PD-L1 Expression Is an Independent Marker for Lymph Node Metastasis in Middle Eastern Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Tissue Microarray Construction and Immunohistochemistry
2.3. POLE Mutation Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. PD-L1 Protein Expression in Endometrial Cancer and Its Clinico-Pathological Associations
3.3. Prognostic Impact of PD-L1 Expression in Endometrial Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.; Yu, M.; Cronin, K.A. (Eds.) SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Alrawaji, A.; Alshahrani, Z.; Alzahrani, W.; Alomran, F.; Almadouj, A.; Alshehri, S.; Alzahrani, A.; Bazarbashi, S.; Alhashmi, H.; Almutlaq, H.; et al. Cancer Incidence Report Saudi Arabia 2015. In Saudi Cancer Registry; Saudi Health Council 2015: Riyadh, Saudi Arabia, 2018. [Google Scholar]
- Althubiti, M.A.; Eldein, M.M.N. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J. 2018, 39, 1259. [Google Scholar] [CrossRef]
- World Health Organization; Global Health Observatory (GHO) Data. Overweight and Obesity, Adults Aged 18+. World Health Organization [updated 16 June 2015]. Available online: http://www.who.int/gho/ncd/risk_factors/overweight_text/en (accessed on 22 December 2020).
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer 2016, 26. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, M.; Janssens, V. Targeted therapies in type II endometrial cancers: Too little, but not too late. Int. J. Mol. Sci. 2018, 19, 2380. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, rv324–rv328. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.; Jin, L.; Zhang, X. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Li, S.; Mao, L.; Lian, B.; Wang, X. Axitinib in combination with toripalimab, a humanized immunoglobulin G4 monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: An open-label phase IB trial. J. Clin. Oncol. 2019, 37, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.O.; Ogunniyi, A.; Barbee, M.S.; Drilon, A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev. Anticancer Ther. 2016, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Antill, Y.C.; Kok, P.S.; Robledo, K.; Barnes, E.; Friedlander, M.; Baron-Hay, S.E.; Shannon, C.M.; Coward, J.; Beale, P.J.; Goss, G. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J. Clin. Oncol. 2019, 37, 5501. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- McDermott, D.F.; Sosman, J.A.; Sznol, M.; Massard, C.; Gordon, M.S.; Hamid, O.; Powderly, J.D.; Infante, J.R.; Fassò, M.; Wang, Y.V. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 2016, 34, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, A.; Ahvenainen, T.; Pellinen, T.; Vahteristo, P.; Loukovaara, M.; Bützow, R. PD-L1 expression in endometrial carcinoma cells and intratumoral immune cells: Differences across histologic and TCGA-based molecular subgroups. Am. J. Surg. Pathol. 2020, 44, 174–181. [Google Scholar] [CrossRef]
- Asaka, S.; Yen, T.-T.; Wang, T.-L.; Shih, I.-M.; Gaillard, S. T cell-inflamed phenotype and increased Foxp3 expression in infiltrating T-cells of mismatch-repair deficient endometrial cancers. Mod. Pathol. 2019, 32, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Lee, H.S.; Yang, W.; Cho, H.; Chay, D.B.; Cho, S.J.; Hong, S.; Kim, J.-H. Prognostic implication of programmed cell death 1 protein and its ligand expressions in endometrial cancer. Gynecol. Oncol. 2018, 149, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Joehlin-Price, A.S.; Rhoades, J.; Ayoola-Adeola, M.; Miller, K.; Parwani, A.V.; Backes, F.J.; Felix, A.S.; Suarez, A.A. Programmed death ligand 1 expression among 700 consecutive endometrial cancers: Strong association with mismatch repair protein deficiency. Int. J. Gynecol. Cancer 2018, 28, 59–68. [Google Scholar] [CrossRef]
- Bregar, A.; Deshpande, A.; Grange, C.; Zi, T.; Stall, J.; Hirsch, H.; Reeves, J.; Sathyanarayanan, S.; Growdon, W.B.; Rueda, B.R. Characterization of immune regulatory molecules B7-H4 and PD-L1 in low and high grade endometrial tumors. Gynecol. Oncol. 2017, 145, 446–452. [Google Scholar] [CrossRef]
- Inaguma, S.; Wang, Z.; Lasota, J.; Sarlomo-Rikala, M.; McCue, P.A.; Ikeda, H.; Miettinen, M. Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD-L1). Analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am. J. Surg. Pathol. 2016, 40, 1133. [Google Scholar] [CrossRef]
- Siraj, A.; Bavi, P.; Abubaker, J.; Jehan, Z.; Sultana, M.; Al-Dayel, F.; Al-Nuaim, A.; Alzahrani, A.; Ahmed, M.; Al-Sanea, O. Genome-wide expression analysis of Middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J. Pathol. 2007, 213, 190–199. [Google Scholar] [CrossRef]
