Is Blood Eosinophil Count a Biomarker for Chronic Obstructive Pulmonary Disease in a Real-World Clinical Setting? Predictive Property and Longitudinal Stability in Japanese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement
2.3. Statistical Methods
3. Results
3.1. Cross-Sectional Observation at Baseline
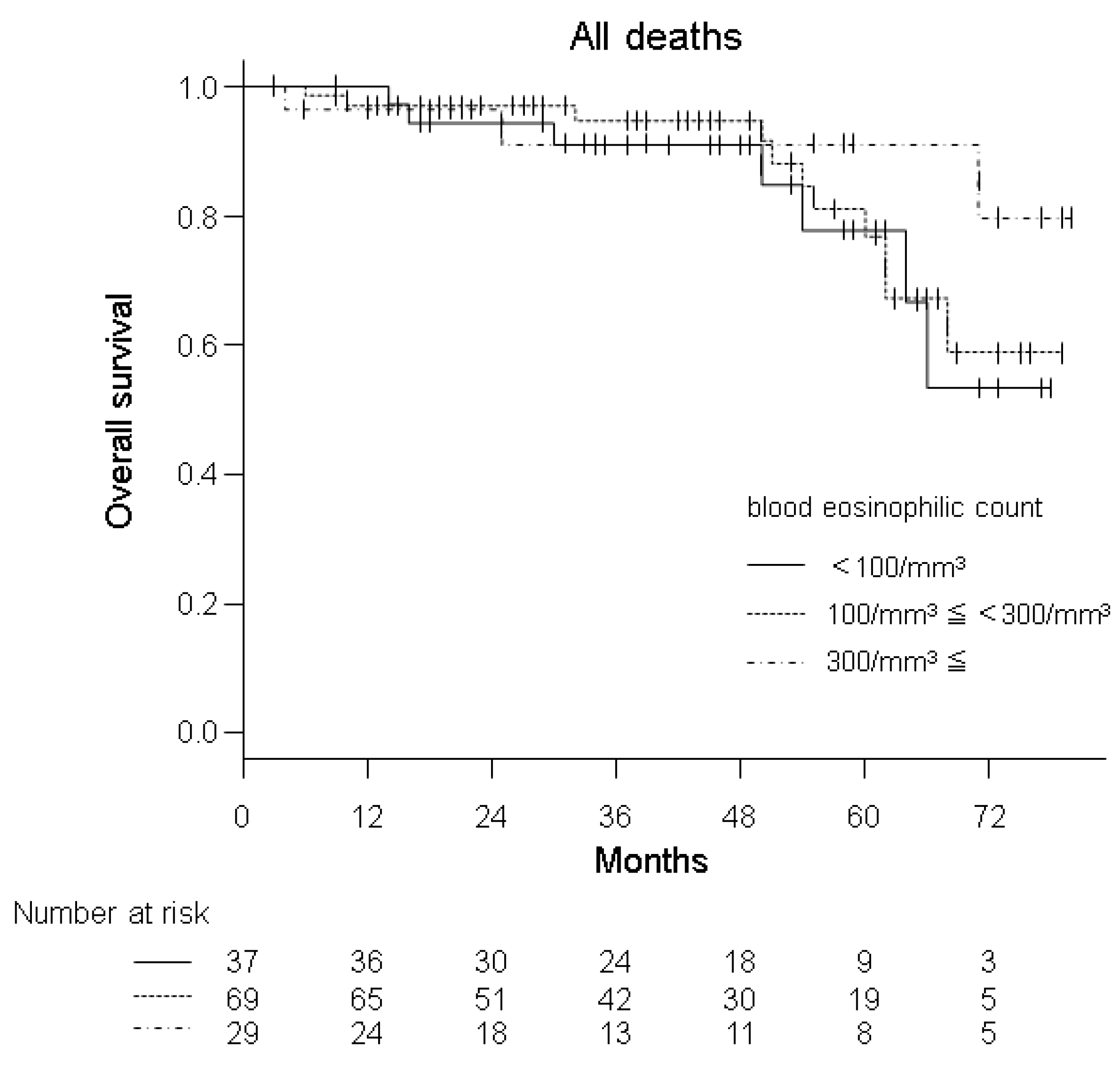
3.2. Predictive Properties of BEC
3.3. Longitudinal Stability of BEC
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
Appendix A
| ICS Administered | ICS Unadministered | Mann–Whitney’s U Test | ||
|---|---|---|---|---|
| n = 96 | n = 39 | p-Value | ||
| Blood eosinophil count | /mm3 | 220 ± 174 | 178 ± 124 | 0.306 |
| FEV1 | Litres | 1.53 ± 0.48 | 2.12 ± 0.46 | <0.001 |
| FEV1 | % pred. | 64.2 ± 20.1 | 79.9 ± 16.4 | <0.001 |
| SGRQ Total Score | (0–100) | 27.5 ± 17.1 | 16.3 ± 11.8 | <0.001 |
| CAT Score | (0–40) | 11.0 ± 7.0 | 5.6 ± 4.6 | <0.001 |
| Data are presented as mean ± SD. ICS, inhaled corticosteroids; SGRQ, the St. George’s Respiratory Questionnaire; | ||||
| CAT, the COPD Assessment Test. The numbers in parentheses denote possible score range. | ||||
Appendix B
| Mean (*) | SD (*) | Median (*) | Max (*) | Min (*) | 75th Percentile (*) | 25th Percentile (*) | Non-Eosinophilic Group | Intermediate Group | Eosinophilic Group | |
|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | 207 | 151 | 169 | 929 | 11 | 271 | 109 | n = 20 (23.3%) | n = 48 (55.8%) | n = 18 (20.9%) |
| Visit 2 | 202 | 125 | 162 | 552 | 9 | 281 | 109 | n = 21 (24.4%) | n = 45 (52.3%) | n = 20 (23.3%) |
| Visit 3 | 210 | 173 | 166 | 971 | 7 | 270 | 99 | n = 22 (25.6%) | n = 47 (54.7%) | n = 17 (19.8%) |
| *: Unit is /mm3 | ||||||||||
References
- Barnes, P.J. Inhaled Corticosteroids Are Not Beneficial in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2000, 161, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M. Inhaled Corticosteroids Are Beneficial in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2000, 161, 341–342. [Google Scholar] [CrossRef]
- Postma, D.S.; Calverley, P. Inhaled corticosteroids in COPD: A case in favour. Eur. Respir. J. 2009, 34, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Barnes, P.J. Inhaled corticosteroids in COPD: The case against. Eur. Respir. J. 2009, 34, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015, 3, 435–442. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Guasconi, A.; Vestbo, J.; Jones, P.; Agusti, A.; Paggiaro, P.; Wedzicha, J.A.; Singh, D. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fagerås, M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: A post-hoc analysis of three randomised trials. Lancet Respir. Med. 2018, 6, 117–126. [Google Scholar] [CrossRef]
- Vestbo, J.; Papi, A.; Corradi, M.; Blazhko, V.; Montagna, I.; Francisco, C.; Cohuet, G.; Vezzoli, S.; Scuri, M.; Singh, D. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomised controlled trial. Lancet 2017, 389, 1919–1929. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef]
