The Prevalence of Liver Steatosis and Fibrosis Assessed by Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter in Apparently Healthy Romanian Medical Students
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. LSM and CAP Assessment
2.3. Anthropometric Measurements
2.4. Statistics
3. Results
3.1. Participants Characteristics
3.2. Participants Characteristics according to Absence or Presence of Liver Steatosis
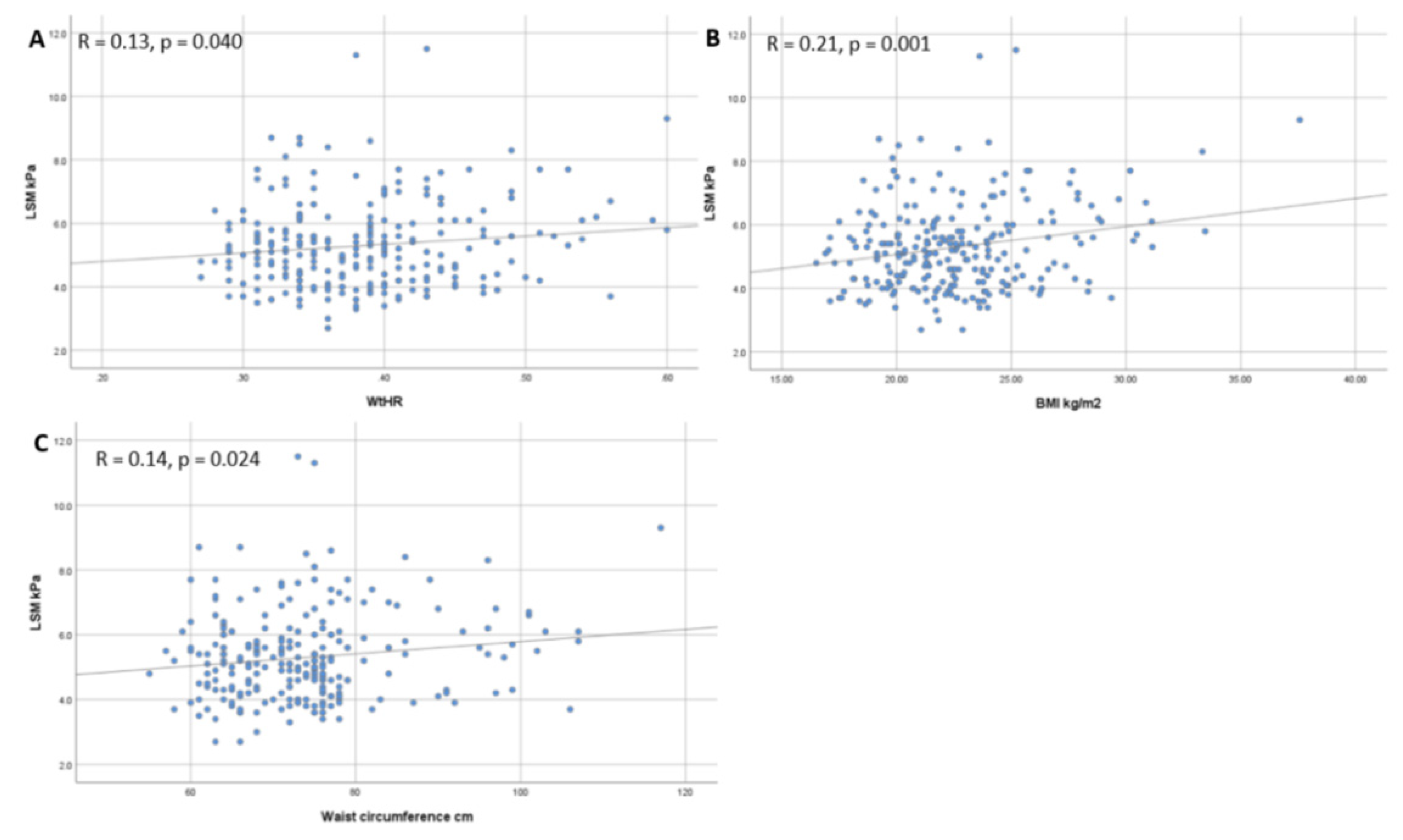
3.3. Correlation between Anthropometric Parameters, CAP and LSM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef]
- Mrad, R.A.; Merjaneh, N.; Mubarak, G.; Lopez, R.; Zein, N.N.; Alkhouri, N. The increasing burden of nonalcoholic fatty liver disease among young adults in the United States: A growing epidemic. Hepatology 2016, 64, 1386–1387. [Google Scholar] [CrossRef]
- Doycheva, I.; Watt, K.D.; Alkhouri, N. Nonalcoholic fatty liver disease in adolescents and young adults: The next frontier in the epidemic. Hepatology 2017, 65, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Trifan, A.; Stanciu, C. Checkmate to liver biopsy in chronic hepatitis C? World J. Gastroenterol. 2012, 18, 5514–5520. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V.; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Asociación Latinoamericana para el Estudio del Hígado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection (Updated Version). Available online: http://www.who.int/hepatitis/publications/hepatitis-cguidelines-2016/en/ (accessed on 15 June 2021).
