Aberrant Left Subclavian Artery-Esophageal Fistula in a Patient with a Prolonged Use of Nasogastric Tube: A Case Report and Literature Review
Abstract
1. Introduction
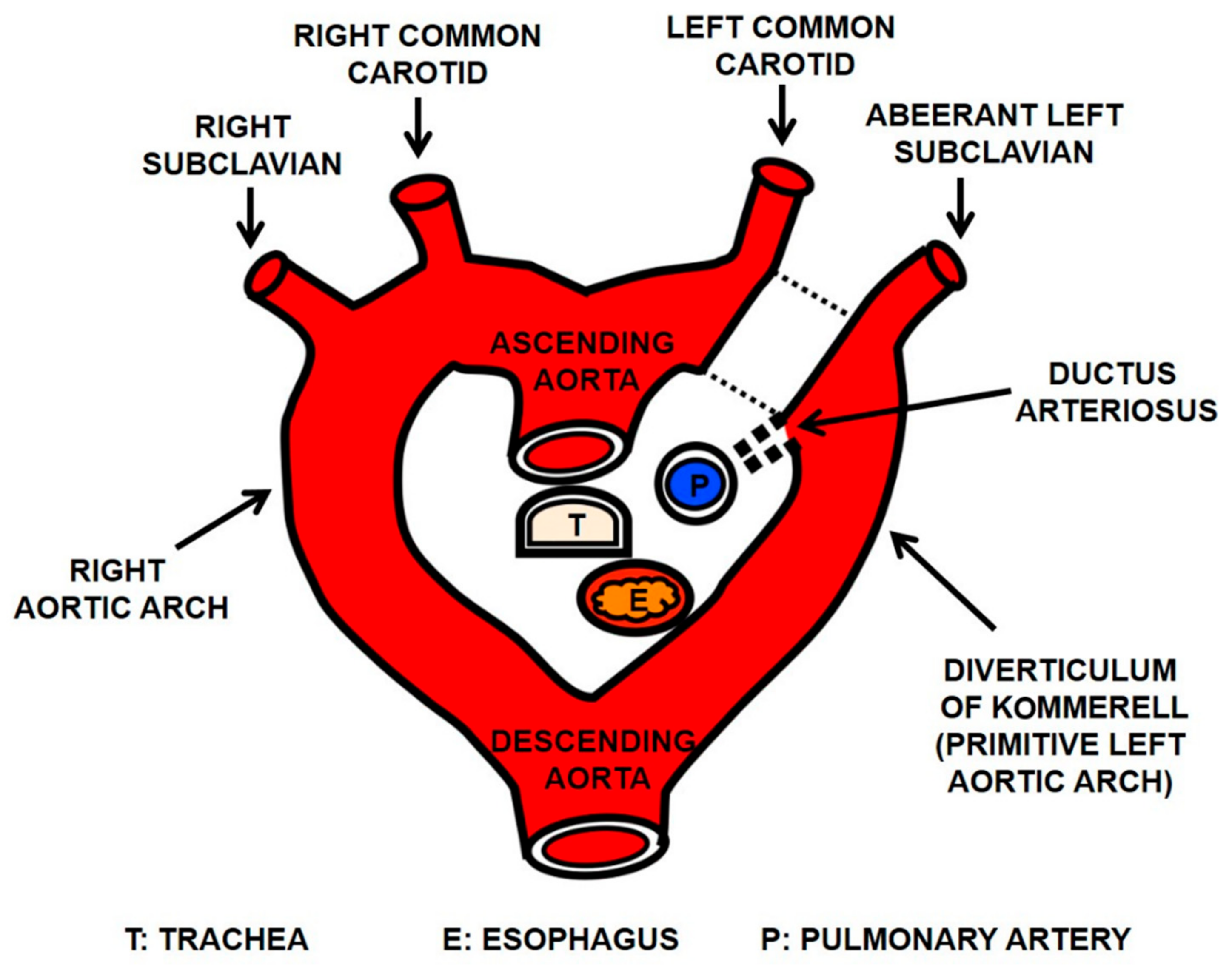
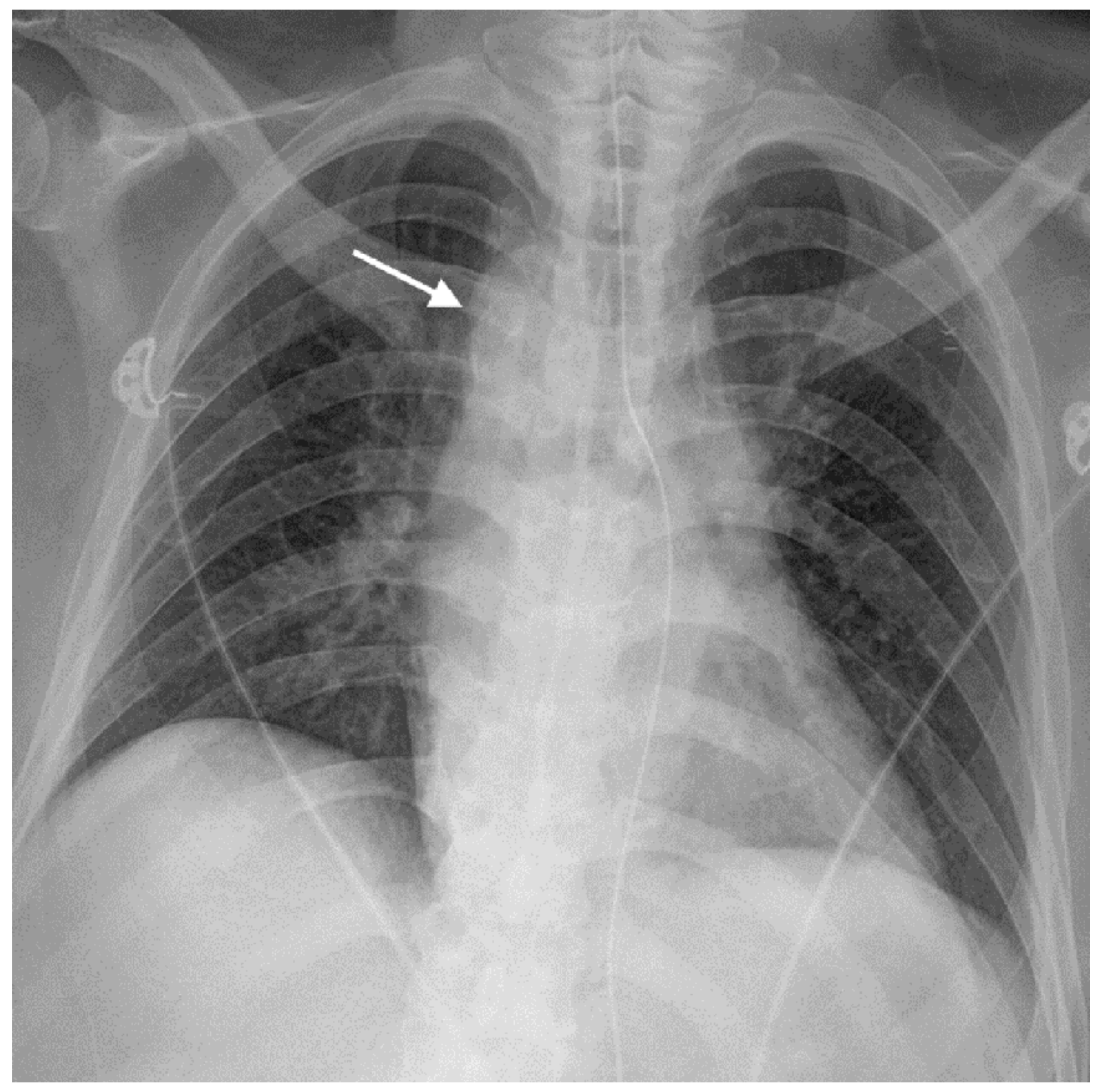
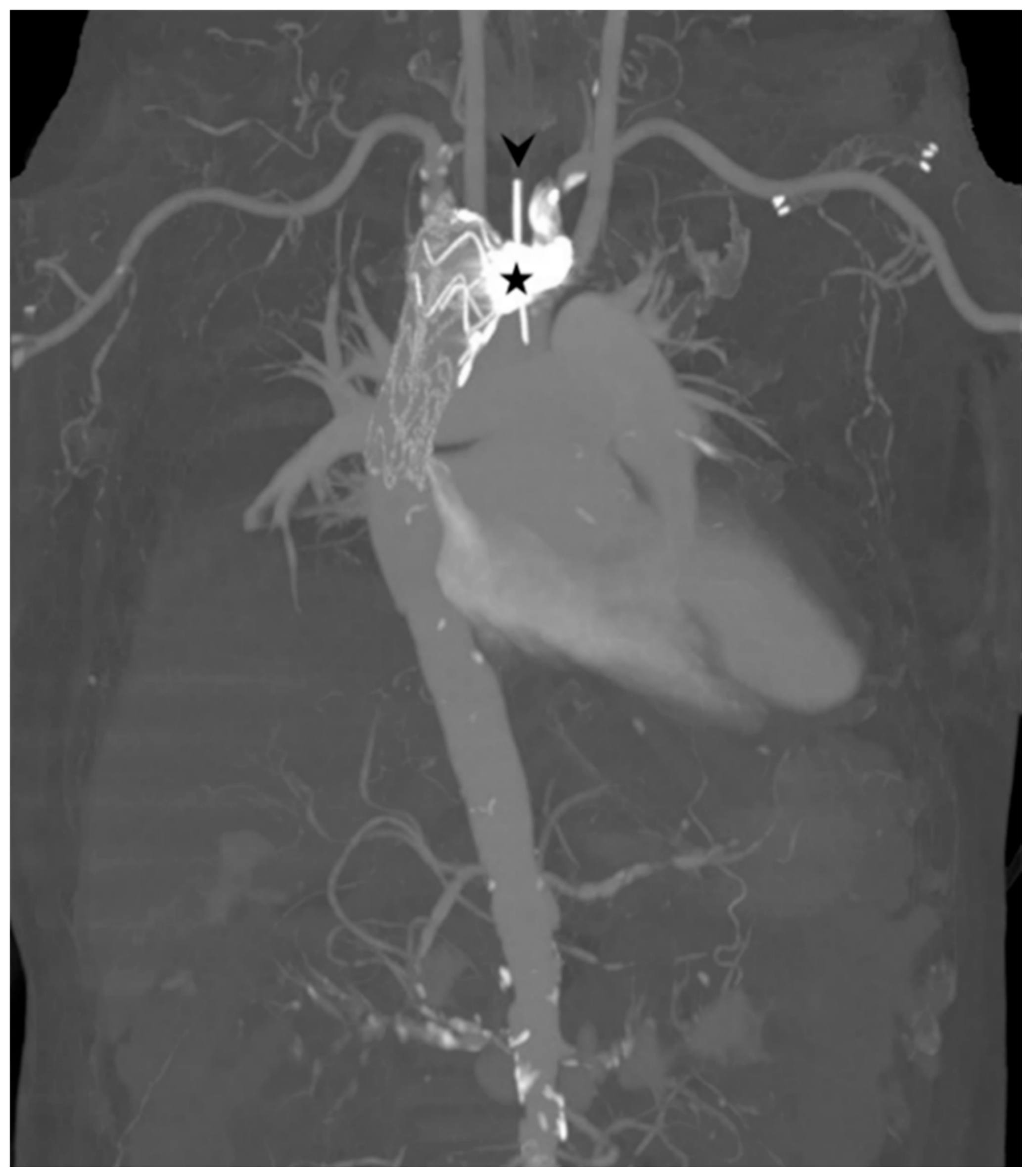
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hollander, J.E.; Quick, G. Aortoesophageal fistula: A comprehensive review of the literature. Am. J. Med. 1991, 91, 279–287. [Google Scholar] [CrossRef]
- Millar, A.; Rostom, A.; Rasuli, P.; Saloojee, N. Upper gastrointestinal bleeding secondary to an aberrant right subclavian artery-esophageal fistula: A case report and review of the literature. Can. J. Gastroenterol. 2007, 21, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Popieluszko, P.; Henry, B.M.; Sanna, B.; Hsieh, W.C.; Saganiak, K.; Kala, P.A.P.; Walocha, J.A.; Tomaszewski, K.A. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J. Vasc. Surg. 2018, 68, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hanneman, K.; Newman, B.; Chan, F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics 2017, 37, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Anastacio, M.; Marques, A. Aberrant right subclavian artery-esophageal fistula: Massive upper gastrointestinal hemorrhage secondary to prolonged intubation. Braz. J. Anesthesiol. 2016, 66, 318–320. [Google Scholar] [CrossRef]
- Turkvatan, A.; Buyukbayraktar, F.G.; Olcer, T.; Cumhur, T. Congenital anomalies of the aortic arch: Evaluation with the use of multidetector computed tomography. Korean J. Radiol. 2009, 10, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Merchant, F.J.; Nichols, R.L.; Bombeck, C.T. Unusual complication of nasogastric esophageal intubation-erosion into an aberrant right subclavian artery. J. Cardiovasc. Surg. 1977, 18, 147–150. [Google Scholar]