- Pascoe, S.; Barnes, N.; Brusselle, G.; Compton, C.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Lange, P.; et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: Analysis of the IMPACT trial. Lancet Respir. Med. 2019, 7, 745–756. [Google Scholar] [CrossRef]
- The Japanese Respiratory Society. The JRS Guidelines for the Management of Chronic Obstructive Pulmonary Disease; The Japanese Respiratory Society location: Tokyo, Japan, 2018. [Google Scholar]
- Barnes, N.; Ishii, T.; Hizawa, N.; Midwinter, D.; James, M.; Hilton, E.; Jones, P.W. The distribution of blood eosinophil levels in a Japanese COPD clinical trial database and in the rest of the world. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 433–440. [Google Scholar] [CrossRef]
- Landis, S.H.; Suruki, R.; Hilton, E.; Compton, C.; Galwey, N.W. Stability of Blood Eosinophil Count in Patients with COPD in the UK Clinical Practice Research Datalink. COPD: J. Chronic Obstr. Pulm. Dis. 2017, 14, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Oshagbemi, O.A.; Burden, A.M.; Braeken, D.C.W.; Henskens, Y.; Wouters, E.F.M.; Driessen, J.H.M.; Der Zee, A.H.M.-V.; De Vries, F.; Franssen, F.M.E. Stability of Blood Eosinophils in Patients with Chronic Obstructive Pulmonary Disease and in Control Subjects, and the Impact of Sex, Age, Smoking, and Baseline Counts. Am. J. Respir. Crit. Care Med. 2017, 195, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; KOLD Study Group; Park, H.Y.; Kang, D.; Cho, J.; Kwon, S.O.; Park, J.H.; Lee, J.S.; Oh, Y.-M.; Sin, D.D.; et al. Serial blood eosinophils and clinical outcome in patients with chronic obstructive pulmonary disease. Respir. Res. 2018, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Southworth, T.; Beech, G.; Foden, P.; Kolsum, U.; Singh, D. The reproducibility of COPD blood eosinophil counts. Eur. Respir. J. 2018, 52, 1800427. [Google Scholar] [CrossRef]
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K.; et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047.e10. [Google Scholar] [CrossRef] [PubMed]
- Long, G.H.; Southworth, T.; Kolsum, U.; Donaldson, G.C.; Wedzicha, J.A.; Brightling, C.E.; Singh, D. The stability of blood Eosinophils in chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Monteagudo, M.; Solntseva, I.; Alcazar, B. Blood Eosinophil Counts and Their Variability and Risk of Exacerbations in COPD: A Population-Based Study. Arch. Bronconeumol. 2021, 57, 13–20. [Google Scholar] [CrossRef]
- Singh, D.; Bafadhel, M.; Brightling, C.E.; Sciurba, F.C.; Curtis, J.L.; Martinez, F.J.; Pasquale, C.B.; Merrill, D.D.; Metzdorf, N.; Petruzzelli, S.; et al. Blood Eosinophil Counts in Clinical Trials for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Van Rossem, I.; Vandevoorde, J.; Hanon, S.; DeRidder, S.; Vanderhelst, E. The stability of blood eosinophils in stable chronic obstructive pulmonary disease: A retrospective study in Belgian primary care. BMC Pulm. Med. 2020, 20, 200. [Google Scholar] [CrossRef]
