regular drinker | 3 | 4.8% | 3 | 5.36% | 6 | 5.0% | |
| Occasional drinker | 39 | 61.9% | 32 | 57.14% | 71 | 59.7% | |
| Age (years) | 43.19 | (12.62) | 47.64 | (14.87) | 45 | (13.86) | |
| Weight (kilograms) | 68.90 | (14.07) | 77.86 | (19.22) | 72.83 | (17.05) | |
| Height (meters) | 1.61 | (0.07) | 1.65 | (0.09) | 1.63 | (0.09) | |
| Body Mass Index | 26.69 | (6.72) | 28.48 | (7.10) | 27 | (6.91) | |
| Items | Component | ||
|---|---|---|---|
| 1 a | 2 a | 3 a | |
| I. Mouth opening under 40 mm | 0.149 | −0.072 | 0.697 |
| II. Deviation in mandibular movement during opening or closing | 0.942 | 0.058 | 0.112 |
| III. Discomfort at masticatory muscle palpation | 0.087 | 0.238 | 0.859 |
| IV. Pain at pressing temporomandibular joint | 0.068 | 0.286 | 0.831 |
| V. Clicks or crackles during joint movement | 0.456 | −0.002 | 0.698 |
| VI. Obstacles or blockages during joint movement | 0.161 | 0.319 | 0.682 |
| VII. Centric relation and intercuspation | 0.471 | 0.657 | 0.312 |
| VIII. Anterior displacement over 1 mm at retrusion from maximum intercuspation | −0.184 | 0.887 | 0.028 |
| IX. Lateral displacement over 1 mm at retrusion | 0.217 | 0.885 | 0.245 |
| Rho Coefficient | p-Value | Correlation | |
|---|---|---|---|
| Fonseca Anamnestic Index | 0.702 | <0.001 | Strong |
| Fonseca Anamnestic Index, Short | 0.675 | <0.001 | Strong |
| Helkimo Clinical Dysfunction Index | 0.710 | <0.001 | Strong |
| Numerical Rating Scale, Orofacial Pain | 0.865 | <0.001 | Strong |
| Headache Impact Test, 6 items | 0.329 | <0.001 | Moderate |
| Dizziness Handicap Inventory, Functional | 0.322 | <0.001 | Moderate |
| Dizziness Handicap Inventory, Emotional | 0.292 | 0.001 | Poor |
| Dizziness Handicap Inventory, Physical | 0.389 | <0.001 | Moderate |
| Dizziness Handicap Inventory, Total Score | 0.377 | <0.001 | Moderate |
| Neck Disability Index | 0.423 | <0.001 | Moderate |
| Numerical Rating Scale, Neck Pain | 0.399 | <0.001 | Moderate |
| Physical Component Summary | −0.076 | 0.414 | Poor |
| Mental Component Summary | −0.197 | 0.032 | Poor |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | 95% CI | −LR | 95% CI | +PV | 95% CI | −PV | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >1 | 90.48 | 80.4–96.4 | 85.71 | 73.8–93.6 | 6.33 | 3.3–12.1 | 0.11 | 0.05–0.2 | 87.7 | 78.9–93.2 | 88.9 | 78.8–94.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-Vera, A.J.; Alonso-Royo, R.; Sánchez-Torrelo, C.M.; Zagalaz-Anula, N.; López-Collantes, J.; Lomas-Vega, R. Psychometric Evaluation of the Krogh-Poulsen Test for the Diagnosis of the Temporomandibular Disorders. Diagnostics 2021, 11, 1876. https://doi.org/10.3390/diagnostics11101876
Ibáñez-Vera AJ, Alonso-Royo R, Sánchez-Torrelo CM, Zagalaz-Anula N, López-Collantes J, Lomas-Vega R. Psychometric Evaluation of the Krogh-Poulsen Test for the Diagnosis of the Temporomandibular Disorders. Diagnostics. 2021; 11(10):1876. https://doi.org/10.3390/diagnostics11101876
Chicago/Turabian StyleIbáñez-Vera, Alfonso Javier, Roger Alonso-Royo, Carmen María Sánchez-Torrelo, Noelia Zagalaz-Anula, Jesús López-Collantes, and Rafael Lomas-Vega. 2021. "Psychometric Evaluation of the Krogh-Poulsen Test for the Diagnosis of the Temporomandibular Disorders" Diagnostics 11, no. 10: 1876. https://doi.org/10.3390/diagnostics11101876
APA StyleIbáñez-Vera, A. J., Alonso-Royo, R., Sánchez-Torrelo, C. M., Zagalaz-Anula, N., López-Collantes, J., & Lomas-Vega, R. (2021). Psychometric Evaluation of the Krogh-Poulsen Test for the Diagnosis of the Temporomandibular Disorders. Diagnostics, 11(10), 1876. https://doi.org/10.3390/diagnostics11101876