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.-M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The controlled attenuation parameter (CAP): A novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 13–20. [Google Scholar] [CrossRef]
- Castera, L.; Forns, X.; Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008, 48, 835–847. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Vergniol, J.; Wong, G.L.-H.; Foucher, J.; Chan, H.L.-Y.; Le Bail, B.; Choi, P.C.-L.; Kowo, M.; Chan, A.W.-H.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Brambilla, P.; Bedogni, G.; Heo, M.; Pietrobelli, A. Waist circumferenceto-height ratio predicts adiposity better than body mass index in children and adolescents. Int. J. Obes. 2013, 37, 943–946. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factssheets/fs311/en/ (accessed on 12 June 2021).
- McCarthy, H.D.; Ashwell, M. A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message e ’keep your waist circumference to less than half your height. Int. J. Obes. 2006, 30, 988–992. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- De Lédinghen, V.; Wong, G.L.-H.; Vergniol, J.; Chan, H.L.-Y.; Hiriart, J.-B.; Chan, A.W.-H.; Chermak, F.; Choi, P.C.-L.; Foucher, J.; Chan, C.K.-M.; et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 31, 848–855. [Google Scholar] [CrossRef]
- Doycheva, I.; Cui, J.; Nguyen, P.; Costa, E.A.; Hooker, J.; Hofflich, H.; Bettencourt, R.; Brouha, S.; Sirlin, C.B.; Loomba, R. Noninvasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment. Pharmacol. Ther. 2016, 43, 83–95. [Google Scholar] [CrossRef]
- Dulai, P.S.; Sirlin, C.B.; Loomba, R. MRI and MRE for noninvasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J. Hepatol. 2016, 65, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- Kaya, E.; Demir, D.; Alahdab, Y.O.; Yilmaz, Y. Prevalence of hepatic steatosis in apparently healthy medical students: A transient elastography study on the basis of a controlled attenuation parameter. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1264–1267. [Google Scholar] [CrossRef]
