A Software Tool for Calculating the Uncertainty of Diagnostic Accuracy Measures
Abstract
:1. Introduction
1.1. Diagnostic Accuracy Measures
- Error-based measures, estimating misclassification rates. These include sensitivity (Se), specificity (Sp), overall diagnostic accuracy (ODA), Youden’s index (J), Euclidean distance (ED) and concordance probability (CZ).
- Information-based measures, assisting the interpretation of each single test result. These include positive predictive value (PPV), negative predictive value (NPV), likelihood ratio for positive result (LR+) and likelihood ratio for negative result (LR−).
- Association-based measures, estimating the strength of the association between the test results and the reference diagnostic method. These include diagnostic odds ratio (DOR).
- Defined conditionally on
- The true disease condition status: sensitivity, specificity, overall diagnostic accuracy, diagnostic odds ratio, likelihood ratio for positive result, likelihood ratio for negative result, Youden’s index, Euclidean distance and concordance probability.
- The test outcome: positive predictive value and negative predictive value.
- As prevalence
- Invariant: sensitivity, specificity, diagnostic odds ratio, likelihood ratio for positive result, likelihood ratio for negative result, Youden’s index, Euclidean distance and concordance probability.
- Dependent: positive predictive value, negative predictive value and overall diagnostic accuracy.
1.2. Uncertainty of Diagnostic Accuracy Measures
1.2.1. Measurement Uncertainty