- Bavi, P.; Jehan, Z.; Atizado, V.; Al-Dossari, H.; Al-Dayel, F.; Tulbah, A.; Amr, S.S.; Sheikh, S.S.; Ezzat, A.; El-Solh, H. Prevalence of fragile histidine triad expression in tumors from Saudi Arabia: A tissue microarray analysis. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1708–1718. [Google Scholar] [CrossRef][Green Version]
- Mesnage, S.; Auguste, A.; Genestie, C.; Dunant, A.; Pain, E.; Drusch, F.; Gouy, S.; Morice, P.; Bentivegna, E.; Lhomme, C. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann. Oncol. 2017, 28, 651–657. [Google Scholar] [CrossRef]
- Siraj, A.K.; Prabhakaran, S.; Bavi, P.; Bu, R.; Beg, S.; Hazmi, M.A.; Al-Rasheed, M.; Al-Assiri, M.; Sairafi, R.; Al-Dayel, F. Prevalence of Lynch syndrome in a Middle Eastern population with colorectal cancer. Cancer 2015, 121, 1762–1771. [Google Scholar] [CrossRef]
- Siraj, A.K.; Parvathareddy, S.K.; Bu, R.; Iqbal, K.; Siraj, S.; Masoodi, T.; Concepcion, R.M.; Ghazwani, L.O.; AlBadawi, I.; Al-Dayel, F. Germline POLE and POLD1 proofreading domain mutations in endometrial carcinoma from Middle Eastern region. Cancer Cell Int. 2019, 19, 334. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; von Pawel, J.; Park, K.; Rittmeyer, A.; Gandara, D.R.; Aix, S.P.; Han, J.-Y.; Gadgeel, S.M.; Hida, T.; Cortinovis, D.L. Updated efficacy analysis including secondary population results for OAK: A randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non–small cell lung cancer. J. Thorac. Oncol. 2018, 13, 1156–1170. [Google Scholar] [CrossRef]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.-P. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Liu, J.F.; Gordon, M.; Veneris, J.; Braiteh, F.; Balmanoukian, A.; Eder, J.P.; Oaknin, A.; Hamilton, E.; Wang, Y.; Sarkar, I. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol. 2019, 154, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Sungu, N.; Yildirim, M.; Desdicioglu, R.; Başaran Aydoğdu, Ö.; Kiliçarslan, A.; Tatli Doğan, H.; Kiliç Yazgan, A.; Akyol, M.; Erdoğan, F. Expression of immunomodulatory molecules PD-1, PD-L1, and PD-L2, and their relationship with clinicopathologic characteristics in endometrial cancer. Int. J. Gynecol. Pathol. 2019, 38, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, M.; Lataifeh, I.; Jaradat, I.; Abdeen, G.; Otay, L.; Badran, O.; Abu Sheikha, A.; Dayyat, A.; El Khaldi, M.; Ashi Al-Loh, S. Undifferentiated endometrial carcinoma, an immunohistochemical study including PD-L1 testing of a series of cases from a single cancer center. Int. J. Gynecol. Pathol. 