Diffusion Is Directional: Innovative Diffusion Tensor Imaging to Improve Prostate Cancer Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. mpMRI Acquisition
2.3. mpMRI Analysis
2.4. DTI Acquisition
2.5. DTI Image Processing and Analysis
2.6. Pathology
2.7. Statistical Analysis
3. Results
3.1. Patients
3.2. DTI Metrics
3.3. Diagnostic Accuracy
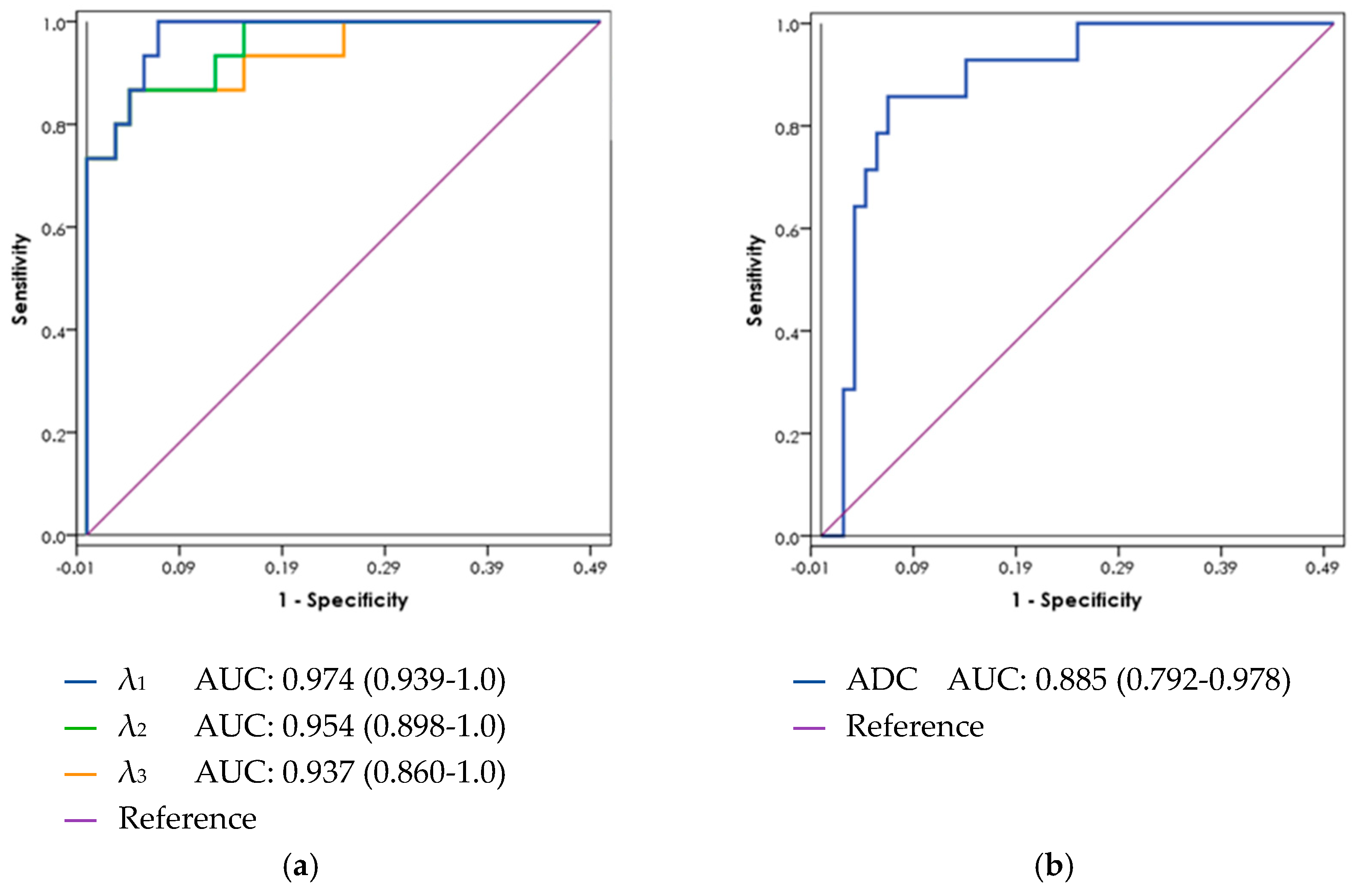
3.4. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamoen, E.H.; de Rooij, M.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur. Urol. 2015, 67, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Fütterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Yap, S.A.; Lawrentschuk, N.; Klotz, L.; Haider, M.; Hersey, K.; Finelli, A.; Zlotta, A.; Trachtenberg, J.; Fleshner, N. Impact of Multiparametric Endorectal Coil Prostate Magnetic Resonance Imaging on Disease Reclassification Among Active Surveillance Candidates: A Prospective Cohort Study. J. Urol. 2012, 187, 1247–1252. [Google Scholar] [CrossRef]
- Quon, J.S.; Moosavi, B.; Khanna, M.; Flood, T.A.; Lim, C.S.; Schieda, N. False positive and false negative diagnoses of prostate cancer at multi-parametric prostate MRI in active surveillance. Insights Imaging 2015, 6, 449–463. [Google Scholar] [CrossRef]
- Rud, E.; Klotz, D.; Rennesund, K.; Baco, E.; Berge, V.; Lien, D.; Svindland, A.; Lundeby, E.; Berg, R.E.; Eri, L.M.; et al. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014, 114, E32–E42. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; A Margolis, D.J.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging–Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Furman-Haran, E.; Eyal, E.; Shapiro-Feinberg, M.; Nissan, N.; Grobgeld, D.; Weisenberg, N.; Degani, H. Advantages and drawbacks of breast DTI. Eur. J. Radiol. 2012, 81, S45–S47. [Google Scholar] [CrossRef]