1.2.2. Sampling Uncertainty
2. Materials and Methods
2.1. Computational Methods
- There is a reference (“gold standard”) diagnostic method classifying correctly a subject as diseased or non-diseased [22].
- Measurement uncertainty is normally distributed and homoscedastic in the diagnostic threshold’s range.
- The sampling is simple random.
- If the measurement is above the threshold the patient is classified as test-positive, otherwise as test-negative.
2.1.1. Calculation of Diagnostic Accuracy Measures
2.1.2. Calculation of Uncertainty of Diagnostic Accuracy Measures
Measurement Uncertainty
Sampling Uncertainty of Means and Standard Deviations
Combined Uncertainty of Means and Standard Deviations
Sampling Uncertainty of Prevalence Rate
Combined Uncertainty of Diagnostic Accuracy Measures
Expanded Uncertainty of Diagnostic Accuracy Measures
2.2. The Program
3. Results
3.1. Flowchart of the Program
3.2. Interface of the Program
3.2.1. Plots vs. Diagnostic Threshold Module
Diagnostic Accuracy Measures Standard Uncertainty Plots Submodule
Diagnostic Accuracy Measures Relative Standard Uncertainty Plots Submodule
Confidence Intervals of Diagnostic Accuracy Measures Plots Submodule
3.2.2. Plots vs. Measurement Uncertainty Module
Diagnostic Accuracy Measures Standard Uncertainty Plots Submodule
Diagnostic Accuracy Measures Relative Standard Uncertainty Plots Submodule
Confidence Intervals of Diagnostic Accuracy Measures Plots Submodule
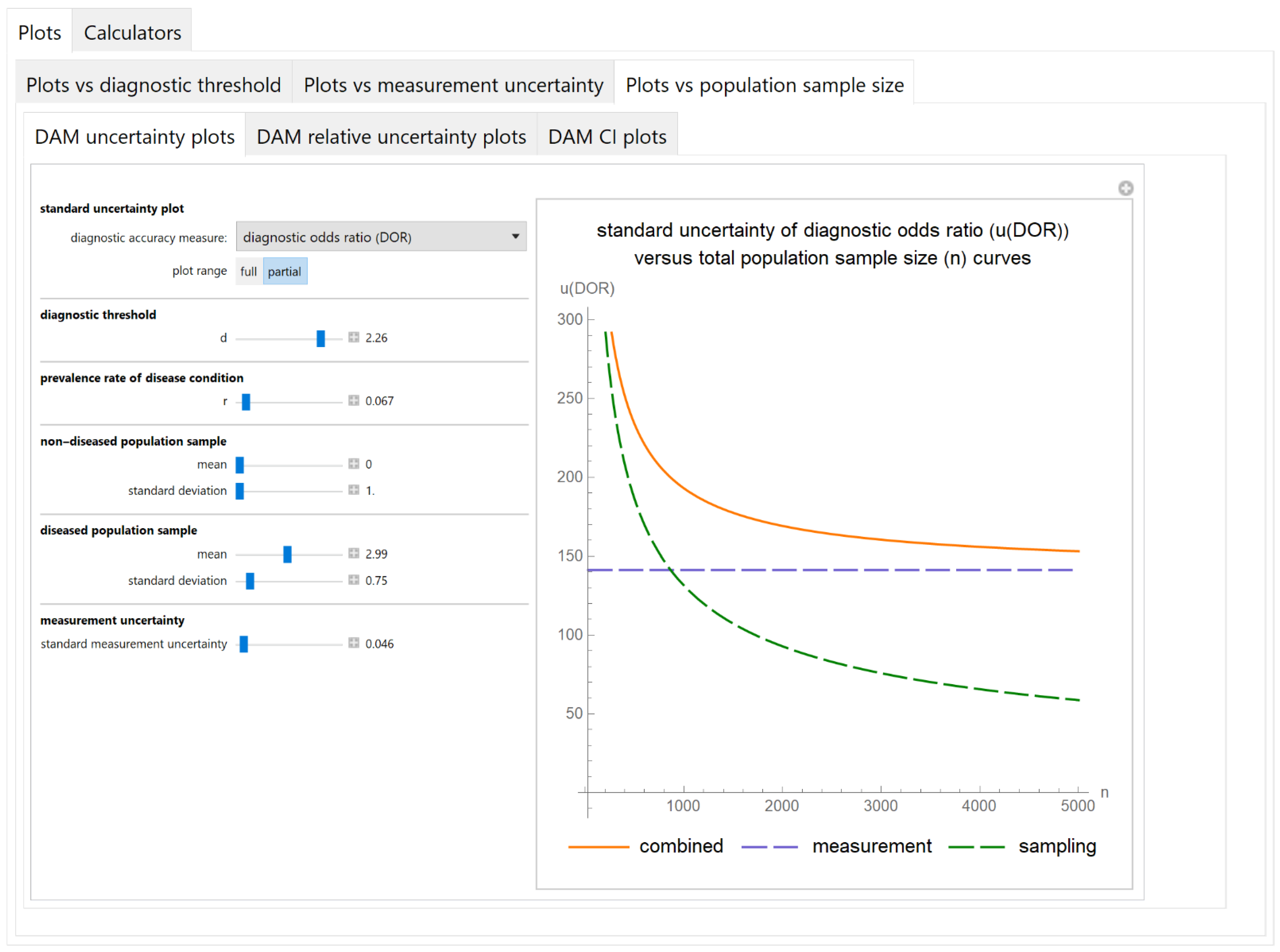
3.2.3. Plots vs. Population Sample Size Module