- Abeysekera, K.W.M.; Fernandes, G.S.; Hammerton, G.; Portal, A.J.; Gordon, F.H.; Heron, J.; Hickman, M. Prevalence of steatosis and fibrosis in young adults in the UK: A population-based study. Lancet Gastroenterol. Hepatol. 2020, 5, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Petta, S.; Di Marco, V.; Pipitone, R.M.; Grimaudo, S.; Buscemi, C.; Craxì, A.; Buscemi, S. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: Genetic and metabolic risk factors in a general population. Liver Int. 2018, 38, 2060–2068. [Google Scholar] [CrossRef]
- Shaheen, T.; Elsayed, M.E.; Megahed, M.A.; Attia, H.H.; Ahmed, H.E.; Osama, H. 214-LB: Vibration-Controlled Transient Elastography Reveals Alarming Prevalence of Nonalcoholic Fatty Liver Disease and Fibrosis among Young Adults in Egypt. Diabetes 2019, 68. [Google Scholar] [CrossRef]
- You, S.C.; Kim, K.J.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, W.J.; Han, K.H. Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J. Gastroenterol. 2015, 21, 1158–1166. [Google Scholar] [CrossRef]
- Koehler, E.M.; Plompen, E.P.; Schouten, J.N.; Hansen, B.E.; Darwish Murad, S.; Taimr, P.; Leebeek, F.W.; Hofman, A.; Stricker, B.H.; Castera, L.; et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology 2016, 63, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Roulot, D.; Costes, J.L.; Buyck, J.F.; Warzocha, U.; Gambier, N.; Czernichow, S.; Le Clesiau, H.; Beaugrand, M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011, 60, 977–984. [Google Scholar] [CrossRef]
- Roman, G.; Bala, C.; Creteanu, G.; Graur, M.; Morosanu, M.; Amorin, P.; Pîrcalaboiu, L.; Radulian, G.; Timar, R.; Cadariu, A.A. Obesity and health-related lifestyle factors in the general population in romania: A cross sectional study. Acta Endocrinol. 2015, 11, 64–71. [Google Scholar] [CrossRef]
- Popa, S.; Moţa, M.; Popa, A.; Moţa, E.; Serafinceanu, C.; Guja, C.; Catrinoiu, D.; Hâncu, N.; Lichiardopol, R.; Bala, C.; et al. Prevalence of overweight/obesity, abdominal obesity and metabolic syndrome and atypical cardiometabolic phenotypes in the adult Romanian population: PREDATORR study. J. Endocrinol. Investig. 2016, 39, 1045–1053. [Google Scholar] [CrossRef]


| Overall Cohort n, 426 | Men n, 137 | Women n, 289 | p-Value | |
|---|---|---|---|---|
| Age (years) | 22.22 ± 1.7 | 22.45 ± 1.8 | 22.11 ± 1.6 | 0.144 |
| Females, n (%) | 289 (67.8) | - | - | |
| Weight (kg) | 65.84 ± 13.37 | 74.09 ± 13.29 | 61.95 ± 11.56 | <0.001 |
| Height (cm) | 170 ± 8.56 | 176 ± 10.2 | 167 ± 10.7 | <0.001 |
| Body mass index (kg/m2) | 22.59 ± 3.34 | 23.71 ± 3.33 | 22.07 ± 3.22 | <0.001 |
