Clinical Analysis of Early-Stage Pancreatic Cancer and Proposal for a New Diagnostic Algorithm: A Multicenter Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging and Pathological Diagnosis
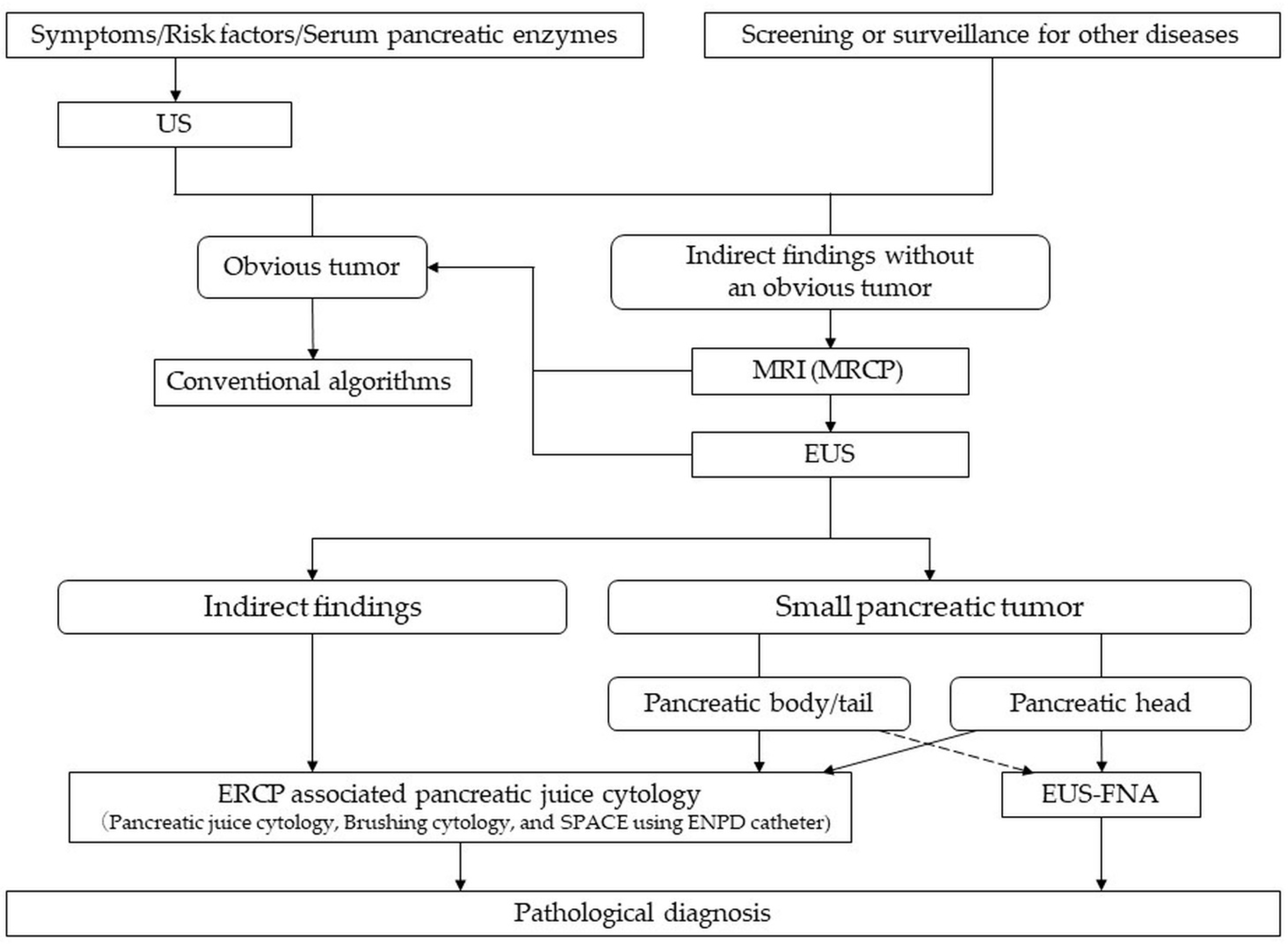
2.2.1. Imaging Diagnosis
2.2.2. Pathological Diagnosis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Reasons for Medical Examination
3.3. Examination
3.3.1. Blood Tests
3.3.2. Imaging Diagnosis
3.4. Preoperative Pathological Diagnosis
3.5. Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Center for Cancer Control and Information Services. Available online: https://ganjoho.jp/reg_stat/statistics/stat/summary (accessed on 4 December 2020).