Diagnostic Accuracy Measures Standard Uncertainty Plots Submodule
Diagnostic Accuracy Measures Relative Standard Uncertainty Plots Submodule
Confidence Intervals of Diagnostic Accuracy Measures Plots Submodule
3.2.4. Diagnostic Accuracy Measures Standard Uncertainty Calculator Module
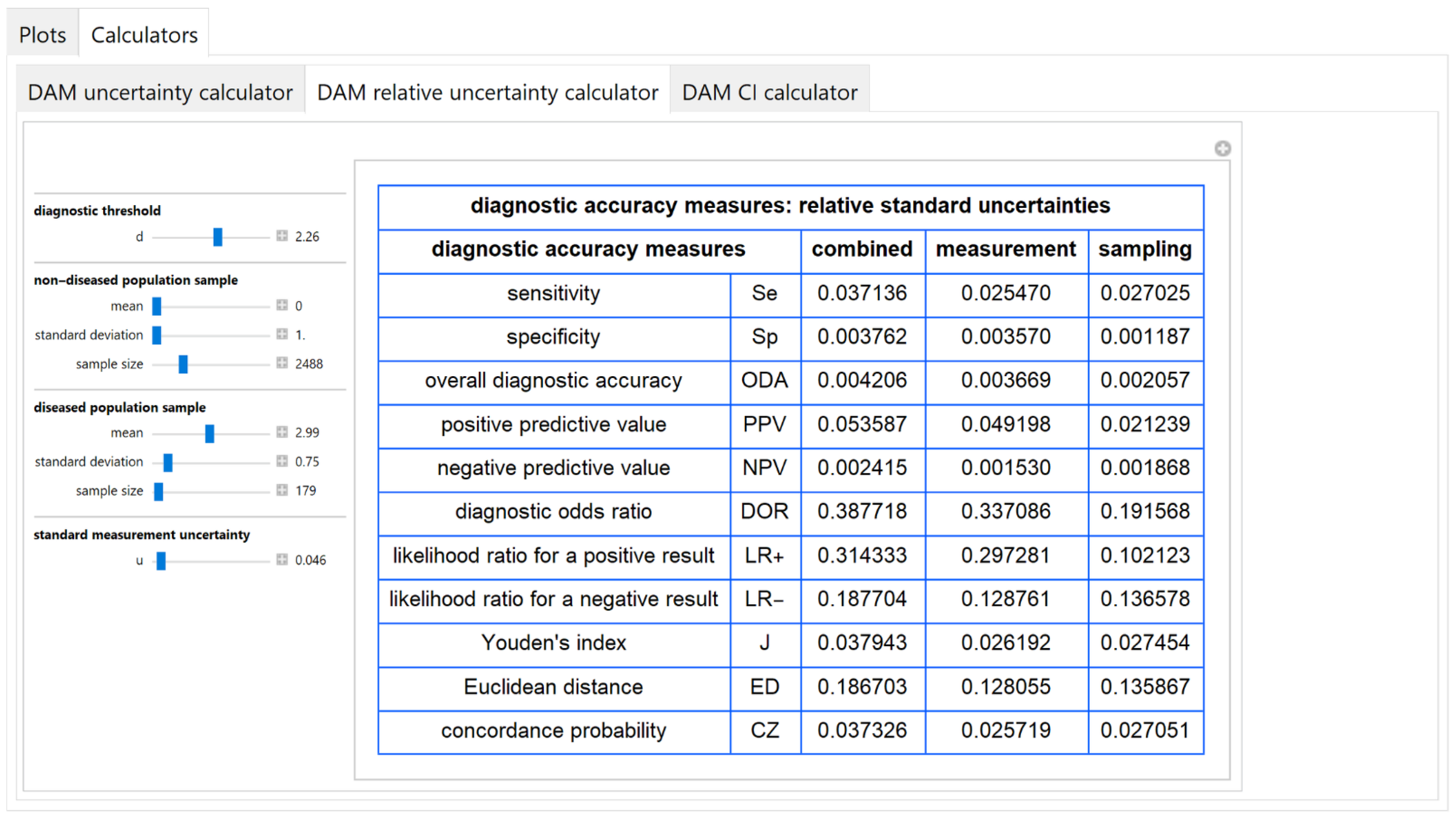
3.2.5. Diagnostic Accuracy Measures Relative Standard Uncertainty Calculator Module
3.2.6. Diagnostic Accuracy Measures Confidence Intervals Calculator Module
3.3. Illustrative Example
- In the chart of Figure 22
- Little effect on specificity, overall diagnostic accuracy and negative predictive value,
- Intermediate effect on sensitivity, positive predictive value, Youden’s index and concordance probability,
4. Discussion
- (1)
- The assumptions used for the calculations:
- The existence of a “gold standard” diagnostic method. If a “gold standard” does not exist, there are alternative approaches for the estimation of diagnostic accuracy measures [39].
- The normality of either the measurements or their applicable transforms [23,24,40,41], however, this is usually valid. There is related literature on the distribution of measurements of diagnostic tests, in the context of reference intervals and diagnostic thresholds or clinical decision limits [42,43,44,45,46].
- The simple random sampling.
- The measurement uncertainty homoscedasticity in the diagnostic thresholds range. Nevertheless, if measurement uncertainty is heteroscedastic, thus skewing the measurements distribution, appropriate transformations may restore homoscedasticity [49].
