Validating METCAM/MUC18 as a Novel Biomarker to Predict the Malignant Potential of Prostate Cancer at an Early Stage by Using a Modified Gold Nanoparticles-Based Lateral Flow Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Recombinant METCAM/MUC18 Proteins
2.3. Preparation of Human Serum Samples
2.4. Preparation of Biotinylated Chicken Antibody
2.5. Western Blot Analysis
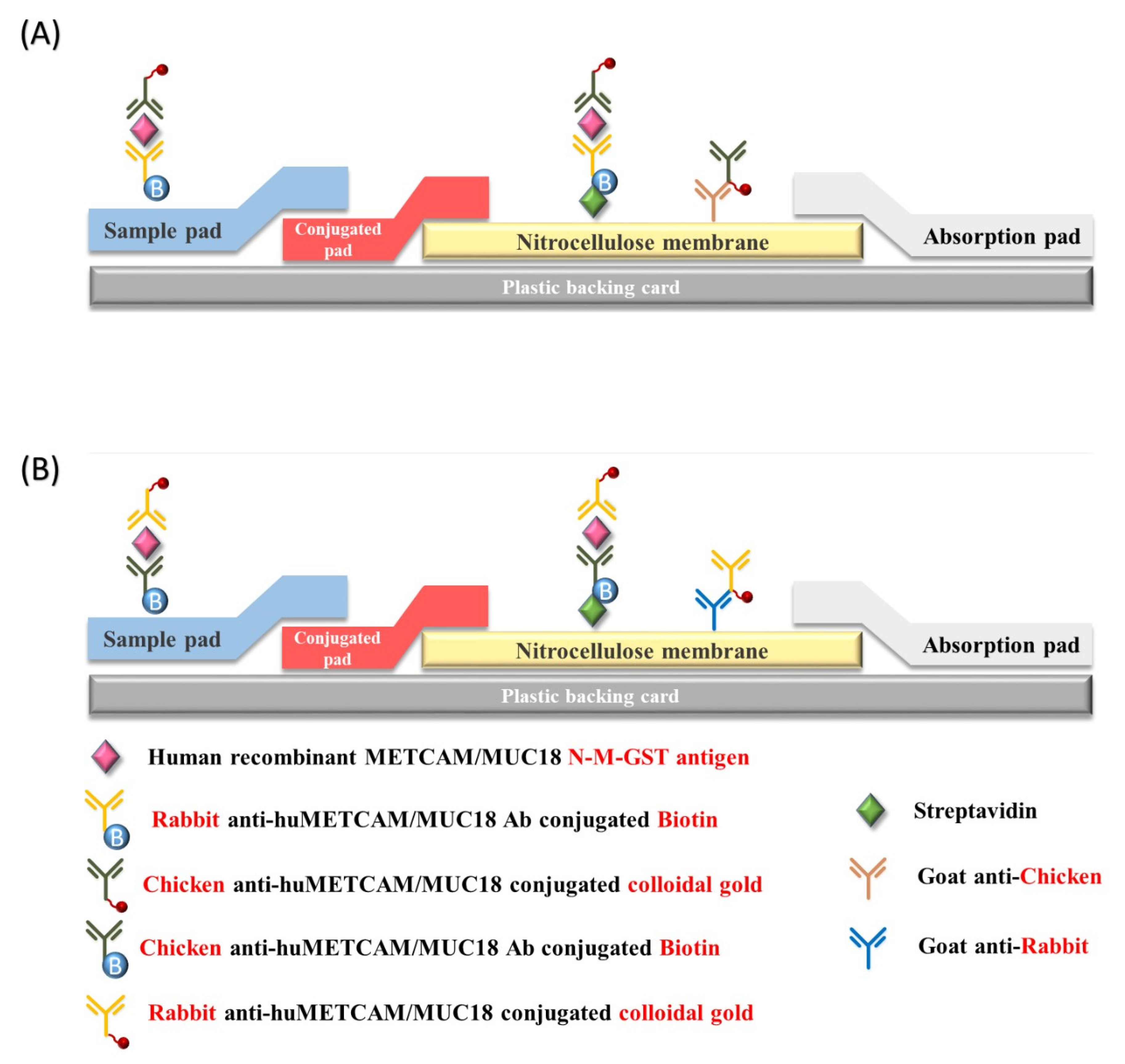
2.6. The Modified Lateral Flow Immunoassay (LFIA)
2.7. Statistical Analysis
3. Results
3.1. Identification of Antibodies for Modified LFIA
3.2. The First Antibody Combination: A Commercial Biotinylated Rabbit Antibody (EPP11278) and the Nano-Gold Conjugated Home-Made Chicken Antibody
3.2.1. The Standard Curve of Recombinant Human METCAM/MUC18 Proteins
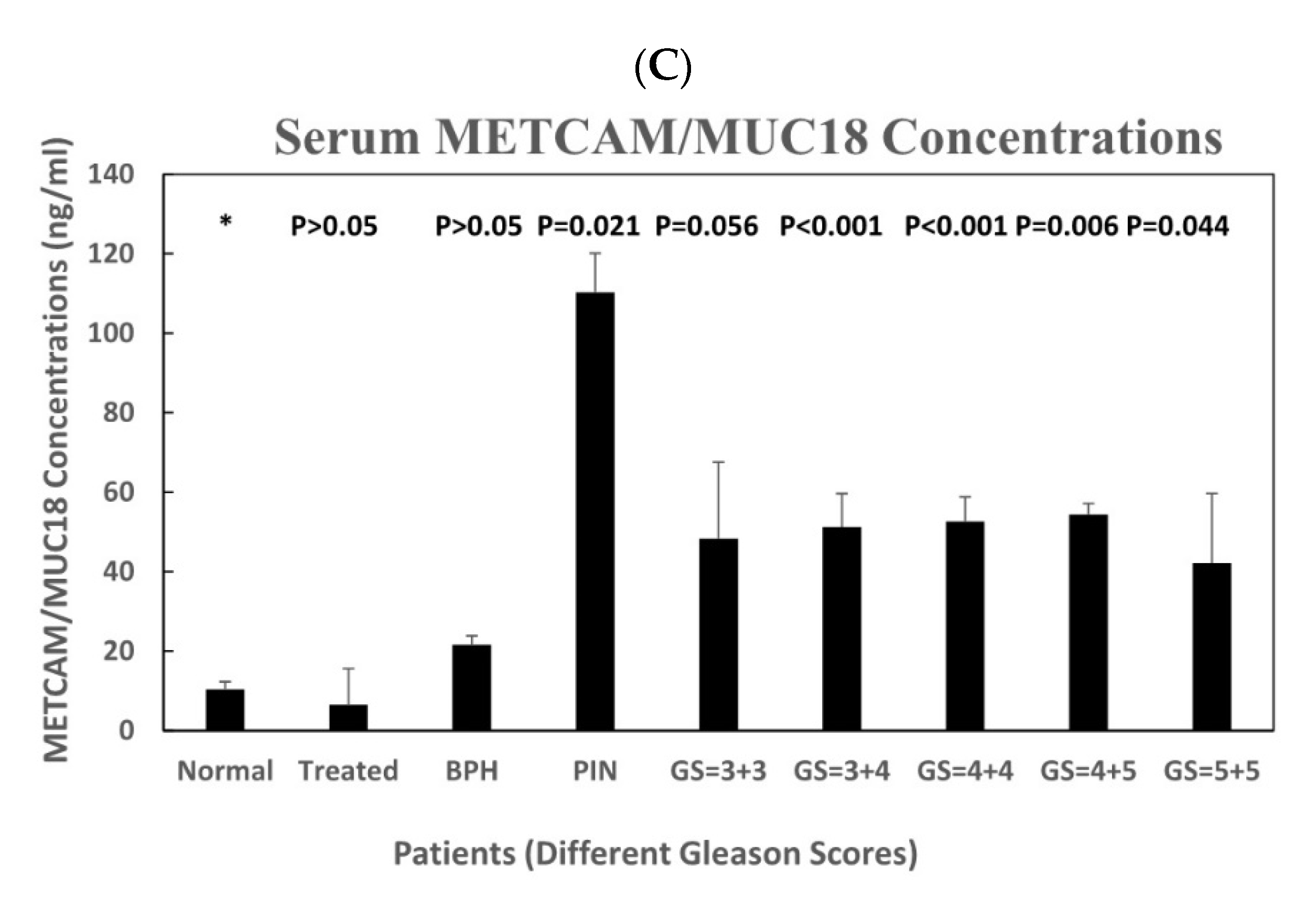
3.2.2. The Serum METCAM/MUC18 Concentrations in Various Human Serum Samples
3.2.3. The Relation of Serum MECTAM/MUC18 Concentrations with PSA
3.3. The Second Antibody Combination: The Home-Made Biotinylated Chicken Antibody and a Nano-Gold Conjugated Commercial Rabbit Antibody (EPP11278 or MBS2529469)
3.3.1. The Standard Curve of Recombinant Human METCAM/MUC18 Proteins
3.3.2. The Serum METCAM/MUC18 Concentrations in Various Human Serum Samples
3.3.3. The Relation of Serum MECTAM/MUC18 Concentrations with PSA
3.4. The Relation of Serum PSA Concentration in Various Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.P., Jr.; Banks, E.R.; Humphreys, S.; McRoberts, J.W.; Rangneker, V.M. Identification of bone marrow micro-metastases in patients with prostate cancer. Cancer 1994, 74, 2533–2540. [Google Scholar] [CrossRef]
- Catalona, W.J.; Scott, W.W. Carcinoma of the prostate: A review. J. Urol. 1978, 119, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Yang, D.; Yang, C.; Mao, L. Current state of biomarkers for the diagnosis and assessment of treatment efficacy of prostate cancer. Discov. Med. 2019, 27, 235–243. [Google Scholar] [PubMed]