- Watanabe, M.; Suzuki, K.; Fujinaga, K.; Yamamoto, A.; Fujioka, M.; Katayama, N.; Imai, H. Postmortem diagnosis of massive gastrointestinal bleeding in a patient with aberrant right subclavian artery-esophageal fistula. Acute Med. Surg. 2016, 3, 139–142. [Google Scholar] [CrossRef][Green Version]
- Joynt, M.R.; Grifka, R.G. Spontaneous aberrant right subclavian arterio-oesophageal fistula in a previously healthy child. Cardiol. Young 2015, 25, 1425–1427. [Google Scholar] [CrossRef]
- Edwards, B.S.; Edwards, W.D.; Connolly, D.C.; Edwards, J.E. Arterial-esophageal fistulae developing in patients with anomalies of the aortic arch system. Chest 1984, 86, 732–735. [Google Scholar] [CrossRef]
- Livesay, J.J.; Michals, A.A.; Dainko, E.C. Anomalous right subclavian arterial esophageal fistula: An unusual complication of tracheostomy. Tex. Heart Inst. J. 1982, 9, 105–108. [Google Scholar] [PubMed]
- Feugier, P.; Lemoine, L.; Gruner, L.; Bertin-Maghit, M.; Rousselet, B.; Chevalier, J.M. Arterioesophageal fistula: A rare complication of retroesophageal subclavian arteries. Ann. Vasc. Surg. 2003, 17, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Shires, C.B.; Rohrer, M.J. Anomalous Right Subclavian Artery-Esophageal Fistulae. Case Rep. Vasc. Med. 2018, 2018, 7541904. [Google Scholar] [CrossRef] [PubMed]
- Jungck, E.; Puschel, K. Erosion hemorrhage from an esophago-aortic fistula in congenital anomaly of the thoracic aorta as a fatal complication of a stomach tube. Anaesthesist 1983, 32, 498–500. [Google Scholar] [PubMed]
- Ikeda, T.; Yokota, Y.; Ando, F.; Okamoto, F.; Otani, S.; Nakanishi, K.; Sugita, T.; Nishimori, H.; Makino, S.; Yoshikawa, E. A case of an aberrant subclavian artery-esophageal fistula due to prolonged nasogastric intubation. Kyobu Geka 1991, 44, 1045–1047. [Google Scholar]
- Kudose, S.; Pineda, J.; Saito, J.M.; Dehner, L.P. Aberrant Right Subclavian Artery-Esophageal Fistula in 20-Year-Old with VATER Association. J. Pediatr. Intensive Care 2017, 6, 127–131. [Google Scholar]