- (2)
- (3)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Notation
- Populations
- : Non-diseased population
- : Diseased population
- Test outcomes
- : Negative test result
- : Positive test result
- TN: True negative test result
- TP: True positive test result
- FN: False negative test result
- FP: False positive test result
- Parameters
- : Mean of the measurand of a test in a sample of population P
- : Standard deviation of the measurand of a test in a sample of population P
- : Size of a sample of population P
- : Size of a sample of total population
- : Size of a measurements sample
- r: Prevalence rate of the disease
- d: Diagnostic threshold of a test
- : Standard measurement uncertainty of a test
- p: Confidence level
- v: Degrees of freedom
- : Effective degrees of freedom
- Diagnostic accuracy measures: Abbreviations
- Se: Sensitivity
- Sp: Specificity
- PPV: Positive predictive value
- NPV: Negative predictive value
- ODA: Overall diagnostic accuracy
- DOR: Diagnostic odds ratio
- LR+: Likelihood ratio for a positive test result
- LR−: Likelihood ratio for a negative test result
- J: Youden’s index
- ED: Euclidean distance of a receiver operating characteristic curve point from the point (0,1)
- CZ: Concordance probability
- Diagnostic accuracy measures: Functions
- : Sensitivity of a test
- : Specificity of a test
- : Overall diagnostic accuracy of a test
- : Positive predictive value of a test
- : Negative predictive value of a test
- : Likelihood ratio for a positive test result
- : Likelihood ratio for a negative test result
- : Diagnostic odds ratio of a test
- : Euclidean distance of a test
- : Youden’s index of a test
- : Concordance probability of a test
- Other functions and relations
- u(x): Standard uncertainty of
- us(x): Standard sampling uncertainty of
- um(x): Standard measurement uncertainty of
- uc(x): Standard combined uncertainty of
- ui(x): The ith component of the standard combined uncertainty of
- : Standard combined uncertainty of the diagnostic accuracy measure
- : Cumulative distribution function of the standard normal distribution evaluated at
- : Cumulative distribution function of a normal distribution with mean and standard deviation , evaluated at
- : Cumulative distribution function of the Student’s t-distribution with degrees of freedom, evaluated at
- : Error function, evaluated at
- : Complementary error function, evaluated at
- : Probability of an event
- : Probability of an event given the event
- : Confidence interval of at confidence level
- : The inverse function
Appendix B
Appendix B.1. Uncertainty Propagation Rules
Appendix B.2. Definitions and Calculations
Appendix B.2.1. Error Function
Appendix B.2.2. Complementary Error Function
Appendix B.2.3. Standard Normal Distribution Cumulative Density Function
Appendix B.2.4. Normal Distribution Cumulative Density Function
Appendix B.2.5. Prevalence Rate (r)
Appendix B.2.6. Sensitivity (Se)
Appendix B.2.7. Specificity (Sp)
Appendix B.2.8. Overall Diagnostic Accuracy (ODA)
Appendix B.2.9. Positive Predictive Value (PPV)
Appendix B.2.10. Negative Predictive Value (NPV)
Appendix B.2.11. Diagnostic Odds Ratio (DOR)
Appendix B.2.12. Likelihood Ratio for a Positive Result (LR+)
Appendix B.2.13. Likelihood Ratio for a Negative Result (LR−)
Appendix B.2.14. Yuden’s Index (J)
Appendix B.2.15. Euclidean Distance (ED)
Appendix B.2.16. Concordance Probability (CZ)
Appendix C
Software Availability and Requirements
- Program name: Diagnostic Uncertainty
- Project home page: https://www.hcsl.com/Tools/Uncertainty/ (accessed 24 February 2021)
- Operating systems: Microsoft Windows, Linux, Apple iOS
- Programming language: Wolfram Language
- Other software requirements: Wolfram Player®, freely available at: https://www.wolfram.com/player/ (accessed 12 February 2021) or Wolfram Mathematica®
- System requirements: Intel® i7™ or equivalent CPU and 16 GB of RAM