| Waist circumference (cm) | 73.7 ± 10.29 | 78.79 ± 11.35 | 71.31 ± 8.82 | <0.001 |
| Abdominal obesity, n (%) | 32 (7.5%) | 17 (12.4%) | 13 (4.5%) | <0.001 |
| Waist-to-height ratio | 0.427 ± 0.06 | 0.442 ± 0.06 | 0.42 ± 0.05 | 0.159 |
| Non-overweight, n (%) | 348 (81.7) | 102 (74.5) | 246 (85.1) | 0.046 |
| Overweight, n (%) | 63 (14.8) | 27 (19.7) | 36 (12.5) | 0.004 |
| Obese, n (%) | 15 (3.5) | 8 (5.8) | 7 (2.4) | 0.037 |
| Liver steatosis, n (%) | 74 (17.4) | 39 (28.5) | 35 (12.1) | 0.011 |
| Steatosis degree, n (%) | 0.026 | |||
| 0 | 352 (82.6) | 98 (71.5) | 254 (87.9) | |
| 1 | 32 (7.5) | 18 (13.1) | 14 (4.8) | |
| 2 | 13 (3.1) | 5 (3.7) | 8 (2.8) | |
| 3 | 29 (6.8) | 16 (11.7) | 13 (4.5) | |
| Fibrosis stage, n (%) | 0.186 | |||
| 0 | 277 (65) | 79 (57.6) | 198 (68.5) | |
| 1 | 136 (31.9) | 50 (36.5) | 86 (29.8) | |
| 2 | 10 (2.4) | 6 (4.4) | 4 (1.4) | |
| 3 | 3 (0.7) | 2 (1.5) | 1 (0.3) | |
| CAP, dB/m | 215.76 ± 48.38 | 234.49 ± 47.38 | 206.95 ± 46.42 | <0.001 |
| LSM, kPa | 5.29 ± 1.35 | 5.36 ± 1.2 | 5.26 ± 1.42 | 0.582 |
| M-probe, n (%) | 402 (94.4) | 128 (93.4) | 274 (94.8) | 0.410 |
| XL-probe, n (%) | 24 (5.6) | 9 (6.7) | 15 (5.2) | 0.372 |
| Subjects, n = 13 | Increased, n (%) | |
|---|---|---|
| Age (years) | 22.7 ± 1.5 | - |
| Males, n (%) | 8 (61.5) | |
| Body mass index (kg/m2) | 24.58 ± 3.41 | 8 (61.5) |
| Waist-to-height-ratio | 0.462 ± 0.07 | 5 (38.5) |
| Platelet count (G/L) | 287 ± 72.45 | 0 (0) |
| ALT (IU/L) | 24.7 ± 14.9 | 3 (23.1) |
| AST (IU/L) | 26.3 ± 11.4 | 4 (30.7) |
| GGT (IU/L) | 25.1 ± 16.6 | 2 (15.3) |
| ALP (IU/L) | 62.7 ± 20.2 | 0 (0) |
| Total bilirubin (mg/dL) | 0.68 ± 0.25 | 0 (0) |
| Fasting glucose (mg/dL) | 88.3 ± 17.1 | 6 (46.1) |
| Total cholesterol (mg/dL) | 208.5 ± 38.3 | 5 (38.5) |
| Triglycerides (mg/dL) | 131.6 ± 52.9 | 7 (53.8) |
| LDL-c (mg/dL) | 112.1 ± 26.6 | 3 (23.1) |
| No Hepatic Steatosis n, 352 | Hepatic Steatosis n, 74 | p-Value | |
|---|---|---|---|
| Age (years) | 22.18 ± 1.61 | 22.36 ± 1.73 | 0.565 |
| Males, n (%) | 98 (27.8) | 39 (52.7) | 0.031 |
| Weight (kg) | 63.14 ± 11.37 | 75.09 ± 16.06 | <0.001 |
| Height (cm) | 170 ± 10.5 | 171 ± 10.8 | 0.061 |
| Body mass index (kg/m2) | 22.14 ± 3.04 | 24.89 ± 3.91 | <0.001 |
| Waist circumference (cm) | 71.9 ± 8.82 | 81.23 ± 12.94 | <0.001 |