- Belkin, R.I.; Keller, F.S.; Everts, E.C.; Rosch, J. Aberrant right subclavian artery--esophageal fistula: A cause of overwhelming upper gastrointestinal hemorrhage. Cardiovasc. Interv. Radiol. 1984, 7, 87–89. [Google Scholar] [CrossRef]
- Chapman, J.R.; Sedghi, S.; Christie, B.D.; Nakayama, D.K.; Wynne, J.L. Aberrant right subclavian artery-esophageal fistula. Am. Surg. 2010, 76, 1430–1432. [Google Scholar] [CrossRef]
- Vanden Eynden, F.; Deviere, J.; Laureys, M.; de Canniere, D. Erosion of a retroesophageal subclavian artery by an esophageal prosthesis. J. Thorac. Cardiovasc. Surg. 2006, 131, 1183–1184. [Google Scholar] [CrossRef]
- Feugier, P.; Lemoine, L.; Beaudoin, N.; Chevalier, J.M. Aberrant right subclavian arterioesophageal fistula: Endovascular occlusion via a transbrachial approach. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 77–78. [Google Scholar] [CrossRef]
- Fuentes, S.; Cano, I.; Lopez, M.; Moreno, C.; Tejedor, R.; Marianeschi, S.; García, E.; Gómez, A. Arterial-esophageal fistula: A severe complication in children with cardiovascular abnormalities. Pediatr. Surg. Int. 2010, 26, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, P.C.; Newman, K.D.; Ceithaml, E. Successful management of aberrant subclavian artery-esophageal fistula in an infant. Ann. Thorac. Surg. 1989, 47, 308–309. [Google Scholar] [CrossRef]
- Hirakata, R.; Hasuo, K.; Yasumori, K.; Yoshida, K.; Masuda, K. Arterioenteric fistulae: Diagnosis and treatment by angiography. Clin. Radiol. 1991, 43, 328–330. [Google Scholar] [CrossRef]
- Hosn, M.A.; Haddad, F.; El-Merhi, F.; Safadi, B.; Hallal, A. Repair of an aberrant subclavian arterioesophageal fistula following esophageal stent placement. World J. Gastrointest. Surg. 2014, 6, 117–121. [Google Scholar] [CrossRef][Green Version]
- Inman, J.C.; Kim, P.; McHugh, R. Retroesophageal subclavian artery--esophageal fistula: A rare complication of a salivary bypass tube. Head Neck 2008, 30, 1120–1123. [Google Scholar] [CrossRef]