- License: Attribution—Noncommercial—ShareAlike 4.0 International Creative Commons License
References
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Lippi, G.; Simundic, A.-M.; Plebani, M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. The Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Leeflang, M.M.G.; Spijker, R.; Hooft, L.; Van den Bruel, A.; Emperador, D.; Dittrich, S. Diagnosis of SARS-CoV-2 infection and COVID-19: Accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database Syst. Rev. 2020, 26, 1896. [Google Scholar] [CrossRef]
- Axell-House, D.B.; Lavingia, R.; Rafferty, M.; Clark, E.; Amirian, E.S.; Chiao, E.Y. The estimation of diagnostic accuracy of tests for COVID-19: A scoping review. J. Infect. 2020, 81, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Lisboa Bastos, M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.-P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef]
- Smith, A.F.; Shinkins, B.; Hall, P.S.; Hulme, C.T.; Messenger, M.P. Toward a Framework for Outcome-Based Analytical Performance Specifications: A Methodology Review of Indirect Methods for Evaluating the Impact of Measurement Uncertainty on Clinical Outcomes. Clin. Chem. 2019, 65, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Theodorsson, E. Uncertainty in Measurement and Total Error: Tools for Coping with Diagnostic Uncertainty. Clin. Lab. Med. 2017, 37, 15–34. [Google Scholar] [CrossRef]
- Padoan, A.; Sciacovelli, L.; Aita, A.; Antonelli, G.; Plebani, M. Measurement uncertainty in laboratory reports: A tool for improving the interpretation of test results. Clin. Biochem. 2018, 57, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chatzimichail, T.; Hatjimihail, A.T. A Software Tool for Exploring the Relation between Diagnostic Accuracy and Measurement Uncertainty. Diagnostics 2020, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.K.; Cooper, N.J.; Quinn, T.J.; Lees, R.; Sutton, A.J. Network meta-analysis of diagnostic test accuracy studies identifies and ranks the optimal diagnostic tests and thresholds for health care policy and decision-making. J. Clin. Epidemiol. 2018, 99, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Haeckel, R.; Wosniok, W.; Gurr, E.; Peil, B. Supplements to a recent proposal for permissible uncertainty of measurements in laboratory medicine. J. Lab. Med. 2016. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef]
- Bossuyt, P.M.M. Interpreting diagnostic test accuracy studies. Semin. Hematol. 2008, 45, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ayyub, B.M.; Klir, G.J. Uncertainty Modeling and Analysis in Engineering and the Sciences; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Kallner, A.; Boyd, J.C.; Duewer, D.L.; Giroud, C.; Hatjimihail, A.T.; Klee, G.G.; Lo, S.F.; Pennello, G.; Sogin, D.; Tholen, D.W.; et al. Expression of Measurement Uncertainty in Laboratory Medicine, Approved Guideline; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2012. [Google Scholar]
- Oosterhuis, W.P.; Theodorsson, E. Total error vs. measurement uncertainty: Revolution or evolution? Clin. Chem. Lab. Med. 2016, 54, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.H.; Ellison, S.L.R.; Rostron, P. (Eds.) Eurachem/EUROLAB/ CITAC/Nordtest/AMC Guide. In Measurement Uncertainty Arising from Sampling: A Guide to Methods and Approaches, 2nd ed.; Eurachem: Vilnius, Lithuania, 2019; 109p. [Google Scholar]
- Ellison, S.L.R.; Williams, A. Eurachem CITAC Guide. In Quantifying Uncertainty in Analytical Measurement; Eurachem: Vilnius, Lithuania, 2019; 133p. [Google Scholar]
- McLeod, A.I. Simple Random Sampling. In Encyclopedia of Statistical Sciences, 2nd ed.; Kotz, S., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; Volume 12, pp. 7740–7742. [Google Scholar]