2018, 37, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Engerud, H.; Berg, H.F.; Myrvold, M.; Halle, M.K.; Bjorge, L.; Haldorsen, I.S.; Hoivik, E.A.; Trovik, J.; Krakstad, C. High degree of heterogeneity of PD-L1 and PD-1 from primary to metastatic endometrial cancer. Gynecol. Oncol. 2020, 157, 260–267. [Google Scholar] [CrossRef]
- Mo, Z.; Liu, J.; Zhang, Q.; Chen, Z.; Mei, J.; Liu, L.; Yang, S.; Li, H.; Zhou, L.; You, Z. Expression of PD-1, PD-L1 and PD-L2 is associated with differentiation status and histological type of endometrial cancer. Oncol. Lett. 2016, 12, 944–950. [Google Scholar] [CrossRef]
- Kamiya, S.; Kato, J.; Kamiya, T.; Yamashita, T.; Sumikawa, Y.; Hida, T.; Horimoto, K.; Sato, S.; Takahashi, H.; Sawada, M. Association between PD-L1 expression and lymph node metastasis in cutaneous squamous cell carcinoma. Asia-Pac. J. Clin. Oncol. 2020, 16, e108–e112. [Google Scholar] [CrossRef]
- Slater, N.A.; Googe, P.B. PD-L1 expression in cutaneous squamous cell carcinoma correlates with risk of metastasis. J. Cutan. Pathol. 2016, 43, 663–670. [Google Scholar] [CrossRef]
- García-Pedrero, J.M.; Martínez-Camblor, P.; Diaz-Coto, S.; Munguia-Calzada, P.; Vallina-Alvarez, A.; Vazquez-Lopez, F.; Rodrigo, J.P.; Santos-Juanes, J. Tumor programmed cell death ligand 1 expression correlates with nodal metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J. Am. Acad. Dermatol. 2017, 77, 527–533. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Sloan, E.A.; Ring, K.L.; Willis, B.C.; Modesitt, S.C.; Mills, A.M. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including lynch syndrome-associated and MLH1 promoter hypermethylated tumors. Am. J. Surg. Pathol. 2017, 41, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Bhangoo, M.S.; Boasberg, P.; Mehta, P.; Elvin, J.A.; Ali, S.M.; Wu, W.; Klempner, S.J. Tumor mutational burden guides therapy in a treatment refractory POLE-mutant uterine carcinosarcoma. Oncologist 2018, 23, 518. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J. Association of polymerase e–mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, Y.; Luo, R.; Xu, J.; Feng, J.; Wang, M. Prognostic and clinicopathological role of PD-L1 in endometrial cancer: A meta-analysis. Front. Oncol. 2020, 10, 632. [Google Scholar] [CrossRef] [PubMed]