- Jain, K.K.; Braze, A.J.; Shapiro, M.A.; Perez-Tamayo, R.A. Aberrant right subclavian artery-esophageal fistula and severe gastrointestinal bleeding after surgical correction of scimitar syndrome. Tex. Heart Inst. J. 2012, 39, 571–574. [Google Scholar]
- Lehmann, B.; Clemetson, I.; Fantin, A.C.; Henning, P.; Kipfer, B.; Mühlethaler, R.; Vetsch, G.; Dinkel, H.-P. Arterioesophageal fistula secondary to rupture of an aberrant right subclavian artery aneurysm: A rare differential diagnosis in upper gastrointestinal bleeding. Endoscopy 2006, 38, 762. [Google Scholar] [CrossRef][Green Version]
- Lo, A.; Baird, R.; De Angelis, P.; Lévesque, D.; Morinville, V.; di Abriola, G.F.; Caldero, T.; Laberge, J.-M.; Dall’Oglio, L. Arterioesophageal fistula after stenting for esophageal atresia. J. Pediatr. Gastroenterol. Nutr. 2013, 56, e30–e31. [Google Scholar] [CrossRef]
- Magagna, P.; Abbiate, N.; Mansi, G.; D’Onofrio, A.; Auriemma, S.; Piccin, C.; Savastano, S.; Fabbri, A. Endovascular treatment of aberrant right subclavian (lusorian) artery to oesophagus fistula: A case report. Vasc. Endovasc. Surg. 2008, 42, 394–396. [Google Scholar] [CrossRef]
- Merlo, A.; Farber, M.; Ohana, E.; Pascarella, L.; Crowner, J.; Long, J. Aberrant Right Subclavian Artery to Esophageal Fistula: A Rare Case and Its Management. Ann. Thorac. Surg. 2020, 110, e85–e86. [Google Scholar] [CrossRef]
- Stone, W.M.; Brewster, D.C.; Moncure, A.C.; Franklin, D.P.; Cambria, R.P.; Abbott, W.M. Aberrant right subclavian artery: Varied presentations and management options. J. Vasc. Surg. 1990, 11, 812–817. [Google Scholar] [CrossRef]
- Morisaki, A.; Hirai, H.; Sasaki, Y.; Hige, K.; Bito, Y.; Suehiro, S. Aortoesophageal fistula after endovascular repair for aberrant right subclavian artery aneurysm. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Robie, D.K.; Davis, S.L.; Cooley, D.A.; Klish, W.J.; Skolkin, M.D.; Kearney, D.L.; Jaksic, T. Survival after aberrant right subclavian artery-esophageal fistula: Case report and literature review. J. Vasc. Surg. 1996, 24, 271–275. [Google Scholar] [CrossRef]