- Murphy, L.; Watson, R.W. Patented prostate cancer biomarkers. Nat. Rev. Urol. 2012, 9, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A.; Rezaeian, I.; Singireddy, S.; Cavallo-Medved, D.; Porter, L.A.; Rueda, L. Transcriptomics signature from next-generation sequencing data reveal new transcriptomic biomarkers related to prostate cancer. Cancer Inform. 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koharr, I.; Petrovics, G.; Srivastava, S. A rich array of prostate cancer molecular biomarkers: Opportunities and challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, O.; Alkhateeb, A.; Zheng, J.Z.; Kandalam, S.; Leung, C.; Atikukke, G.; Cavallo-Medved, D.; Palanisamy, N.; Rueda, L. A hierarchical machine learning model to discover Gleason grade-specific biomarkers in prostate cancer. Diagnostics 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Wu, M.W.H.; Wang, S.W.; Liu, Z.; Peng, Q.; Qu, P.; Yang, H.; Petros, J.A.; Lim, S.D.; Amin, M.B.; et al. Isolation and characterization of the major form of human MUC18 cDNA gene and correlation of MUC18 over-expression in prostate cancer cells and tissues with malignant progression. Gene 2001, 279, 17–31. [Google Scholar] [CrossRef]
- Wu, G.J.; Varma, V.A.; Wu, M.W.H.; Yang, H.; Wang, S.W.C.; Liu, Z.; Qu, P.; Petros, J.A.; Lim, S.D.; Amin, M.B. Expression of a human cell adhesion molecule, MUC18, in prostate cancer cell lines and tissues. Prostate 2001, 48, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J. Chapter 7: The role of MUC18 in prostate carcinoma. In Immunohistochemistry and in Situ Hybridization of Human Carcinomas. Volume 2. Molecular Pathology of Lung Carcinoma, Breast Carcinoma, and Prostate Carcinoma; Hayat, M.A., Ed.; Elsevier Sciences/Academic Press: New York, NY, USA, 2004; pp. 347–358. [Google Scholar]
- Wu, G.J.; Peng, Q.; Fu, P.; Wang, S.W.C.; Chiang, C.F.; Dillehay, D.L.; Wu, M.W.H. Ectopical expression of human MUC18 increases metastasis of human prostate cancer cells. Gene 2004, 327, 201–213. [Google Scholar] [CrossRef]