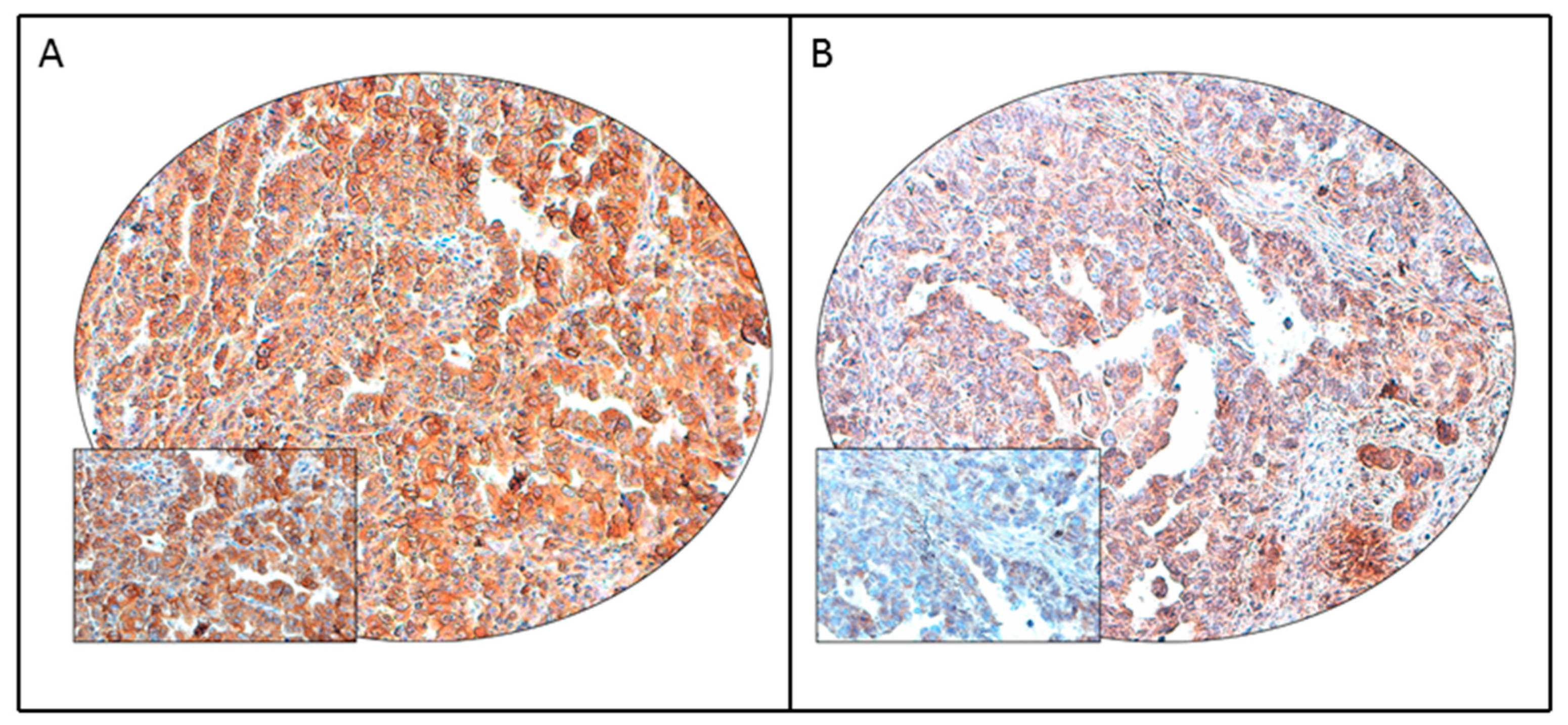
| Clinico-Pathological Parameter | n (%) |
|---|---|
| Age | |
| Median | 59.3 |
| Range(IQR) ^ | 53.0–66.2 |
| Histologic subtype | |
| Type I | 387 (88.0) |
| Type II | 53 (12.0) |
| Myometrial invasion | |
| High | 221 (50.2) |
| Low | 219 (49.8) |
| Histological grade | |
| Well differentiated | 148 (33.6) |
| Moderately differentiated | 147 (33.4) |
| Poorly differentiated | 130 (29.6) |
| Unknown | 15 (3.4) |
| pT | |
| T1 | 308 (70.0) |
| T2 | 55 (12.5) |
| T3 | 58 (13.2) |
| T4 | 19 (4.3) |
| pN | |
| N0 | 410 (93.2) |
| N1-2 | 30 (6.8) |
| pM | |
| M0 | 417 (94.8) |
| M1 | 23 (5.2) |
| Tumor Stage | |
| I | 285 (64.8) |
| II | 48 (10.9) |
| III | 70 (15.9) |
| IV | 37 (8.4) |
| Primary treatment | |
| Surgery | 440 (100.0) |
| Adjuvant chemotherapy | 84 (19.1) |
| Paclitaxel and Carboplatin | 69 (15.7) |
| Carboplatin only | 15 (3.4) |
| Adjuvant Radiotherapy | 175 (39.8) |
| Total | PD-L1 Positive | PD-L1 Negative | p Value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| No. of patients | 440 | 83 | 18.9 | 357 | 81.1 | ||
| Age (years) | |||||||
| ≤60 | 236 | 53.6 | 42 | 17.8 | 194 | 82.2 | 0.5387 |
| >60 | 204 | 46.4 | 41 | 20.1 | 163 | 79.9 | |
| Histologic subtype | |||||||
| Type I | 387 | 87.9 | 63 | 16.3 | 324 | 83.7 | 0.0005 * |
| Type II | 53 | 12.1 | 20 | 37.7 | 33 | 62.3 | |
| Myometrial invasion | |||||||
| High | 221 | 50.2 | 49 | 22.2 | 172 | 77.8 | 0.0741 |
| Low | 219 | 49.8 | 34 | 15.5 | 185 | 84.5 | |
| Grade | |||||||
| Grade 1 | 148 | 34.8 | 22 | 14.9 | 126 | 85.1 | 0.0893 |
| Grade 2 | 147 | 34.6 | 24 | 16.3 | 123 | 83.7 | |
| Grade 3 | 130 | 30.6 | 32 | 24.6 | 98 | 75.4 | |
| pT | |||||||
| T1 | 308 | 70.0 | 53 | 17.2 | 255 | 82.8 | 0.0570 |
| T2 | 55 | 12.5 | 14 | 25.5 | 41 | 74.5 | |
| T3 | 58 | 13.2 | 8 | 13.8 | 50 | 86.2 | |
| T4 | 19 | 4.3 | 8 | 42.1 | 11 | 57.9 | |
| pN | |||||||
| N0 | 410 | 93.2 | 72 | 17.6 | 338 | 82.4 | 0.0172 * |
| N1-N2 | 30 | 6.8 | 11 | 36.7 | 19 | 63.3 | |
| pM | |||||||
| M0 | 417 | 94.8 | 77 | 18.5 | 340 | 81.5 | 0.3822 |
| M1 | 23 | 5.2 | 6 | 26.1 | 17 | 73.9 | |
| Tumor Stage | |||||||
| I | 285 | 64.8 | 47 | 16.5 | 238 | 83.5 | 0.2443 |
| II | 48 | 10.9 | 11 | 22.9 | 37 | 77.1 | |
| III | 70 | 15.9 | 14 | 20.0 | 56 | 80.0 | |
| IV | 37 | 8.4 | 11 | 29.7 | 26 | 70.3 | |
| MMR IHC | |||||||
| dMMR | 53 | 12.1 | 8 | 15.1 | 45 | 84.9 | 0.4435 |
