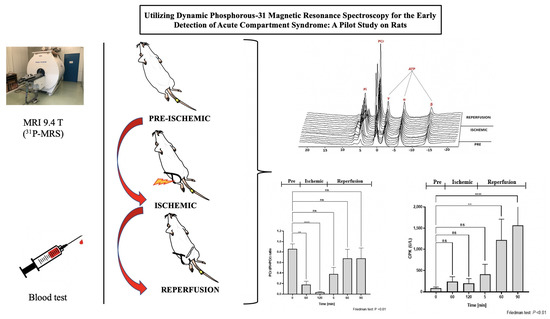
Utilizing Dynamic Phosphorous-31 Magnetic Resonance Spectroscopy for the Early Detection of Acute Compartment Syndrome: A Pilot Study on Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Creation of the Fastened Zip-Tie Rat Model
2.3. Experimental Procedure
2.4. Assessing 1H-MRI and 31P-MRS
2.5. Examining Blood Samples
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Ethics Approval
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Köstler, W.; Strohm, P.C.; Südkamp, N.P. Acute compartment syndrome of the limb. Injury 2004, 35, 1221–1227. [Google Scholar] [CrossRef]
- Cone, J.; Inaba, K. Lower extremity compartment syndrome. Trauma Surg. Acute Care Open 2017, 2, e000094. [Google Scholar] [CrossRef]
- MacKenzie, J.S.; Banskota, B.; Sirisreetreerux, N.; Shafiq, B.; Hasenboehler, E.A. A review of the epidemiology and treatment of orthopaedic injuries after earthquakes in developing countries. World J. Emerg. Surg. WJES 2017, 12, 9. [Google Scholar] [CrossRef]
- Pechar, J.; Lyons, M.M. Acute Compartment Syndrome of the Lower Leg: A Review. J. Nurse Pract. 2016, 12, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Galyfos, G.; Gkovas, C.; Kerasidis, S.; Stamatatos, I.; Stefanidis, I.; Giannakakis, S.; Geropapas, G.; Kastrisios, G.; Papacharalampous, G.; Maltezos, C. Acute Compartment Syndrome of the Lower Extremity: Update on Proper Evaluation and Management. J. Vasc. Endovasc. Surg. 2016, 1. [Google Scholar] [CrossRef]
- Long, B.; Koyfman, A.; Gottlieb, M. Evaluation and Management of Acute Compartment Syndrome in the Emergency Department. J. Emerg. Med. 2019, 56, 386–397. [Google Scholar] [CrossRef]
- Shadgan, B.; Menon, M.; O’Brien, P.J.; Reid, W.D. Diagnostic techniques in acute compartment syndrome of the leg. J. Orthop. Trauma 2008, 22, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yin, Y.; Jin, L.; Zhang, R.; Hou, Z.; Zhang, Y. Acute compartment syndrome: Cause, diagnosis, and new viewpoint. Medicine 2019, 98, e16260. [Google Scholar] [CrossRef]
- Von Keudell, A.G.; Weaver, M.J.; Appleton, P.T.; Bae, D.S.; Dyer, G.S.M.; Heng, M.; Jupiter, J.B.; Vrahas, M.S. Diagnosis and treatment of acute extremity compartment syndrome. Lancet 2015, 386, 1299–1310. [Google Scholar] [CrossRef]
- Olinic, D.M.; Stanek, A.; Tătaru, D.A.; Homorodean, C.; Olinic, M. Acute Limb Ischemia: An Update on Diagnosis and Management. J. Clin. Med. 2019, 8, 1215. [Google Scholar] [CrossRef]
- Bott, A.R.; Roszpopa, J.; Johnston, P.; Grant, I. Simultaneous 4 Compartment Pressure Monitoring in Diagnosis of Chronic Exertional Compartment Syndrome of the Forearm. Tech. Hand Up. Extrem. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ali, S.; Mahdi, A.A.; Rn, S. Serum Creatine Kinase: A Predictive Biomarker for Diagnosis of Acute Compartment Syndrome in the Paediatric Population. J. Orthop. Bone Res. 2018, 1, 103. [Google Scholar]
- Valdez, C.; Schroeder, E.; Amdur, R.; Pascual, J.; Sarani, B. Serum creatine kinase levels are associated with extremity compartment syndrome. J. Trauma Acute Care Surg. 2013, 74, 441–445, discussion 445–447. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Vrouenraets, B.C.; Kroon, B.B.; Klaase, J.M.; Bonfrer, J.M.; Nieweg, O.E.; van Slooten, G.W.; van Dongen, J.A. Value of laboratory tests in monitoring acute regional toxicity after isolated limb perfusion. Ann. Surg. Oncol. 1997, 4, 88–94. [Google Scholar] [CrossRef]