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan pancreatic cancer registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Hur, C.; Tramontano, A.C.; Dowling, E.C.; Brooks, G.A.; Jeon, A.; Brugge, W.R.; Gazelle, G.S.; Kong, C.Y.; Pandharipande, P.V. Early pancreatic ductal adenocarcinoma survival is dependent on size: Positive implications for future targeted screening. Pancreas 2016, 45, 1062–1066. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: A prospective study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 mm) pancreatic adenocarcinomas: Analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Yamao, K.; Takenaka, M.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Kamata, K.; Minaga, K.; Matsumoto, I.; et al. Partial pancreatic parenchymal atrophy is a new specific finding to diagnose small pancreatic cancer (≤10 mm) including carcinoma in situ: Comparison with localized benign main pancreatic duct stenosis patients. Diagnostics 2020, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Nakahodo, J.; Kikuyama, M.; Nojiri, S.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Horiguchi, S.I.; Honda, G. Focal parenchymal atrophy of pancreas: An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology 2020, 20, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Japan Pancreas Society. General Rules for the Study of Pancreatic Cancer, 7th ed.; Kanehara & Co: Tokyo, Japan, 2016; pp. 12–57. [Google Scholar]
- Satoh, T.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Muro, H.; Hanada, K. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (SPACE). Clin. J. Gastroenterol. 2017, 10, 541–545. [Google Scholar] [CrossRef]
- Cotton, P.B.; Lehman, G.; Vennes, J.; Geenen, J.E.; Russell, R.C.; Meyers, W.C.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: A Synopsis. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Hanada, K.; Minami, T.; Shimizu, A.; Fukuhara, M.; Yano, S.; Sasaki, K.; Koda, M.; Sugiyama, K.; Yonehara, S.; Yanagisawa, A. Roles of ERCP in the early diagnosis of pancreatic cancer. Diagnostics 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Minami, T.; Ikemoto, J.; Kanemitsu, K.; Hino, F. Effective screening for early diagnosis of pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 929–939. [Google Scholar] [CrossRef]
- Sharma, C.; Eltawil, K.M.; Renfrew, P.D.; Walsh, M.J.; Molinari, M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990–2010. World J. Gastroenterol. 2011, 17, 867–897. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kim, M.H.; Lee, T.Y.; Kwon, S.; Oh, H.C.; Lee, S.S.; Seo, D.W.; Lee, S.K. Clinicopathological aspects of 542 cases of pancreatic cancer: A special emphasis on small pancreatic cancer. J. Korean Med. Sci. 2007, 22, S79–S85. [Google Scholar] [CrossRef]
- Hanada, K.; Amano, H.; Abe, T. Early diagnosis of pancreatic cancer: Current trends and concerns. Ann. Gastroenterol. Surg. 2017, 1, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Kikuyama, M.; Kitano, M. Advances in early detection of pancreatic cancer. Diagnostics 2019, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Kurihara, K.; Hanada, K.; Shimizu, A. Endoscopic ultrasonography diagnosis of early pancreatic cancer. Diagnostics 2020, 10, 1086. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Yasuda, I.; Iwashita, T.; Doi, S.P.; Nakashima, M.; Moriwaki, H. Role of EUS in the early detection of small pancreatic cancer. Dig. Endosc. 2011, 23, 22–25. [Google Scholar] [CrossRef]