- Nissan, N.; Golan, T.; Furman-Haran, E.; Apter, S.; Inbar, Y.; Ariche, A.; Bar-Zakay, B.; Goldes, Y.; Schvimer, M.; Grobgeld, D.; et al. Diffusion Tensor Magnetic Resonance Imaging of the Pancreas. PLoS ONE 2014, 9, e115783. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Uribe, C.F.; Jones, E.C.; Chang, S.D.; Goldenberg, S.L.; Reinsberg, S.A.; Kozlowski, P. In vivo 3T and ex vivo 7T diffusion tensor imaging of prostate cancer: Correlation with histology. Magn. Reson. Imaging 2015, 33, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Onay, A.; Ertas, G.; Vural, M.; Acar, O.; Saglican, Y.; Coskun, B.; Akpek, S. Evaluation of Peripheral Zone Prostate Cancer Aggressiveness Using the Ratio of Diffusion Tensor Imaging Measures. Contrast Media Mol. Imaging 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, C.K.; Park, B.K.; Ha, S.Y.; Kwon, G.Y.; Kim, B. Diffusion-Tensor MRI at 3 T: Differentiation of Central Gland Prostate Cancer From Benign Prostatic Hyperplasia. Am. J. Roentgenol. 2014, 202, W254–W262. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, M.; Li, S.; Zhao, X.; Zhang, C.; Luo, X.; Zhou, C. Detection of prostate cancer in peripheral zone: Comparison of MR diffusion tensor imaging, quantitative dynamic contrast-enhanced MRI, and the two techniques combined at 3.0 T. Acta Radiol. 2014, 55, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gürses, B.; Kabakci, N.; Kovanlikaya, A.; Firat, Z.; Bayram, A.; Uluð, A.M.; Kovanlikaya, I.; Uluğ, A.M.; Uluo, A.M. Diffusion tensor imaging of the normal prostate at 3 Tesla. Eur. Radiol. 2007, 18, 716–721. [Google Scholar] [CrossRef]
- Sinha, S.; Sinha, U. In vivo diffusion tensor imaging of the human prostate. Magn. Reson. Med. 2004, 52, 530–537. [Google Scholar] [CrossRef]
- Hectors, S.J.; Semaan, S.; Song, C.; Lewis, S.; Haines, G.K.; Tewari, A.; Rastinehad, A.R.; Taouli, B. Advanced Diffusion-weighted Imaging Modeling for Prostate Cancer Characterization: Correlation with Quantitative Histopathologic Tumor Tissue Composition—A Hypothesis-generating Study. Radiology 2018, 286, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Ertas, G. Detection of high GS risk group prostate tumors by diffusion tensor imaging and logistic regression modelling. Magn. Reson. Imaging 2018, 50, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lemberskiy, G.; Rosenkrantz, A.B.; Veraart, J.; Taneja, S.S.; Novikov, D.S.; Fieremans, E. Time-Dependent Diffusion in Prostate Cancer. Investig. Radiol. 