| pMMR | 387 | 87.9 | 75 | 19.4 | 312 | 80.6 | |
| POLE mutation | |||||||
| Present | 2 | 0.5 | 0 | 0.0 | 2 | 100.0 | 0.3576 |
| Absent | 429 | 99.5 | 82 | 19.1 | 347 | 80.9 | |
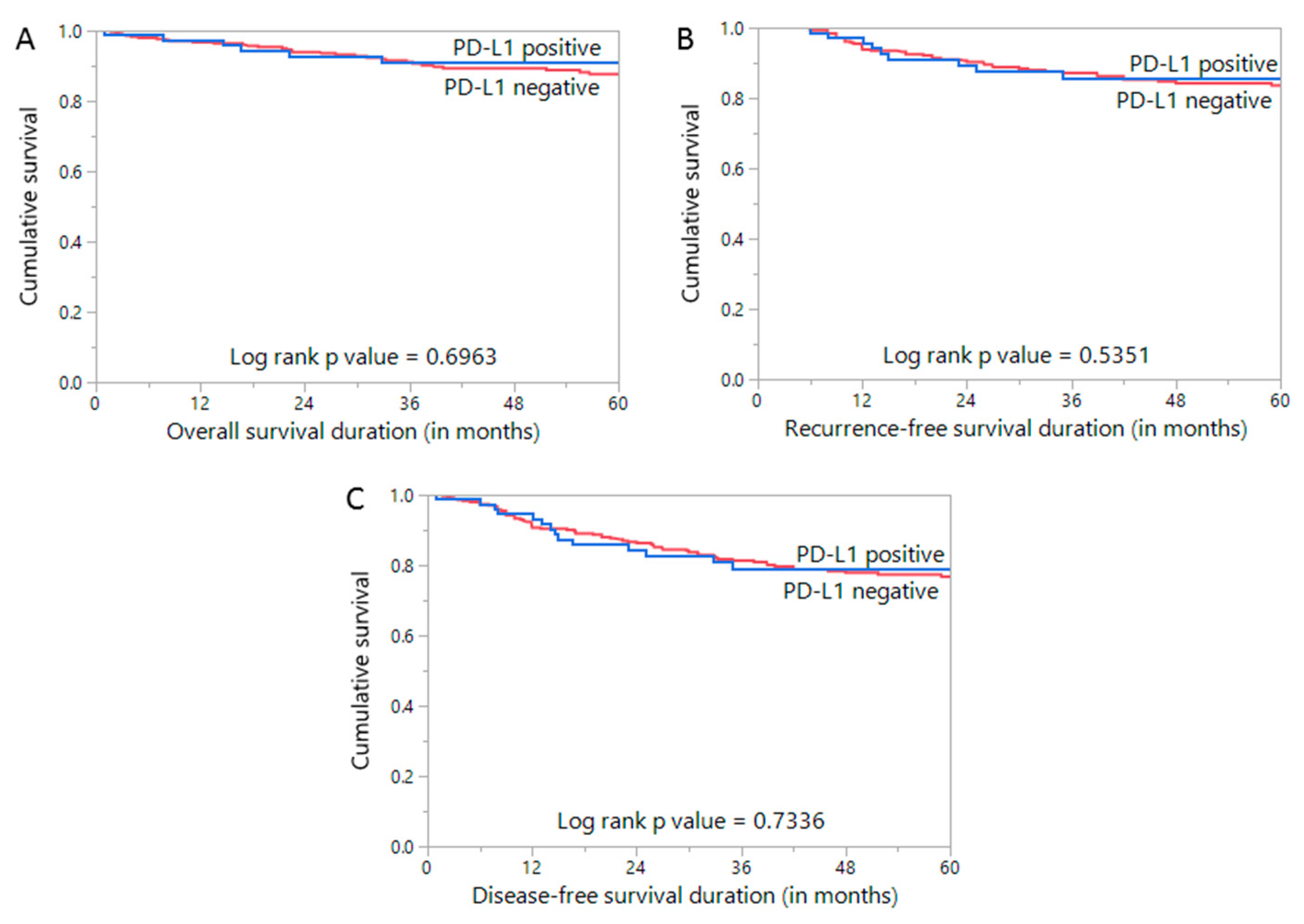
| 5 year overall survival | 90.9 | 87.0 | 0.6963 | ||||
| 5 year recurrence-free survival | 85.7 | 83.6 | 0.5351 | ||||
| 5 year disease-free survival | 78.9 | 76.8 | 0.7336 | ||||
| Clinico-Pathological Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| Age>60 years (vs. ≤60 years) | 0.65 | 0.29–1.47 | 0.3020 |
| Histologic grade | |||
| Grade 3 (vs. Grade 1–2) | 2.14 | 0.92–4.96 | 0.0755 |
| Myometrial invasion | |||
| High (vs. Low) | 2.84 | 1.01–7.94 | 0.0473 * |
| pT | |||
| T3-4 (vs. T1-2) | 2.42 | 0.90–6.49 | 0.0801 |
| pM | |||
| M1 (VS. M0) | 0.63 | 0.03–11.74 | 0.7548 |
| Tumor Stage | |||
| IV (vs I–III) | 0.28 | 0.03–2.75 | 0.2766 |
| MMR statusd | |||
| MMR (vs. pMMR) | 1.20 | 0.41–3.58 | 0.7376 |
| PD-L1 expression | |||
| Positive (vs. Negative) | 2.94 | 1.26–6.84 | 0.0123 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siraj, A.K.; Parvathareddy, S.K.; Annaiyappanaidu, P.; Siraj, N.; Al-Rasheed, M.; Al-Badawi, I.A.; Al-Dayel, F.; Al-Kuraya, K.S. PD-L1 Expression Is an Independent Marker for Lymph Node Metastasis in Middle Eastern Endometrial Cancer. Diagnostics 2021, 11, 394. https://doi.org/10.3390/diagnostics11030394
Siraj AK, Parvathareddy SK, Annaiyappanaidu P, Siraj N, Al-Rasheed M, Al-Badawi IA, Al-Dayel F, Al-Kuraya KS. PD-L1 Expression Is an Independent Marker for Lymph Node Metastasis in Middle Eastern Endometrial Cancer. Diagnostics. 2021; 11(3):394. https://doi.org/10.3390/diagnostics11030394
Chicago/Turabian StyleSiraj, Abdul K., Sandeep Kumar Parvathareddy, Padmanaban Annaiyappanaidu, Nabil Siraj, Maha Al-Rasheed, Ismail A. Al-Badawi, Fouad Al-Dayel, and Khawla S. Al-Kuraya. 2021. "PD-L1 Expression Is an Independent Marker for Lymph Node Metastasis in Middle Eastern Endometrial Cancer" Diagnostics 11, no. 3: 394. https://doi.org/10.3390/diagnostics11030394
APA StyleSiraj, A. K., Parvathareddy, S. K., Annaiyappanaidu, P., Siraj, N., Al-Rasheed, M., Al-Badawi, I. A., Al-Dayel, F., & Al-Kuraya, K. S. (2021). PD-L1 Expression Is an Independent Marker for Lymph Node Metastasis in Middle Eastern Endometrial Cancer. Diagnostics, 11(3), 394. https://doi.org/10.3390/diagnostics11030394