- Kumagi, T.; Terao, T.; Yokota, T.; Azemoto, N.; Kuroda, T.; Imamura, Y.; Uesugi, K.; Kisaka, Y.; Tanaka, Y.; Shibata, N.; et al. Early detection of pancreatic cancer in patients with chronic liver disease under hepatocellular carcinoma surveillance. Mayo Clin. Proc. 2019, 94, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ohtsuka, T.; Matsunaga, T.; Watanabe, Y.; Tamura, K.; Ideno, N.; Aso, T.; Miyazaki, T.; Osoegawa, T.; Aishima, S.; et al. Predictors and diagnostic strategies for early-stage pancreatic ductal adenocarcinoma a retrospective study. Pancreas 2015, 44, 1148–1154. [Google Scholar] [CrossRef]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: Establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef]
- Cheng, C.L.; Sherman, S.; Watkins, J.L.; Barnett, J.; Freeman, M.; Geenen, J.; Ryan, M.; Parker, H.; Frakes, J.T.; Fogel, E.L.; et al. Risk factors for post-ERCP pancreatitis: A prospective multicenter study. Am. J. Gastroenterol. 2006, 101, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; DiSario, J.A.; Nelson, D.B.; Fennerty, M.B.; Lee, J.G.; Bjorkman, D.J.; Overby, C.S.; Aas, J.; Ryan, M.E.; Bochna, G.S.; et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest. Endosc. 2001, 54, 425–434. [Google Scholar] [CrossRef]
- Testoni, P.A.; Mariani, A.; Giussani, A.; Vailati, C.; Masci, E.; Macarri, G.; Ghezzo, L.; Familiari, L.; Giardullo, N.; Mutignani, M.; et al. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: A prospective multicenter study. Am. J. Gastroenterol. 2010, 105, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Mouri, T.; Sasaki, T.; Serikawa, M.; Ishigaki, T.; Ishii, Y.; Shimizu, A.; Tsuboi, T.; Kurihara, K.; Tatsukawa, Y.; Miyaki, E.; et al. A comparison of 4-Fr with 5-Fr endoscopic nasopancreatic drainage catheters: A randomized, controlled trial. J. Gastroenterol. Hepatol. 2016, 31, 1783–1789. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.L.; Zhao, Y.P.; Dai, M.H.; Zhang, T.P. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology 2013, 13, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Banafea, O.; Mghanga, F.P.; Zhao, J.F.; Zhao, R.F.; Zhu, L.R. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Teraoka, Y.; Ikemoto, J.; Kanemitsu, K.; Hino, F.; Fukuda, T.; Yonehara, S. Diagnostic strategies for early pancreatic cancer. J. Gastroenterol. 2015, 50, 147–154. [Google Scholar] [CrossRef]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ. Endosc. Int. Open 2019, 7, E585–E593. [Google Scholar] [CrossRef]
- Yane, K.; Kuwatani, M.; Yoshida, M.; Goto, T.; Matsumoto, R.; Ihara, H.; Okuda, T.; Taya, Y.; Ehira, N.; Kudo, T.; et al. Non-negligible rate of needle tract seeding after endoscopic ultrasound-guided fine-needle aspiration for patients undergoing distal pancreatectomy for pancreatic cancer. Dig. Endosc. 2020, 32, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Takenaka, M.; Katanuma, A.; Kitano, M.; Yamashita, Y.; Kamata, K.; Yamao, K.; Watanabe, T.; Maguchi, H.; Kudo, M. Needle tract seeding: An overlooked rare complication of endoscopic ultrasound-guided fine-needle aspiration. Oncology 2017, 93, 107–112. [Google Scholar] [CrossRef]
- Japan Pancreas Society. Available online: http://suizou.org/pdf/guide2019_P176-179.pdf (accessed on 29 October 2019).