2017, 52, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Reischauer, C.; Wilm, B.J.; Froehlich, J.M.; Gutzeit, A.; Prikler, L.; Gablinger, R.; Boesiger, P.; Wentz, K.-U. High-resolution diffusion tensor imaging of prostate cancer using a reduced FOV technique. Eur. J. Radiol. 2011, 80, e34–e41. [Google Scholar] [CrossRef]
- Gurses, B.; Taşdelen, N.; Yencilek, F.; Kılıckesmez, N.O.; Alp, T.; Firat, Z.; Albayrak, M.S.; Uluğ, A.M.; Gürmen, A.N.; Fırat, Z. Diagnostic utility of DTI in prostate cancer. Eur. J. Radiol. 2011, 79, 172–176. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Greer, P.B.; Simpson, J.; Denham, J.; Lau, P.; Dowling, J.; Hondermarck, H.; Ramadan, S. Characterization of prostate cancer using diffusion tensor imaging: A new perspective. Eur. J. Radiol. 2019, 110, 112–120. [Google Scholar] [CrossRef]
- Eyal, E.; Shapiro-Feinberg, M.; Furman-Haran, E.; Grobgeld, D.; Golan, T.; Itzchak, Y.; Catane, R.; Papa, M.; Degani, H. Parametric Diffusion Tensor Imaging of the Breast. Investig. Radiol. 2012, 47, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Lanzman, R.S.; Wittsack, H.-J. Diffusion tensor imaging in abdominal organs. NMR Biomed. 2015, 30, e3434. [Google Scholar] [CrossRef]
- Reisert, M.; Weigel, M.; Eyal, E.; Grobgeld, D.; Degani, H.; Hennig, J. Diffusion Tensor Based Reconstruction of the Ductal Tree. In Proceedings of the International Society for Magnetic Resonance in Medicine, Montreal, QC, Canada, 7–13 May 2011; Volume 19, p. 1011. [Google Scholar]
- McNeal, J.E. The zonal anatomy of the prostate. Prostate 1981, 2, 35–49. [Google Scholar] [CrossRef]