- Yoon, J.-K.; Lee, J.-K.; Lee, C.-H.; Hwang, Y.I.; Kim, H.; Park, D.; Hwang, K.-E.; Kim, S.-H.; Jung, K.-S.; Yoo, K.H.; et al. The Association Between Eosinophil Variability Patterns and the Efficacy of Inhaled Corticosteroids in Stable COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Greulich, T.; Mager, S.; Lucke, T.; Koczulla, A.R.; Bals, R.; Fähndrich, S.; Jörres, R.A.; Alter, P.; Kirsten, A.-M.; Vogelmeier, C.F.; et al. Longitudinal stability of blood eosinophil count strata in the COPD COSYCONET cohort. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2999–3002. [Google Scholar] [CrossRef]
- Kusunose, M.; Oga, T.; Nakamura, S.; Hasegawa, Y.; Nishimura, K. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: Are they independent entities? BMJ Open Respir. Res. 2017, 4, e000196. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nakamura, M.; Kida, K.; Kambe, M.; Takahashi, K.; Fujimura, M. Reference values for spirogram and blood gas analysis in Japanese adults. J. Jpn. Respir. Soc. 2001, 39, S1–S17. [Google Scholar]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Leidy, N.K. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Tsuda, T.; Suematsu, R.; Kamohara, K.; Kurose, M.; Arakawa, I.; Tomioka, R.; Kawayama, T.; Hoshino, T.; Aizawa, H. Development of the Japanese version of the COPD Assessment Test. Respir. Investig. 2012, 50, 34–39. [Google Scholar] [CrossRef]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M.; Littlejohns, P. A Self-complete Measure of Health Status for Chronic Airflow Limitation: The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992, 145, 1321–1327. [Google Scholar] [CrossRef]
- Hajiro, T.; Nishimura, K.; Tsukino, M.; Ikeda, A.; Koyama, H.; Izumi, T. Comparison of Discriminative Properties among Disease-specific Questionnaires for Measuring Health-related Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 785–790. [Google Scholar] [CrossRef]
- Hyland, M.E.; Sodergren, S.C. Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Qual. Life Res. 1996, 5, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Oga, T.; Ikeda, A.; Hajiro, T.; Tsukino, M.; Koyama, H. Comparison of Health-Related Quality of Life Measurements Using a Single Value in Patients with Asthma and Chronic Obstructive Pulmonary Disease. J. Asthma 2008, 45, 615–620. [Google Scholar] [CrossRef]
- Yorke, J.; Moosavi, S.H.; Shuldham, C.; Jones, P.W. Quantification of dyspnoea using descriptors: Development and initial testing of the Dyspnoea-12. Thorax 2009, 65, 21–26. [Google Scholar] [CrossRef]
- Anthonisen, N.R.; Wright, E.C.; Hodgkin, J.E. Prognosis in Chronic Obstructive Pulmonary Disease 1–3. Am. Rev. Respir. Dis. 1986, 133, 14–20. [Google Scholar] [CrossRef]
- Gupta, N.; Pinto, L.; Aaron, S.D.; Marciniuk, D.D.; O’Donnell, D.E.; Walker, B.L.; Fitzgerald, J.M.; Sin, D.; Marciniuk, D.; O’Donnell, D.; et al. The COPD Assessment Test. Chest 2016, 150, 1069–1079. [Google Scholar] [CrossRef]