- Bloch, D.A. Comparing Two Diagnostic Tests against the Same “Gold Standard” in the Same Sample. Biometrics 1997, 53, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J. A generalised Box–Cox transformation for the parametric estimation of clinical reference intervals. J. Appl. Stat. 2012, 39, 2231–2245. [Google Scholar] [CrossRef]
- Atkinson, A.B. The box-cox transformation: Review and extensions. Stat. Sci. 2020, in press. [Google Scholar]
- Hund, E.; Massart, D.L.; Smeyers-Verbeke, J. Operational definitions of uncertainty. Trends Analyt. Chem. 2001, 20, 394–406. [Google Scholar] [CrossRef]
- White, G.H. Basics of estimating measurement uncertainty. Clin. Biochem. Rev. 2008, 29, S53–S60. [Google Scholar] [PubMed]
- Agresti, A.; Coull, B.A. Approximate is Better than “Exact” for Interval Estimation of Binomial Proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
- Joint Committee for Guides in Metrology. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement; BIPM: Sèvres, France, 2008; 120p. [Google Scholar]
- Farrance, I.; Frenkel, R. Uncertainty of Measurement: A Review of the Rules for Calculating Uncertainty Components through Functional Relationships. Clin. Biochem. Rev. 2012, 33, 49–75. [Google Scholar] [PubMed]
- Wilson, B.M.; Smith, B.L. Taylor-Series and Monte-Carlo-method uncertainty estimation of the width of a probability distribution based on varying bias and random error. Meas. Sci. Technol. 2013, 24, 035301. [Google Scholar] [CrossRef]
- Welch, B.L. The Generalization of ‘Student’s’ Problem when Several Different Population Variances are Involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, F.E. An approximate distribution of estimates of variance components. Biometrics 1946, 2, 110–114. [Google Scholar] [CrossRef]
- Wolfram Research Inc. An Elementary Introduction to the Wolfram Language, 2nd ed.; Wolfram Research Inc.: Champaign, IL, USA, 2017; 340p. [Google Scholar]
- Wolfram Research Inc. Mathematica, Ver. 12.2; Wolfram Research Inc.: Champaign, IL, USA, 2020. [Google Scholar]
- Lim, T.-O.; Bakri, R.; Morad, Z.; Hamid, M.A. Bimodality in blood glucose distribution: Is it universal? Diabetes Care 2002, 25, 2212–2217. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Berg, E.G.; Saraco, M.; Petersen, M.P.; Uelmen, S.; Robinson, S. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Kupchak, P.; Wu, A.H.B.; Ghani, F.; Newby, L.K.; Ohman, E.M.; Christenson, R.H. Influence of imprecision on ROC curve analysis for cardiac markers. Clin. Chem. 2006, 52, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Biswas, B.; Budd, J.R.; Durham, P.; Gorman, R.T.; Gwise, T.E.; Halim, A.-B.; Hatjimihail, A.T.; Hilden, J.; Song, K. Assessment of the Diagnostic Accuracy of Laboratory Tests Using Receiver Operating Characteristic Curves, 2nd ed.; Approved Guideline; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2011. [Google Scholar]
- Collins, J.; Albert, P.S. Estimating diagnostic accuracy without a gold standard: A continued controversy. J. Biopharm. Stat. 2016, 26, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Sakia, R.M. The Box-Cox Transformation Technique: A Review. J. R. Stat. Soc. Ser. D Stat. 1992, 41, 169–178. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Series B Stat. Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Solberg, H.E. Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin. Chim. Acta 1987, 170, S13–S32. [Google Scholar] [CrossRef]
- Pavlov, I.Y.; Wilson, A.R.; Delgado, J.C. Reference interval computation: Which method (not) to choose? Clin. Chim. Acta 2012, 413, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Sikaris, K. Application of the stockholm hierarchy to defining the quality of reference intervals and clinical decision limits. Clin. Biochem. Rev. 2012, 33, 141–148. [Google Scholar] [PubMed]
- Daly, C.H.; Liu, X.; Grey, V.L.; Hamid, J.S. A systematic review of statistical methods used in constructing pediatric reference intervals. Clin. Biochem. 2013, 46, 1220–1227. [Google Scholar] [CrossRef]