- Xu, J.; Humphrey, P.A.; Kibel, A.S.; Snyder, A.Z.; Narra, V.R.; Ackerman, J.J.H.; Song, S.-K. Magnetic resonance diffusion characteristics of histologically defined prostate cancer in humans. Magn. Reson. Med. 2009, 61, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Manenti, G.; Carlani, M.; Mancino, S.; Colangelo, V.; Di Roma, M.; Squillaci, E.; Simonetti, G. Diffusion Tensor Magnetic Resonance Imaging of Prostate Cancer. Investig. Radiol. 2007, 42, 412–419. [Google Scholar] [CrossRef]
- Kim, C.K.; Jang, S.M.; Park, B.K. Diffusion tensor imaging of normal prostate at 3 T: Effect of number of diffusion-encoding directions on quantitation and image quality. Br. J. Radiol. 2012, 85, e279–e283. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Pu, Y.-S.; Chueh, S.-C.; Shun, C.-T.; Chu, W.-C.; Tseng, W.-Y.I. Diffusion MRI predicts transrectal ultrasound biopsy results in prostate cancer detection. J. Magn. Reson. Imaging 2011, 33, 356–363. [Google Scholar] [CrossRef]
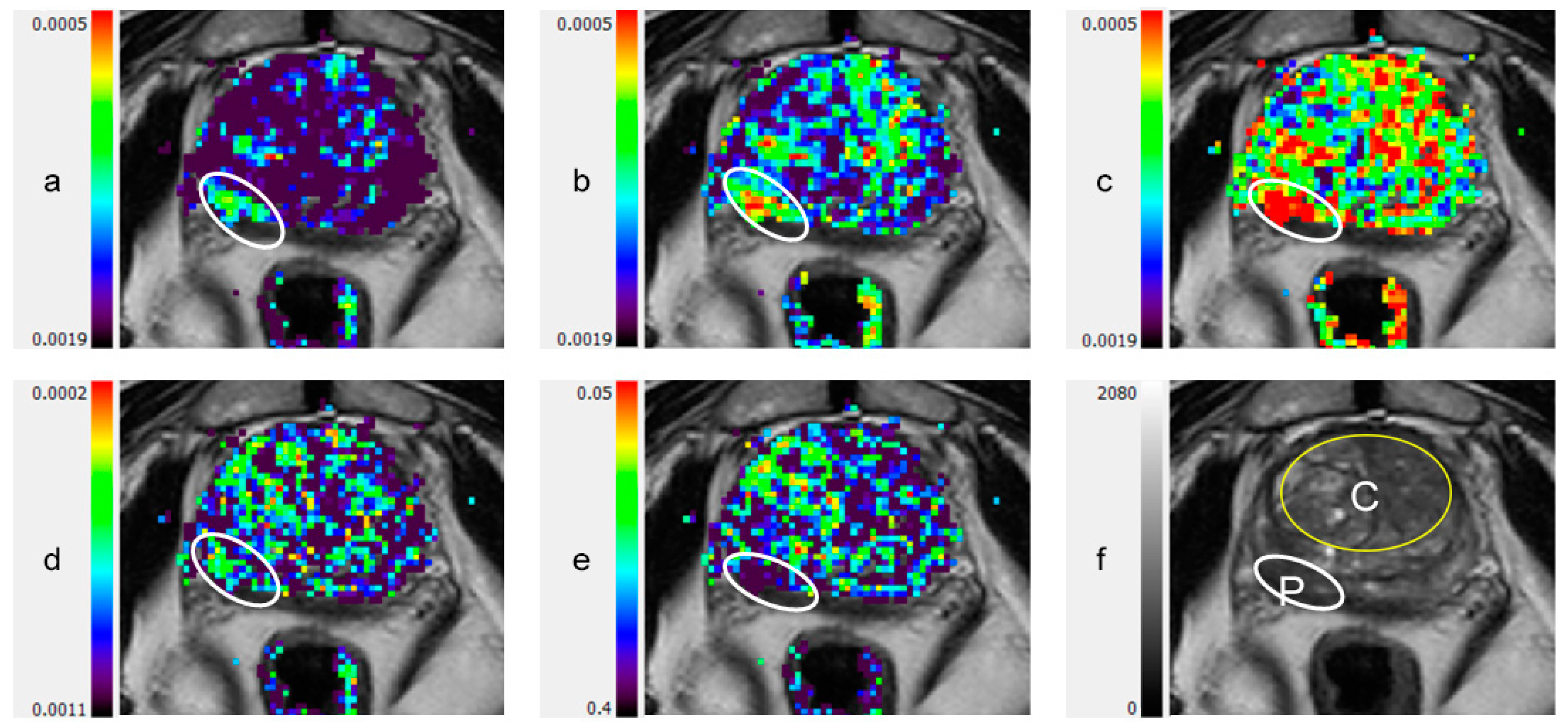

| All | NC | PCa | Significance * | |
|---|---|---|---|---|
| n = 42 | n = 26 | n = 16 | ||
| Age, median [IQR] | 61.7 [54.4–68.4] | 61.5 [51.1–65.4] | 63.3 [55.5–71.0] | p = 0.21 |
| PSA (ng/mL), median [IQR] | 4.8 [1.3–10.7] | 2.3 [1.0–6.8] | 7.8 [4.2–11.2] | p = 0.29 |
| Prostate volume by MRI (mL), median [IQR] | 49.5 [34.8–70.0] | 47.0 [33.2–69.0] | 51.0 [35.0–91.0] | p = 0.26 |
| PSA density (ng/mL/mL), median [IQR] | 0.064 [0.035–0.158] | 0.055 [0.026–0.125] | 0.108 [0.056–0.218] | p = 0.11 |
| Suspicious DRE | 16% | 16% | 16% | p = 1 |
| Family History of prostate and related cancers | 26% | 30% | 19% | p = 0.39 |
| PCa: n (%) | ||||
| Gleason 6 (3 + 3) | 8 (50%) | |||
| Gleason 7 (3 + 4) | 5 (31%) | |||
| Gleason 7 (4 + 3) | 0 | |||
| Gleason 8 (4 + 4) | 1 (6%) | |||
| Gleason 9 (4 + 5) | 2 (13%) | |||
| Lesion in peripheral zone | 11 (69%) | |||
| Lesion in central gland | 5 (31%) |
| Parameter | Peripheral Zone | Central Gland * | Mean Difference | 2-Tailed Significance | Significance (Bonferroni-Corrected) | |
|---|---|---|---|---|---|---|
| DTI: | λ1, | 2.34 ± 0.25 | 2.03 ± 0.12 | 0.315 | <0.0001 | <0.0001 |
| λ2 | 1.87 ± 0.26 | 1.51 ± 0.13 | 0.357 | <0.0001 | <0.0001 | |