- Oga, T.; Nishimura, K.; Tsukino, M.; Sato, S.; Hajiro, T. Analysis of the Factors Related to Mortality in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 544–549. [Google Scholar] [CrossRef]
- Bikov, A.; Lange, P.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Cowans, N.J.; Crim, C.; Dixon, I.J.; Martinez, F.J.; et al. FEV1 is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.; Celli, B.R.; De-Torres, J.P.; Martínez-Gonzalez, C.; Cosio, B.G.; Pinto-Plata, V.; De Lucas-Ramos, P.; Divo, M.; Fuster, A.; Peces-Barba, G.; et al. Prevalence of persistent blood eosinophilia: Relation to outcomes in patients with COPD. Eur. Respir. J. 2017, 50, 1701162. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-M.; Lee, K.S.; Hong, Y.; Hwang, S.C.; Kim, J.Y.; Kim, D.K.; Yoo, K.H.; Lee, J.-H.; Kim, T.-H.; Lim, S.Y.; et al. Blood eosinophil count as a prognostic biomarker in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Zysman, M.; Deslee, G.; Caillaud, D.; Chanez, P.; Escamilla, R.; Court-Fortune, I.; Nesme-Meyer, P.; Perez, T.; Paillasseur, J.-L.; Pinet, C.; et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1819–1824. [Google Scholar] [CrossRef]
- Tashiro, H.; Kurihara, Y.; Takahashi, K.; Sadamatsu, H.; Haraguchi, T.; Tajiri, R.; Takamori, A.; Kimura, S.; Sueoka-Aragane, N. Clinical features of Japanese patients with exacerbations of chronic obstructive pulmonary disease. BMC Pulm. Med. 2020, 20, 318. [Google Scholar] [CrossRef] [PubMed]
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Kostikas, K.; Fang, J.; Tian, H.; Jones, B.; Morgan, C.L.; Fogel, R.; Gutzwiller, F.S.; Cao, H. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir. Res. 2019, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hastie, A.T.; Martinez, F.J.; Curtis, J.L.; Doerschuk, C.M.; Hansel, N.N.; Christenson, S.; Putcha, N.; Ortega, V.E.; Li, X.; Barr, R.G.; et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017, 5, 956–967. [Google Scholar] [CrossRef]
- Wu, H.-X.; Zhuo, K.-Q.; Cheng, D.-Y. Prevalence and Baseline Clinical Characteristics of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-Analysis and Systematic Review. Front. Med. 2019, 6, 282. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Bikov, A.; Foden, P.; LaHousse, L.; Brusselle, G.; Singh, D.; Vestbo, J. Change in blood eosinophils following treatment with inhaled corticosteroids may predict long-term clinical response in COPD. Eur. Respir. J. 2020, 55, 1902119. [Google Scholar] [CrossRef] [PubMed]

| Median | IQR | Max | Min | Correlations | |||
|---|---|---|---|---|---|---|---|
| With BEC | |||||||
| rs | p Value | ||||||
| Age | years | 74.0 | 71.0–80.0 | 89 | 51 | - | - |
| BMI | kg/m2 | 22.6 | 20.3–24.4 | 35.7 | 14 | - | - |
| Cumulative Smoking | pack-years | 53.0 | 37.5–73.5 | 204 | 10 | - | - |
| FVC | % pred. | 97.1 | 82.2–108.5 | 141.1 | 53.7 | - | - |
| FEV1 | Litres | 1.70 | 1.34–2.09 | 3.13 | 0.53 | - | - |
| FEV1/FVC | % | 58.8 | 48.4–64.5 | 69.9 | 25 | - | - |