- Yu, J.; Li, A.; Hong, S.M.; Hruban, R.H.; Goggins, M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res. 2012, 18, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Ohtsuka, T.; Kono, H.; Nagayoshi, Y.; Ideno, N.; Aso, T.; Kozono, S.; Ohuchida, K.; Takahata, S.; Nakamura, M.; et al. A Minimally invasive and simple Screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas 2013, 42, 187–192. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 96) | Stage 0 (n = 40) | Stage IA (n = 56) | |
|---|---|---|---|
| Sex (men/women) | 47/49 | 25/15 | 22/34 |
| Age, mean ± SD (range) | 71 ± 9.4 (39–88) | 72 ± 8.3 (52–86) | 71 ± 10.2 (39–88) |
| Location, head/body/tail/multiple | 30/50/12/4 | 10/23/6/1 | 20/27/6/3 |
| Risk Factors, n (%) * | |||
| DM | 26/96 (27) | 8/40 (20) | 18/56 (32) |
| Tobacco use | 29/95 (31) | 15/40 (38) | 14/55 (25) |
| Heavy alcohol consumption | 24/94 (26) | 15/39 (38) | 9/55 (16) |
| Obesity (> BMI 30 kg/m2) | 3/96 (3.1) | 2/40 (5.0) | 1/56 (1.8) |
| Past history of acute pancreatitis | 6/93 (6.5) | 1/39 (2.6) | 5/54 (9.3) |
| Chronic pancreatitis | 7/94 (7.4) | 5/40 (13) | 2/54 (3.7) |
| IPMN/Pancreatic cyst | 27/95 (28) | 15/40 (38) | 12/55 (22) |
| Family history of pancreatic cancer | 2/84 (2.4) | 1/38 (2.6) | 1/46 (2.2) |
| Familial pancreatic cancer | 0/96 (0) | 0/38 (0) | 0/46 (0) |
| Hereditary pancreatic cancer syndrome | 0/96 (0) | 0/38 (0) | 0/46 (0) |
| Hepatitis B virus infection | 3/93 (3.2) | 0/39 (0) | 3/54 (5.6) |
| All Patients (n = 96) | Stage 0 (n = 40) | Stage IA (n = 56) | |
|---|---|---|---|
| Symptoms | 27/96 (28) | 13/40 (33) | 14/56 (25) |
| Abdominal pain/Back pain/Loss weight/Jaundice/Others | 18/4/3/1/4 | 10/1/2/1/2 | 8/3/1/0/2 |
| Abnormalities identified on medical health check-ups | 26/96 (27) | 8/40 (20) | 18/56 (32) |
| Abnormal findings on US | 18/26 (69) | 5/8 (63) | 13/18 (72) |
| Detection of tumors/Indirect imaging findings | 4/14 | 0/5 | 4/9 |
| Abnormal findings on CT | 2/26 (7.7) | 0/8 (0) | 2/18 (11) |
| Detection of tumors/Indirect imaging findings | 1/1 | 0/0 | 1/1 |
| Elevated serum pancreatic enzyme levels | 3/26 (11) | 2/8 (25) | 1/18 (5.6) |
| Onset or exacerbation of DM | 3/26 (11) | 1/8 (13) | 2/18 (11) |
| Abnormalities incidentally detected during screening or surveillance for other disease | 35/96 (36) | 16/40 (40) | 19/56 (34) |
| Abnormal findings on US | 4/35 (11) | 3/16 (19) | 1/19 (5.3) |
| Detection of tumors/Indirect imaging findings | 0/4 | 0/3 | 0/1 |
| Abnormal findings on CT | 19/35 (54) | 9/16 (56) | 10/19 (53) |