- Wu, G.J. METCAM/MUC18 and cancer metastasis. Curr. Genom. 2005, 6, 333–349. [Google Scholar] [CrossRef]
- Wu, G.J.; Fu, P.; Chiang, C.F.; Hess, W.J.; Greenberg, N.M.; Wu, M.W.H. Increased expression of MUC18 correlates with the metastatic progression of mouse prostate adenocarcinoma in the TRAMP model. J. Urol. 2005, 173, 1778–1783. [Google Scholar] [CrossRef]
- Wu, G.J.; Wu, M.W.H.; Liu, Y. Enforced expression of human METCAM/MUC18 increases the tumorigenesis of human prostate cancer cells in nude mice. J. Urol. 2011, 185, 1504–1512. [Google Scholar] [CrossRef]
- Wu, G.J. Human METCAM/MUC18 as a Novel Biomarker to Drive and its Specific SiRNAs to Block the Malignant Progression of Prostate Cancer. J. Cell Sci. Ther. 2015, 6, 5. [Google Scholar] [CrossRef]
- Wu, G.J. Chapter 1. METCAM/MUC18 promotes tumor progression and metastasis in most human cancers. In Tumor Progression and Metastasis; Lasfar, A., Ed.; InTech-Open Access Publisher: London, UK, 2020; ISBN 978-1-78985-350-6. [Google Scholar]
- Pong, Y.H.; Su, Y.R.; Lo, H.W.; Ho, C.K.; Chu, C.T.; Chen-Yang, Y.W.; Tsai, V.F.S.; Wu, J.C.; Wu, G.J. METCAM/MUC18 is a new early diagnostic biomarker for the malignant potential of prostate cancer: Validation with Western blot method, enzyme-linked immunosorbent assay and lateral flow immunoassay. Cancer Biomark. 2020, 27, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Parolo, C.; de la Escosura-Muniz, A.; Merkoci, A. Enhanced lateral flow immunoassay using gold nanoparticle loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef]
- Wei, Y.C. Comparing Magnetic Beads-Enriched Array Display Immunoassay with the two Gold Nanoparticles-Based Lateral Flow Immunoassay for Determination of the Prostate Cancer Biomarker-METCAM/MUC18 Concentration in Human Serum. Master’s Thesis, Chung Yuan Christian University, Taoyuan, Taiwan, 2018. [Google Scholar]
- Chuang, Y.H. Using Magnetic Beads-Enriched Array Display Immunoassay and the Gold Nanoparticles-Based Lateral Flow Immunoassay for Determination of the Prostate Cancer Biomarker-METCAM/MUC18 Concentration in Human Serum. Master’s Thesis, Chung Yuan Christian University, Taoyuan, Taiwan, 2019. [Google Scholar]