- Ozarda, Y.; Sikaris, K.; Streichert, T.; Macri, J.; IFCC Committee on Reference intervals and Decision Limits (C-RIDL). Distinguishing reference intervals and clinical decision limits—A review by the IFCC Committee on Reference Intervals and Decision Limits. Crit. Rev. Clin. Lab. Sci. 2018, 55, 420–431. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968; Volume 34, 163p. [Google Scholar]
- Petersen, P.H.; Horder, M. 2.3 Clinical test evaluation. Unimodal and bimodal approaches. Scand. J. Clin. Lab. Invest. 1992, 52, 51–57. [Google Scholar] [CrossRef]
- Analytical Methods Committee, A.N. Why do we need the uncertainty factor? Anal. Methods 2019, 11, 2105–2107. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval Estimation for a Binomial Proportion. Stat. Sci. 2001, 16, 101–117. [Google Scholar]




















| Populations | |||
| Non-diseased | Diseased | ||
| Test Results | Negative | true negative (TN) | false negative (FN) |
| Positive | false positive (FP) | true positive (TP) | |
| Measure | Natural Frequency Definition | Probability Definition |
| Sensitivity (Se) | ||
| Specificity (Sp) | ||
| Positive Predictive Value (PPV) | ||
| Negative Predictive Value (NPV) | ||
| Overall Diagnostic Accuracy (ODA) | ||
| Diagnostic Odds Ratio (DOR) | ||
| Likelihood Ratio for a Positive Result (LR+) | ||
| Likelihood Ratio for a Negative Result (LR−) | ||
| Juden’s Index (J) | ||
| Euclidean Distance (ED) | ||
| Concordance Probability (CZ) |
| Settings | Figure 3 | Figure 4 | Figure 5 | Figure 6 and Figure 7 | Figure 8 | Figure 9 and Figure 10 | Figure 11 | Figure 12 and Figure 13 | Figure 14 |
|---|---|---|---|---|---|---|---|---|---|
| - | - | 0.95 | - | 0.95 | - | 0.95 | - | 0.95 | |
| 1.1–2.5 | 0–4.0 | 2.26 | 2.26 | 2.26 | 2.26 | 2.26 | 2.26 | 2.26 | |
| - | - | - | - | - | 0.067 | 0.067 | - | - | |
| 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | |
| 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |
| 179 | 179 | 179 | 179 | 179 | - | - | 179 | 179 | |
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| 2488 | 2488 | 2488 | 2488 | 2488 | - | - | 2488 | 2488 | |
| - | - | - | - | - | 30–5000 | 30–5000 | - | - | |
| 0.046 | 0.046 | 0.046 | 0–0.15 | 0–0.15 | 0.046 | 0.046 | 0.046 | 0.046 | |
| - | - | 80 | - | 80 | - | 80 | - | 80 |
| Settings | Figure 15 and Figure 16 | Figure 17 | Figure 18 | Figure 19 | Figure 20 | Figure 21 | Figure 22 |
|---|---|---|---|---|---|---|---|
| - | 0.95 | - | 0.95 | - | 0.95 | - | |
| 0.0–4.0 | 0.0–4.0 | 2.26 | - | 2.26 | - | 2.26 | |
| - | - | - | - | 0.067 | 0.067 | - | |
| 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | 2.99 | |
| 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |
| 179 | 179 | 179 | 179 | - | - | 179 | |
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| 2488 | 2488 | 2488 | 2488 | - | - | 2488 | |
| n | - | - | - | - | 30–5000 | 30–5000 | - |
| 0.046 | 0.046 | 0–0.15 | 0–0.15 | 0.046 | 0.046 | 0.046 | |
| - | 80 | - | 80 | - | 80 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimichail, T.; Hatjimihail, A.T. A Software Tool for Calculating the Uncertainty of Diagnostic Accuracy Measures. Diagnostics 2021, 11, 406. https://doi.org/10.3390/diagnostics11030406
Chatzimichail T, Hatjimihail AT. A Software Tool for Calculating the Uncertainty of Diagnostic Accuracy Measures. Diagnostics. 2021; 11(3):406. https://doi.org/10.3390/diagnostics11030406
Chicago/Turabian StyleChatzimichail, Theodora, and Aristides T. Hatjimihail. 2021. "A Software Tool for Calculating the Uncertainty of Diagnostic Accuracy Measures" Diagnostics 11, no. 3: 406. https://doi.org/10.3390/diagnostics11030406
APA StyleChatzimichail, T., & Hatjimihail, A. T. (2021). A Software Tool for Calculating the Uncertainty of Diagnostic Accuracy Measures. Diagnostics, 11(3), 406. https://doi.org/10.3390/diagnostics11030406