| RV § | % pred. | 117.1 | 94.3–139.3 | 718.9 | 28.4 | 0.172 | 0.047 |
| RV/TLC § | % | 44.6 | 38.3–51.1 | 85.1 | 18.1 | - | - |
| DLco ¶ | % pred. | 52.4 | 39.4–63.2 | 156.1 | 10.7 | - | - |
| PaO2 (1) | mmHg | 78.2 | 71.4–83.1 | 101.5 | 52.1 | −0.171 | 0.047 |
| BNP (2) | pg/mL | 25.5 | 10.8–49.9 | 229.9 | 5.7 | - | - |
| SGRQ Total Score | (0–100) | 21.0 | 9.4–35.1 | 77.3 | 1.2 | - | - |
| CAT Score | (0–40) | 8.0 | 4.0–14.0 | 28 | 0 | - | - |
| Hyland Scale Score | (0–100) | 65.0 | 55.0–75.0 | 95 | 20 | - | - |
| D-12 Total Score§ | (0–36) | 1.0 | 0.0–2.0 | 24 | 0 | - | - |
| Non-Eosinophilic Group | Intermediate Group | Eosinophilic Group | |||||
|---|---|---|---|---|---|---|---|
| n = 37 | n = 69 | n = 29 | |||||
| Blood Eosinophil Count (/mm3) | <100 | ≥100 and <300 | ≥300 | ||||
| Age | years | 74.0 | (72.0–80.0) | 74.0 | (72.0–80.0) | 73.0 | (69.0–79.0) |
| BMI | kg/m2 | 22.6 | (19.5–24.2) | 22.8 | (20.8–24.9) | 21.8 | (20.5–23.8) |
| Cumulative Smoking | pack-years | 54.0 | (37.5–78.8) | 51.0 | (38.0–63.0) | 50.0 | (40.0–71.8) |
| FVC | % pred. | 100.4 | (87.2–108.5) | 97.4 | (82.2–109.8) | 91.8 | (78.0–104.3) |
| FEV1 | Liters | 1.62 | (1.38–1.98) | 1.71 | (1.38–2.10) | 1.62 | (1.21–2.07) |
| FEV1/FVC | % | 59.1 | (48.4–66.7) | 60.8 | (51.6–64.4) | 55.8 | (43.8–63.4) |
| RV § | % pred. | 123.9 | (93.1–137.2) | 109.7 | (91.7–137.5) | 121.9 | (115.4–147.7) *** |
| RV/TLC § | % | 44.4 | (39.8–49.7) | 43.3 | (37.6–50.6) ‡‡ | 44.9 | (41.0–56.0) *** |
| DLco ¶ | % pred. | 48.5 | (39.3–59.2) | 55.3 | (41.1–66.8) ** | 46.7 | (33.7–64.3) *** |
| PaO2 (1) | mmHg | 79.3 | (72.8–87.1) | 77.2 | (70.7–81.8) | 75.8 | (70.8–82.1) |
| BNP (2) | pg/mL | 27.8 | (10.6–46.4) | 25.1 | (14.1–49.9) | 20.4 | (8.4–46.7) |
| SGRQ Total Score | (0–100) | 19.7 | (9.4–28.5) | 21.5 | (8.9–34.8) | 25.3 | (16.3–40.8) |
| CAT Score | (0–40) | 8.0 | (3.0–12.0) | 8.0 | (4.0–12.0) | 9.0 | (5.0–15.0) |
| Hyland Scale Score | (0–100) | 65.0 | (60.0–75.0) | 70.0 | (65.0–80.0) | 65.0 | (50.0–75.0) |
| D-12 Total Score § | (0–36) | 0.5 | (0.0–2.0) * | 0.0 | (0.0–1.0) | 1.0 | (0.0–2.0) |
| All Deaths (n = 135) | AECOPD (n = 130) | Admission Due to AECOPD (n = 132) | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |||
| Blood eosinophil count | /mm3 | 0.999 (0.995–1.002) | 0.352 | 1.000 (0.999–1.001) | 0.915 | 1.000 (0.998–1.002) | 0.869 | |
| Three different groups of blood eosinophil count | <100/mm3 (Ref.) | 1 | 1 | 1 | ||||
| ≥100/mm3 and <300/mm3 | 0.849 (0.329–2.192) | 0.735 | 1.285 (0.735–2.247) | 0.379 | 1.289 (0.564–2.946) | 0.547 | ||
| ≥300/mm3 | 0.461 (0.118–1.803) | 0.266 | 1.503 (0.773–2.921) | 0.230 | 1.445 (0.542–3.854) | 0.462 | ||
| Age | years | 1.098 (1.025–1.176) | 0.007 | 1.040 (1.000–1.081) | 0.050 | 1.091 (1.027–1.158) | 0.005 | |
| FEV1 | Litres | 0.293 (0.126–0.679) | 0.004 | 0.318 (0.195–0.519) | <0.001 | 0.127 (0.061–0.263) | <0.001 | |
| SGRQ Total Score | (0–100) | 1.023 (1.001–1.047) | 0.043 | 1.028 (1.014–1.043) | <0.001 | 1.047 (1.027–1.067) | <0.001 | |