- McMillan, T.E.; Gardner, W.T.; Schmidt, A.H.; Johnstone, A.J. Diagnosing acute compartment syndrome-where have we got to? Int. Orthop. 2019, 43, 2429–2435. [Google Scholar] [CrossRef]
- Mabvuure, N.T.; Malahias, M.; Hindocha, S.; Khan, W.; Juma, A. Acute compartment syndrome of the limbs: Current concepts and management. Open Orthop. J. 2012, 6, 535–543. [Google Scholar] [CrossRef]
- Shah, P.K.; Ye, F.; Liu, M.; Jayaraman, A.; Baligand, C.; Walter, G.; Vandenborne, K. In vivo (31)P NMR spectroscopy assessment of skeletal muscle bioenergetics after spinal cord contusion in rats. Eur. J. Appl. Physiol. 2014, 114, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Miki, N.; Ikata, T.; Takai, H.; Takata, S.; Koga, K.; Sogabe, T. Effects of denervation on energy metabolism of rat hindlimb muscles: Application of 31P-MRS and 19F-MRS. J. Orthop. Sci. 1999, 4, 370–375. [Google Scholar] [CrossRef]
- Baligand, C.; Todd, A.G.; Lee-McMullen, B.; Vohra, R.S.; Byrne, B.J.; Falk, D.J.; Walter, G.A. (13)C/(31)P MRS Metabolic Biomarkers of Disease Progression and Response to AAV Delivery of hGAA in a Mouse Model of Pompe Disease. Mol. Ther. Methods Clin. Dev. 2017, 7, 42–49. [Google Scholar] [CrossRef]
- Greiner, A.; Esterhammer, R.; Messner, H.; Biebl, M.; Mühlthaler, H.; Fraedrich, G.; Jaschke, W.R.; Schocke, M.F. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J. Vasc. Surg. 2006, 43, 978–986. [Google Scholar] [CrossRef][Green Version]
- Kemp, G.J. Quantifying Skeletal Muscle Mitochondrial Function In Vivo by 31P Magnetic Resonance Spectroscopy. In Mitochondrial Dysfunction Caused by Drugs and Environmental Toxicants; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 443–456. [Google Scholar] [CrossRef]
- Mehta, P.; Morrow, M.; Russell, J.; Madhuripan, N.; Habeeb, M. Magnetic Resonance Imaging of Musculoskeletal Emergencies. Semin. Ultrasound CT MRI 2017, 38, 439–452. [Google Scholar] [CrossRef]
- Miller, R.G.; Boska, M.D.; Moussavi, R.S.; Carson, P.J.; Weiner, M.W. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. Comparison of aerobic and anaerobic exercise. J. Clin. Investig. 1988, 81, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.I.; Schewzow, K.; Fiedler, G.B.; Goluch, S.; Laistler, E.; Wolzt, M.; Moser, E.; Meyerspeer, M. Exercising calf muscle T(2) * changes correlate with pH, PCr recovery and maximum oxidative phosphorylation. NMR Biomed. 2014, 27, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Soussi, B.; Idström, J.-P.; Bylund-Fellenius, A.-C.; Scherstén, T. Dynamics of skeletal muscle energetics during ischemia and reperfusion assessed by in vivo 31P NMR. NMR Biomed. 1990, 3, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Ikata, T.; Sairyo, K.; Matsuura, T.; Sasa, T.; Koga, K.; Fukunaga, M. Evaluation of disuse atrophy of rat skeletal muscle based on muscle energy metabolism assessed by 31P-MRS. J. Physiol. Anthropol. Appl. Hum. Sci. 2001, 20, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, G.B.; Meyerspeer, M.; Schmid, A.I.; Goluch, S.; Schewzow, K.; Laistler, E.; Mirzahosseini, A.; Niess, F.; Unger, E.; Wolzt, M.; et al. Localized semi-LASER dynamic (31)P magnetic resonance spectroscopy of the soleus during and following exercise at 7 T. Magma 2015, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Klepochova, R.; Valkovic, L.; Hochwartner, T.; Triska, C.; Bachl, N.; Tschan, H.; Trattnig, S.; Krebs, M.; Krssak, M. Differences in Muscle Metabolism Between Triathletes and Normally Active Volunteers Investigated Using Multinuclear Magnetic Resonance Spectroscopy at 7T. Front. Physiol. 