- Hsieh, C.C. Using a Gold Nanoparticles-Based Lateral Flow Immunoassay and a Magnetic Beads-Enriched Array Display Immunoassay with a High Affinity Bond for Determining the Prostate Cancer Biomarker-METCAM/MUC18 Concentration in Human Serum. Master’s Thesis, Chung Yuan Christian University, Taoyuan, Taiwan, 2020. [Google Scholar]
- Yang, H.; Wang, S.W.C.; Liu, Z.; Wu, M.W.H.; Armstrong, C.; Wu, G.J. Isolation and characterization of murine MUC18 cDNA gene, and correlation of MUC18 expression in murine melanoma cell lines with metastatic ability. Gene 2001, 265, 133–145. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. (Eds.) In situ hybridization and immunohistochemistry. In Current Protocols in Molecular Biology; Section 14; Green Publishing Associates and Wiley-Interscience Press: New York, NY, USA, 1987. [Google Scholar]
- Wu, G.J. Chapter 2: METCAM/MUC18: A Novel Tumor Suppressor for Some Cancers. In Genes and Cancer; Guy-Joseph, L., Ed.; InTech-Open Access Publisher: London, UK, 2019; ISBN 978-1-78984-427-6. [Google Scholar]
- Eskra, J.N.; Rabizadeh, D.; Pavlovich, C.P.; Catalona, W.J.; Luo, J. Approaches to urinary detection of prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 362–381. [Google Scholar] [CrossRef] [PubMed]











| Antibodies | Size of METCAM/MUC18 (Recognized in WB) | Location of Epitopes (# Amino Acid) | Recombinant Protein of huMETCAM/MUC18 Recognized in WB * | Sources |
|---|---|---|---|---|
| Chicken Ab | 113–150 kDa | 212–374 | M | Home-made |
| Biotinylated chicken Ab | 113–150 kDa | 212–374 | M | Home-made |
| Rabbit Ab (MBS2529469 = EPP11278) | 120 kDa | 26–350 | N | MyBiosource and Elabscience |
| Biotinylated rabbit Ab (EPP11278) | 120 kDa | 26–350 | N | Elabscience |
| Characteristics | Age Range (Years) | Age Median (Years) | No of Cases | Percentage (%) |
|---|---|---|---|---|
| Normal | 28–75 | 52 | 8 | 27 |