- Pop, D.; Venissac, N.; Nadeemy, A.S.; Schneck, A.S.; Aze, O.; Mouroux, J. Lesson to be learned: Beware of lusoria artery during transhiatal esophagectomy. Ann. Thorac. Surg. 2012, 94, 1010–1011. [Google Scholar] [CrossRef]
- Singha, N.K.; Hale, S.J.; Kuhlman, J.E. Arterio-esophageal communication from a ruptured aberrant right subclavian artery aneurysm. CT diagnosis. Clin. Imaging 1998, 22, 117–121. [Google Scholar] [CrossRef]
- Situma, M.; Kubiak, R.; Numanoglu, A.; Wood, R.; Brooks, A.; Millar, A.J. Near-fatal bleeding from an aberrant subclavian artery following colonic interposition for oesophageal atresia. Pediatr. Surg. Int. 2011, 27, 1131–1133. [Google Scholar] [CrossRef]
- Takahashi, S.; Okada, K.; Orihashi, K.; Sueda, T. Arterio-oesophageal fistula caused by aberrant right subclavian artery aneurysm. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 920–922. [Google Scholar] [CrossRef][Green Version]
- Warshauer, D.M.; Walters, T.P.; Detterbeck, F.C. Aberrant left subclavian artery aneurysm with esophago-arterial fistula. CT demonstration. Clin. Imaging 1993, 17, 276–278. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, X.; Chen, S.; Wei, J.; Wei, Z.; Miao, L.; Zhang, X. A Pseudoaneurysm of Aberrant Right Subclavian Artery Caused by Esophageal Stent Placement Because of Esophageal Stricture After Endoscopic Submucosal Dissection. Surg. Laparosc. Endosc. Percutan. Tech. 2019, 29, e69–e71. [Google Scholar] [CrossRef]
- Kullnig, P. Aneurysm of an aberrant right subclavian artery with bleeding into the esophageal wall. Semin. Roentgenol. 1989, 24, 75–76. [Google Scholar] [CrossRef]
- Minyard, A.N.; Smith, D.M. Arterial-esophageal fistulae in patients requiring nasogastric esophageal intubation. Am. J. Forensic Med. Pathol. 2000, 21, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Gossot, D.; Nussaume, O.; Kitzis, M.; Cohen, G.; Chalaux, G.; Andreassian, B. Fatal hematemesis due to erosion of a retro-esophageal right subclavian artery by an esophagogastric tube. Presse Med. 1985, 14, 1655–1656. [Google Scholar] [PubMed]