| Detection of tumors/Indirect imaging findings | 4/15 | 1/8 | 3/7 |
| Elevated serum pancreatic enzyme levels | 2/35 (5.7) | 1/16 (6.3) | 1/19 (5.3) |
| Elevated serum tumor marker level | 1/35 (2.9) | 0/16 (0) | 1/19 (5.3) |
| Onset or exacerbation of DM | 5/35 (14) | 1/16 (6.3) | 4/19 (21) |
| Others | 4/35 (11) | 2/16 (13) | 2/19 (11) |
| Screening primary disease DM/Liver disease/Other cancer/Lung disease/Other | 8/6/12/3/6 | 3/2/5/1/5 | 5/4/7/2/1 |
| Abnormalities during follow-up of pancreatic disease | 6/96 (6.3) | 3/40 (7.5) | 3/56 (5.4) |
| Pancreatic cysts/Acute pancreatitis/Chronic pancreatitis | 3/1/2 | 1/0/2 | 2/1/0 |
| Unknown | 2/96 (2.1) | 0/40 (0) | 2/56 (3.6) |
| All Patients (n = 96) | Stage 0 (n = 40) | Stage IA (n = 56) | |
|---|---|---|---|
| Abnormalities of pancreatic enzymes | 46/94 (49) | 19/39 (49) | 27/55(49) |
| Increased amylase | 19/77 (25) | 9/31 (29) | 10/46(22) |
| Depressed amylase | 5/77 (6.5) | 3/31 (9.7) | 2/46(4.3) |
| Increased pancreatic amylase | 22/74 (30) | 11/37 (30) | 11/37 (30) |
| Depressed pancreatic amylase | 3/74 (4.1) | 1/37 (2.7) | 2/37 (5.4) |
| Increased lipase level | 21/44 (48) | 6/13 (46) | 15/31 (48) |
| Depressed lipase | 1/44 (2.3) | 0/13 (0) | 1/31 (3.2) |
| Increased elastase 1 | 7/24 (29) | 2/6 (33) | 5/18 (28) |
| Abnormal sugar tolerance | 25/93 (27) | 8/39 (21) | 17/54 (31) |
| Increased tumor markers level | |||
| CEA | 6/87 (6.9) | 1/37 (2.7) | 5/50 (10) |
| CA19-9 | 25/94 (27) | 4/38 (11) | 21/56 (38) |
| DUPAN-2 | 9/52 (17) | 1/21 (4.8) | 8/31 (26) |
| SPAN-1 | 6/32 (19) | 1/10 (10) | 5/22 (23) |
| All Patients (n = 96) | Stage 0 (n = 40) | Stage IA (n = 56) | |
|---|---|---|---|
| Performed, US/CT/MRI/EUS/ERCP | 51/92/75/95/86 | 13/39/36/40/38 | 38/53/39/55/48 |
| Detection of pancreatic tumors | |||
| US | 20/51 (39) | 0/13 (0) | 20/38 (53) |
| CT | 37/92 (40) | 6/39 (15) | 31/53 (58) |
| MRI | 18/75 (24) | 3/36 (8.3) | 15/39 (38) |
| EUS | 53/95 (56) | 8/40 (20) | 45/55 (82) |
| Indirect imaging findings | |||
| MPD dilatation | |||
| US | 31/51 (61) | 8/13 (62) | 23/38 (61) |
| CT | 67/92 (73) | 28/39 (72) | 39/53 (74) |
| MRI (MRCP) | 55/75 (73) | 28/36 (78) | 27/39 (69) |
| EUS | 71/92 (77) | 31/40 (78) | 40/52 (77) |
| ERCP | 56/86 (65) | 23/38 (61) | 33/48 (69) |
| MPD stenosis | |||
| MRI (MRCP) | 61/75 (81) | 31/36 (86) | 30/39 (77) |
| EUS | 53/92 (60) | 29/40 (73) | 24/52 (46) |
| ERCP | 75/86 (87) | 31/38 (82) | 44/48 (92) |
| Pancreatic cysts | |||
| US | 13/50 (26) | 5/13 (38) | 8/38 (21) |
| CT | 35/92 (38) | 16/39 (41) | 19/53 (36) |
| MRI (MRCP) | 37/75 (49) | 18/36 (50) | 19/39 (49) |