| CAT Score | (0–40) | 1.067 (1.013–1.125) | 0.015 | 1.066 (1.030–1.103) | <0.001 | 1.144 (1.089–1.203) | <0.001 | |
| Publication Year | Reference | First Author | The Name of the Cohort or Database | Association with Mortality | Association with AECOPD |
|---|---|---|---|---|---|
| 2016 | #45 | Vedel-Krogh S | the Copenhagen General Population Study (n = 4.303) | N.A. | positive |
| 2017 | #41 | Casanova C | the CHAIN cohort (n = 424) and BODE cohort (n = 308) | positive | negative |
| 2017 | #47 | Hastie AT | the SPIROMICS cohort (n = 2.499) | N.A. | negative |
| 2017 | #43 | Zysman M | Initiatives BPCO French cohort (n = 458) | negative | negative |
| 2018 | #16 | Shin SH | the Korean Obstructive Lung Disease cohort (n = 299) | positive | N.A. |
| 2018 | #42 | Oh YM | the Korean Obstructive Lung Disease cohort (n = 395) and COPD in Dusty Area cohort of Kangwon University Hospital (n = 234) | positive | N.A. |
| 2018 | #18 | Yun JH | the COPDGene (n = 1.553) and ECLIPSE (n = 1.895) studies | N.A. | positive |
| 2019 | #46 | Vogelmeier CF | the UK Clinical Practice Research Datalink (n = 15,364) and US Optum Clinformatics™ Data Mart databases (n = 139,465) | N.A. | positive |
| 2020 | #20 | Miravitlles M | a primary care electronic medical record database in Catalonia, Spain (n = 57,209) | N.A. | negative |
| 2020 | #44 | Tashiro H | retrospective medical records at the Saga University Hospital (n = 481) | N.A. | positive |
| The present study | Nishimura K | the hospital-based cohort at NCGG, Japan (n = 135) | negative | negative | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, K.; Kusunose, M.; Sanda, R.; Mori, M.; Shibayama, A.; Nakayasu, K. Is Blood Eosinophil Count a Biomarker for Chronic Obstructive Pulmonary Disease in a Real-World Clinical Setting? Predictive Property and Longitudinal Stability in Japanese Patients. Diagnostics 2021, 11, 404. https://doi.org/10.3390/diagnostics11030404
Nishimura K, Kusunose M, Sanda R, Mori M, Shibayama A, Nakayasu K. Is Blood Eosinophil Count a Biomarker for Chronic Obstructive Pulmonary Disease in a Real-World Clinical Setting? Predictive Property and Longitudinal Stability in Japanese Patients. Diagnostics. 2021; 11(3):404. https://doi.org/10.3390/diagnostics11030404
Chicago/Turabian StyleNishimura, Koichi, Masaaki Kusunose, Ryo Sanda, Mio Mori, Ayumi Shibayama, and Kazuhito Nakayasu. 2021. "Is Blood Eosinophil Count a Biomarker for Chronic Obstructive Pulmonary Disease in a Real-World Clinical Setting? Predictive Property and Longitudinal Stability in Japanese Patients" Diagnostics 11, no. 3: 404. https://doi.org/10.3390/diagnostics11030404
APA StyleNishimura, K., Kusunose, M., Sanda, R., Mori, M., Shibayama, A., & Nakayasu, K. (2021). Is Blood Eosinophil Count a Biomarker for Chronic Obstructive Pulmonary Disease in a Real-World Clinical Setting? Predictive Property and Longitudinal Stability in Japanese Patients. Diagnostics, 11(3), 404. https://doi.org/10.3390/diagnostics11030404