- Buades Reynes, J.; Aguirre Errasti, C.; Bilbao Ercoreca, F.J.; Onate Landa, A. Letter: Aneurysm of aberrant right subclavian artery with esophageal perforation. Chest 1976, 70, 105. [Google Scholar] [CrossRef]
- Lynn, R.B. Kommerell’s diverticulum with esophago-arterial fistula. Can. J. Surg. 1969, 12, 331–333. [Google Scholar] [PubMed]



| References | Sex | Age (year) | Causes | ETT * | NGT † | Endoscopic Findings | Treatment | Outcome | Sentinel Bleed |
|---|---|---|---|---|---|---|---|---|---|
| Lynn (1969) [44] | M | 57 | ARSA aneurysm | N | N | NIA | S | Died | NIA |
| Reynes et al. (1976) [43] | F | 72 | ARSA aneurysm | N | N | N | N | Died | Y |
| Merchant et al. (1977) [7] | F | 17 | NGT | N | Y | NIA | N | Died | NIA |
| Livesay et al. (1982) [11] | M | 25 | ETT/NGT | Y | Y | Mucosal defect in upper esophagus | B & S | Died | N |
| Jungck and Puschel (1983) [14] | M | 6 | ETT/NGT | Y | Y | NIA | B | Died | N |
| Belkin et al. (1984) [17] | M | 27 | NGT | N | Y | Fungating lesion on posterior wall of esophagus | B & S | Died | N |
| Edwards et al. (1984) [10] | M | 79 | ARSA aneurysm | N | N | Extrinsic compression at posterior wall of the esophagus | S | Died | Y |
| F | 36 | NGT | Y | Y | Active hemorrhage in the esophagus | N | Died | Y | |
| Gossot et al. (1985) [42] | F | 72 | ETT/NGT | Y | Y | NIA | NIA | Died | NIA |
| Guzzetta et al. (1989) [22] | F | 4 mo | NGT | Y | Y | N | S | Survived | Y |
| Kullnig (1989) [40] | M | 66 | ARSA aneurysm | N | N | N | N | Died | Y |
| Stone et al. (1990) [31] | M | 72 | ETT | Y | NIA | N | S | Died | N |
| Ikeda et al. (1991) [15] | M | 9 | ETT/NGT | Y | Y | NIA | NIA | Died | NIA |
| Hirakata et al. (1991) [23] | M | 55 | NGT | NIA | Y | N | B | Survived | N |
| Warshauer et al. (1993) [38] | M | 73 | ALSA aneurysm | Y | NIA | Extrinsic compression of esophagus | S | Survived | Y |
| Miller et al. (1996) [33] | F | 11 | ETT/NGT | Y | Y | Focal bleeding in the esophagus | B & S | Survived | N |