| BPH | 68–75 | 72 | 4 | 13.3 |
| PIN | 63–87 | 75 | 2 | 6.7 |
| Prostate carcinoma (total) | 50–93 | 72 | 14 | 47 |
| Radiotherapy | 67–73 | 70 | 2 | 6.7 |
| Pathological grades of Prostate carcinoma | 50–93 | 72 | 14 | 47 |
| Gleason score 3 + 3 | 93 | 93 | 1 | 3.3 |
| Gleason score 3 + 4 or 4 + 3 | 63–85 | 74 | 6 | 20 |
| Gleason score 4 + 4 | 50–75 | 63 | 4 | 13.3 |
| Gleason score 4 + 5 or 5 + 4 | 69–86 | 78 | 2 | 6.7 |
| Gleason score 5 + 5 | 83 | 83 | 1 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-C.; Chuang, Y.-H.; Wei, Y.-C.; Hsieh, C.-C.; Pong, Y.-H.; Su, Y.-R.; Tsai, V.F.-S.; Wu, G.-J. Validating METCAM/MUC18 as a Novel Biomarker to Predict the Malignant Potential of Prostate Cancer at an Early Stage by Using a Modified Gold Nanoparticles-Based Lateral Flow Immunoassay. Diagnostics 2021, 11, 443. https://doi.org/10.3390/diagnostics11030443
Wu J-C, Chuang Y-H, Wei Y-C, Hsieh C-C, Pong Y-H, Su Y-R, Tsai VF-S, Wu G-J. Validating METCAM/MUC18 as a Novel Biomarker to Predict the Malignant Potential of Prostate Cancer at an Early Stage by Using a Modified Gold Nanoparticles-Based Lateral Flow Immunoassay. Diagnostics. 2021; 11(3):443. https://doi.org/10.3390/diagnostics11030443
Chicago/Turabian StyleWu, Jui-Chuang, Yin-Huan Chuang, Yu-Chun Wei, Chia-Chi Hsieh, Yuan-Hung Pong, Yenn-Rong Su, Vincent F.-S. Tsai, and Guang-Jer Wu. 2021. "Validating METCAM/MUC18 as a Novel Biomarker to Predict the Malignant Potential of Prostate Cancer at an Early Stage by Using a Modified Gold Nanoparticles-Based Lateral Flow Immunoassay" Diagnostics 11, no. 3: 443. https://doi.org/10.3390/diagnostics11030443
APA StyleWu, J.-C., Chuang, Y.-H., Wei, Y.-C., Hsieh, C.-C., Pong, Y.-H., Su, Y.-R., Tsai, V. F.-S., & Wu, G.-J. (2021). Validating METCAM/MUC18 as a Novel Biomarker to Predict the Malignant Potential of Prostate Cancer at an Early Stage by Using a Modified Gold Nanoparticles-Based Lateral Flow Immunoassay. Diagnostics, 11(3), 443. https://doi.org/10.3390/diagnostics11030443