| λ3 | 1.39 ± 0.30 | 1.00 ± 0.14 | 0.382 | <0.0001 | <0.0001 | |
| MD | 1.91 ± 0.27 | 1.52 ± 0.12 | 0.351 | <0.0001 | <0.0001 | |
| MA | 0.95 ± 0.11 | 1.01 ± 0.09 | −0.067 | 0.007 | 0.15 | |
| FA | 0.27 ± 0.06 | 0.34 ± 0.04 | −0.076 | <0.0001 | <0.0001 | |
| DWI: | ADC | 2.33 ± 0.06 | 2.15 ± 0.03 | 0.177 | 0.076 | 1.0 |
| (a) Peripheral Zone | ||||||
| Parameter | Normal | PCa | Mean Difference | 2-Tailed Significance | Significance (Bonferroni-Corrected) | |
| DTI: | λ1 | 2.34 ± 0.25 | 1.66 ± 0.30 | 0.684 | <0.0001 | <0.0001 |
| λ2 | 1.87 ± 0.26 | 1.23 ± 0.28 | 0.64 | <0.0001 | <0.0001 | |
| λ3 | 1.39 ± 0.30 | 0.78 ± 0.26 | 0.61 | <0.0001 | <0.0001 | |
| MD | 1.91 ± 0.27 | 1.22 ± 0.28 | 0.644 | <0.0001 | <0.0001 | |
| MA | 0.95 ± 0.11 | 0.87 ± 0.10 | 0.074 | 0.027 | 0.57 | |
| FA | 0.27 ± 0.06 | 0.38 ± 0.06 | −0.104 | <0.0001 | <0.0001 | |
| DWI: | ADC | 2.33 ± 0.06 | 1.50 ± 0.04 | 0.083 | <0.0001 | <0.0001 |
| (b) Central Gland | ||||||
| Parameter | Normal | PCa | Mean Difference | 2-Tailed Significance | Significance (Bonferroni-Corrected) | |
| DTI: | λ1, | 2.03 ± 0.12 | 1.26 ± 0.13 | 0.768 | <0.0001 | <0.0001 |
| λ2 | 1.51 ± 0.13 | 0.85 ± 0.05 | 0.663 | <0.0001 | <0.0001 | |
| λ3 | 1.00 ± 0.14 | 0.46 ± 0.07 | 0.545 | <0.0001 | <0.0001 | |
| MD | 1.52 ± 0.12 | 0.85 ± 0.04 | 0.663 | <0.0001 | <0.0001 | |
| MA | 1.01 ± 0.09 | 0.80 ± 0.18 | 0.21 | 0.0003 | 0.0063 | |
| FA | 0.34 ± 0.04 | 0.45 ± 0.08 | −0.111 | 0.078 | 1.0 | |
| DWI: | ADC | 2.15 ± 0.03 | 1.35 ± 0.03 | 0.08 | <0.0001 | <0.0001 |
| a. DTI Likert score 2 and 3 were considered as DTI-positive | ||||||
| Pathology | ||||||
| Pos | Neg | sensitivity | 87.5% | [71.3–100%] | ||
| DTI Pos | 14 | 4 | 18 | specificity | 84.6% | [70.7–98.5%] |
| DTI Neg | 2 | 22 | 24 | PPV | 77.8% | [58.6–97.0%] |
| 16 | 26 | 42 | NPV | 91.7% | [80.6–100%] | |
| b. mpMRI PI-RADS2 3, 4 and 5 lesions on mpMRI were considered as mpMRI-positive | ||||||
| Pathology | ||||||
| Pos | Neg | sensitivity | 87.5% | [71.3–100%] | ||