| Singha et al. (1998) [35] | M | 82 | ARSA aneurysm | N | N | Bleeding from fungating mass in upper esophagus | N | Died | N |
| Minyard and Smith (2000) [41] | F | 39 | NGT | NIA | Y | Normal esophagus | N | Died | NIA |
| Feugier et al. (2002) [20] | M | 24 | ETT/NGT | Y | Y | NIA | S | Survived | N |
| Eynden (2006) [19] | F | 9 | Esophageal stent | NIA | NIA | Blood oozing around the prosthesis | S & V | Survived | Y |
| Lehmann et al. (2006) [27] | M | 78 | Rupture of an ARSA aneurysm | N | N | Necrotic polypoid lesion in upper esophagus | B | Died | N |
| Milar et al. (2007) [2] | M | 57 | Esophageal carcinoma with esophagectomy and grastric pull-up | N | Y | A small anastomotic ulcer | S & V | Died | Y |
| Inman et al. (2008) [25] | M | 63 | Salivary bypass tube | NIA | Y | Bleeding focus not identified | S, V, & B | Died | Y |
| Magagna et al. (2008) [29] | F | 73 | Laryngeal carcinoma with prolonged radiotherapy | Y | N | Esophagitis and blood clots | B &V | Survived | Y |
| Fuentes et al. (2010) [21] | F | 3 | Esophageal prosthesis insertion | NIA | NIA | N | B, V, & S | Survived | N |
| Chapman et al. (2010) [18] | F | 34 | NGT | Y | Y | Active arterial bleeding at 25 cm from the incisors | B & S | Died | N |
| Situma et al. (2011) [36] | F | 5 mo | Colonic interposition for esophageal atresia with a distal fistula | Y | Y | Blood clots at the colon-gastric junction | S | Survived | N |
| Jain et al. (2012) [26] | F | 57 | ETT/NGT | Y | Y | Vigorous bleeding from ARSA–esophageal fistula | B & S | Survived | N |
| Pop et al. (2012) [34] | M | 67 | Operation for esophageal cancer | NIA | N | Arterioesophageal Fistula | B & V | Died | N |
| Takahashi et al. (2013) [37] | M | 63 | ARSA aneurysm | N | N | Esophageal ulcer covered with fibrinous tissue | V &S | Survived | Y |
| Lo et al. (2013) [28] | NIA | 16 mo | Stent insertion for esophageal atresia | N | Y | NIA | B, V, & | Died | N |