| Abdominal obesity, n (%) | 18 (5.1%) | 14 (18.9%) | <0.001 |
| Waist-to-height ratio | 0.418 ± 0.05 | 0.482 ± 0.09 | <0.001 |
| Non-overweight, n (%) | 311 (88.3) | 37 (50) | 0.029 |
| Overweight, n (%) | 33 (9.4) | 30 (40.5) | <0.001 |
| Obese, n (%) | 8 (2.3) | 7 (9.5) | <0.001 |
| Fibrosis stage, n (%) | 0.024 | ||
| 0 | 243 (69) | 34 (45.9) | |
| 1 | 104 (29.6) | 32 (43.2) | |
| 2 | 5 (1.4) | 5 (6.8) | |
| ≥3 | 0 (0) | 3 (4.1) | |
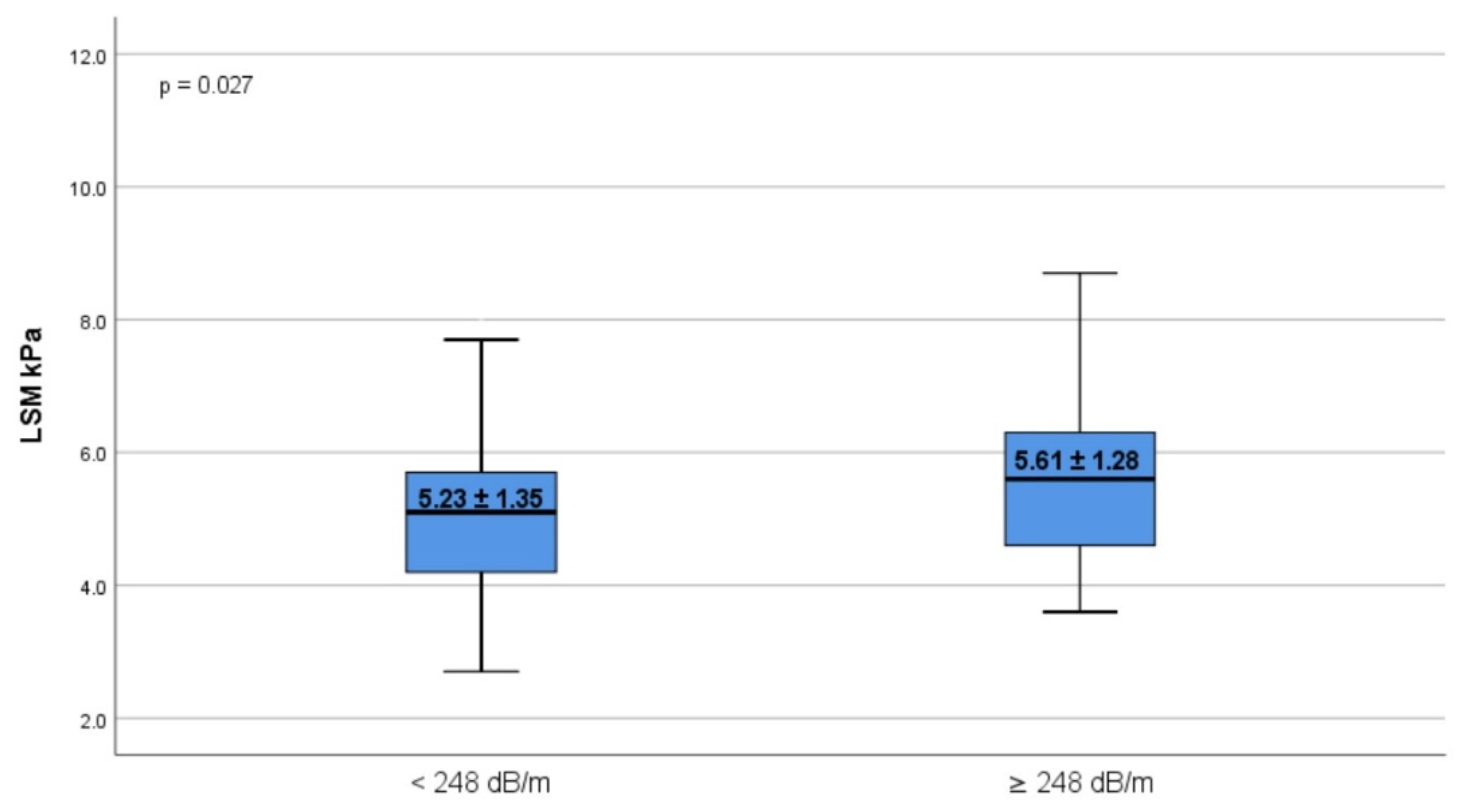
| LSM kPa | 5.23 ± 1.35 | 5.61 ± 1.28 | 0.027 |
| CAP dB/m | 199.16 ± 35.39 | 280.41 ± 38.95 | <0.001 |
| M-probe, n (%) | 341 (96.9) | 61 (82.4) | 0.244 |
| XL-probe, n (%) | 11 (3.1) | 13 (17.6) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastasa, R.; Stanciu, C.; Zenovia, S.; Singeap, A.-M.; Cojocariu, C.; Sfarti, C.; Girleanu, I.; Chiriac, S.; Cuciureanu, T.; Huiban, L.; et al. The Prevalence of Liver Steatosis and Fibrosis Assessed by Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter in Apparently Healthy Romanian Medical Students. Diagnostics 2021, 11, 2341. https://doi.org/10.3390/diagnostics11122341
Nastasa R, Stanciu C, Zenovia S, Singeap A-M, Cojocariu C, Sfarti C, Girleanu I, Chiriac S, Cuciureanu T, Huiban L, et al. The Prevalence of Liver Steatosis and Fibrosis Assessed by Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter in Apparently Healthy Romanian Medical Students. Diagnostics. 2021; 11(12):2341. https://doi.org/10.3390/diagnostics11122341
Chicago/Turabian StyleNastasa, Robert, Carol Stanciu, Sebastian Zenovia, Ana-Maria Singeap, Camelia Cojocariu, Catalin Sfarti, Irina Girleanu, Stefan Chiriac, Tudor Cuciureanu, Laura Huiban, and et al. 2021. "The Prevalence of Liver Steatosis and Fibrosis Assessed by Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter in Apparently Healthy Romanian Medical Students" Diagnostics 11, no. 12: 2341. https://doi.org/10.3390/diagnostics11122341
APA StyleNastasa, R., Stanciu, C., Zenovia, S., Singeap, A.-M., Cojocariu, C., Sfarti, C., Girleanu, I., Chiriac, S., Cuciureanu, T., Huiban, L., Muzica, C.-M., & Trifan, A. (2021). The Prevalence of Liver Steatosis and Fibrosis Assessed by Vibration-Controlled Transient Elastography and Controlled Attenuation Parameter in Apparently Healthy Romanian Medical Students. Diagnostics, 11(12), 2341. https://doi.org/10.3390/diagnostics11122341