| mpMRI Pos | 14 | 16 | 30 | specificity | 38.5% | [19.8–57.2%] |
| mpMRI Neg | 2 | 10 | 12 | PPV | 46.7% | [28.8–64.5%] |
| 16 | 26 | 42 | NPV | 83.3% | [62.3–100%] | |
| c. DTI Likert score 3 on DTI was considered as DTI-positive | ||||||
| Pathology | ||||||
| Pos | Neg | sensitivity | 81.3% | [62.1–100%] | ||
| DTI Pos | 13 | 1 | 14 | specificity | 96.2% | [88.8–100%] |
| DTI Neg | 3 | 25 | 28 | PPV | 92.9% | [79.4–100%] |
| 16 | 26 | 42 | NPV | 89.3% | [77.8–100%] | |
| d. mpMRI PI-RADS2 4 and 5 lesions on mpMRI were considered as mpMRI-positive | ||||||
| Pathology | ||||||
| Pos | Neg | sensitivity | 62.5% | [38.8–86.2%] | ||
| mpMRI P | 10 | 8 | 18 | specificity | 69.2% | [51.5–87.0%] |
| mpMRI N | 6 | 18 | 24 | PPV | 55.6% | [32.6–78.5%] |
| 16 | 26 | 42 | NPV | 75.0% | [57.7–92.3%] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenhar, C.; Degani, H.; Ber, Y.; Baniel, J.; Tamir, S.; Benjaminov, O.; Rosen, P.; Furman-Haran, E.; Margel, D. Diffusion Is Directional: Innovative Diffusion Tensor Imaging to Improve Prostate Cancer Detection. Diagnostics 2021, 11, 563. https://doi.org/10.3390/diagnostics11030563
Shenhar C, Degani H, Ber Y, Baniel J, Tamir S, Benjaminov O, Rosen P, Furman-Haran E, Margel D. Diffusion Is Directional: Innovative Diffusion Tensor Imaging to Improve Prostate Cancer Detection. Diagnostics. 2021; 11(3):563. https://doi.org/10.3390/diagnostics11030563
Chicago/Turabian StyleShenhar, Chen, Hadassa Degani, Yaara Ber, Jack Baniel, Shlomit Tamir, Ofer Benjaminov, Philip Rosen, Edna Furman-Haran, and David Margel. 2021. "Diffusion Is Directional: Innovative Diffusion Tensor Imaging to Improve Prostate Cancer Detection" Diagnostics 11, no. 3: 563. https://doi.org/10.3390/diagnostics11030563
APA StyleShenhar, C., Degani, H., Ber, Y., Baniel, J., Tamir, S., Benjaminov, O., Rosen, P., Furman-Haran, E., & Margel, D. (2021). Diffusion Is Directional: Innovative Diffusion Tensor Imaging to Improve Prostate Cancer Detection. Diagnostics, 11(3), 563. https://doi.org/10.3390/diagnostics11030563