| 15 mo | N | Y | NIA | S | Survived | Y | |||
| Morisaki et al. (2014) [32] | F | 74 | Endovascular repair for ARSA aneurysm | NIA | NIA | esophageal mucosal erosion and fistula | B, V, & S | Died | N |
| Hosn et al. (2014) [24] | F | 29 | Esophageal stent | N | Y | Teflon patch | S | Survived | N |
| Joynt and Grifka (2015) [9] | NIA | 17 mo | Spontaneous fistula | N | N | Small mucosa abnormality with white plaque | V & S | Survived | Y |
| Oliveira et al. (2016) [5] | M | 20 | ETT/NGT | Y | Y | Esophageal ulcer with copious bleeding | S | Survived | N |
| Kudose et al. (2017) [16] | F | 20 | ETT/NGT | Y | Y | NIA | N | Died | N |
| Shires et al. (2018) [13] | F | 41 | Tracheostomy tube/NGT | Y | Y | N | V & S | Died | Y |
| Zheng et al. (2019) [39] | M | 67 | Esophageal stent | NIA | NIA | N | V | Died | N |
| Merlo et al. (2020) [30] | F | 29 | Esophageal stent | Y | Y | Esophageal perforation | V & S | Survived | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jeon, K.N.; Bae, K. Aberrant Left Subclavian Artery-Esophageal Fistula in a Patient with a Prolonged Use of Nasogastric Tube: A Case Report and Literature Review. Diagnostics 2021, 11, 195. https://doi.org/10.3390/diagnostics11020195
Kim S, Jeon KN, Bae K. Aberrant Left Subclavian Artery-Esophageal Fistula in a Patient with a Prolonged Use of Nasogastric Tube: A Case Report and Literature Review. Diagnostics. 2021; 11(2):195. https://doi.org/10.3390/diagnostics11020195
Chicago/Turabian StyleKim, Sungbin, Kyung Nyeo Jeon, and Kyungsoo Bae. 2021. "Aberrant Left Subclavian Artery-Esophageal Fistula in a Patient with a Prolonged Use of Nasogastric Tube: A Case Report and Literature Review" Diagnostics 11, no. 2: 195. https://doi.org/10.3390/diagnostics11020195
APA StyleKim, S., Jeon, K. N., & Bae, K. (2021). Aberrant Left Subclavian Artery-Esophageal Fistula in a Patient with a Prolonged Use of Nasogastric Tube: A Case Report and Literature Review. Diagnostics, 11(2), 195. https://doi.org/10.3390/diagnostics11020195