| EUS | 34/92 (37) | 16/40 (40) | 18/52 (35) |
| Localized pancreatic tissue atrophy | |||
| CT | 30/92 (33) | 12/39 (31) | 18/53 (34) |
| EUS | 7/84 (8.3) | 4/40 (10) | 3/52 (5.8) |
| Hypoechoic area surrounding the MPD stenosis | |||
| EUS | 35/92 (38) | 18/40 (45) | 17/52 (33) |
| All Patients (n = 96) | Stage 0 (n = 40) | Stage IA (n = 56) | |
|---|---|---|---|
| ERCP | 86/96 (90) | 38/40 (95) | 48/56 (86) |
| Confirmation of malignancy | 72/86 (84) | 33/38 (87) | 39/48 (81) |
| Single aspiration of pancreatic juice | 26/68 (38) | 15/34 (44) | 11/34 (32) |
| Brushing cytology of MPD stenosis | 14/20 (70) | 3/3 (100) | 11/17 (65) |
| SPACE | 58/75 (75) | 29/35 (83) | 29/40 (73) |
| EUS-FNA | 18/96 (19) | 3/40 (7.5) | 15/56 (27) |
| Confirmation of malignancy | 15/18 (83) | 1/3 (33) | 14/15 (93) |
| Cytology | 14/18 (78) | 1/3 (33) | 13/15 (87) |
| Biopsy | 10/18 (56) | 1/3 (33) | 9/15 (60) |
| Preoperative confirmation of malignancy | 79/96 (82) | 33/40 (83) | 46/56 (82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikemoto, J.; Serikawa, M.; Hanada, K.; Eguchi, N.; Sasaki, T.; Fujimoto, Y.; Sugiyama, S.; Yamaguchi, A.; Noma, B.; Kamigaki, M.; et al. Clinical Analysis of Early-Stage Pancreatic Cancer and Proposal for a New Diagnostic Algorithm: A Multicenter Observational Study. Diagnostics 2021, 11, 287. https://doi.org/10.3390/diagnostics11020287
Ikemoto J, Serikawa M, Hanada K, Eguchi N, Sasaki T, Fujimoto Y, Sugiyama S, Yamaguchi A, Noma B, Kamigaki M, et al. Clinical Analysis of Early-Stage Pancreatic Cancer and Proposal for a New Diagnostic Algorithm: A Multicenter Observational Study. Diagnostics. 2021; 11(2):287. https://doi.org/10.3390/diagnostics11020287
Chicago/Turabian StyleIkemoto, Juri, Masahiro Serikawa, Keiji Hanada, Noriaki Eguchi, Tamito Sasaki, Yoshifumi Fujimoto, Shinichiro Sugiyama, Atsushi Yamaguchi, Bunjiro Noma, Michihiro Kamigaki, and et al. 2021. "Clinical Analysis of Early-Stage Pancreatic Cancer and Proposal for a New Diagnostic Algorithm: A Multicenter Observational Study" Diagnostics 11, no. 2: 287. https://doi.org/10.3390/diagnostics11020287
APA StyleIkemoto, J., Serikawa, M., Hanada, K., Eguchi, N., Sasaki, T., Fujimoto, Y., Sugiyama, S., Yamaguchi, A., Noma, B., Kamigaki, M., Minami, T., Okazaki, A., Yukutake, M., Ishii, Y., Mouri, T., Shimizu, A., Tsuboi, T., Arihiro, K., & Chayama, K. (2021). Clinical Analysis of Early-Stage Pancreatic Cancer and Proposal for a New Diagnostic Algorithm: A Multicenter Observational Study. Diagnostics, 11(2), 287. https://doi.org/10.3390/diagnostics11020287