2018, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Purvis, L.A.B.; Valkovič, L.; Robson, M.D.; Rodgers, C.T. Feasibility of absolute quantification for (31) P MRS at 7 T. Magn. Reson. Med. 2019, 82, 49–61. [Google Scholar] [CrossRef]
- Van der Kolk, A.G.; Hendrikse, J.; Zwanenburg, J.J.M.; Visser, F.; Luijten, P.R. Clinical applications of 7T MRI in the brain. Eur. J. Radiol. 2013, 82, 708–718. [Google Scholar] [CrossRef]
- Isaacs, B.R.; Mulder, M.J.; Groot, J.M.; van Berendonk, N.; Lute, N.; Bazin, P.-L.; Forstmann, B.U.; Alkemade, A. 3 versus 7 Tesla magnetic resonance imaging for parcellations of subcortical brain structures in clinical settings. PLoS ONE 2020, 15, e0236208. [Google Scholar] [CrossRef] [PubMed]
- Valkovič, L.; Chmelík, M.; Meyerspeer, M.; Gagoski, B.; Rodgers, C.T.; Krššák, M.; Andronesi, O.C.; Trattnig, S.; Bogner, W. Dynamic (31)P-MRSI using spiral spectroscopic imaging can map mitochondrial capacity in muscles of the human calf during plantar flexion exercise at 7 T. NMR Biomed. 2016, 29, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Parasoglou, P.; Xia, D.; Chang, G.; Regatte, R.R. Dynamic three-dimensional imaging of phosphocreatine recovery kinetics in the human lower leg muscles at 3T and 7T: A preliminary study. NMR Biomed. 2013, 26, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Bakermans, A.J.; Abdurrachim, D.; van Nierop, B.J.; Koeman, A.; van der Kroon, I.; Baartscheer, A.; Schumacher, C.A.; Strijkers, G.J.; Houten, S.M.; Zuurbier, C.J.; et al. In vivo mouse myocardial (31)P MRS using three-dimensional image-selected in vivo spectroscopy (3D ISIS): Technical considerations and biochemical validations. NMR Biomed. 2015, 28, 1218–1227. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, X.; Zhu, X.H.; Du, F.; Chen, W. In vivo 31P MRS of human brain at high/ultrahigh fields: A quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn. Reson. Imaging 2006, 24, 1281–1286. [Google Scholar] [CrossRef]
- Lu, M.; Chen, W.; Zhu, X.-H. Field dependence study of in vivo brain (31)P MRS up to 16.4 T. NMR Biomed. 2014, 27, 1135–1141. [Google Scholar] [CrossRef]
- Ohta, H.; Ohki, T.; Kanaoka, Y.; Koizumi, M.; Okano, H.J. Pitfalls of invasive blood pressure monitoring using the caudal ventral artery in rats. Sci. Rep. 2017, 7, 41907. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, Y.; Webb, J.; Gregory, A.; Fatemi, M.; Alizad, A.; Zhao, C. Ultrasound shear wave elastography for measuring intracompartmental pressure of compartment syndrome using a turkey hind limb model. J. Biomech. 2020, 98, 109427. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Kido, C.; Inubushi, T. Observation of rat hind limb skeletal muscle during arterial occlusion and reperfusion by 31P MRS and 1H MRI. Magn. Reson. Imaging 1991, 9, 269–274. [Google Scholar] [CrossRef]
- Irvine Office of Research (University of California). Determination and Justification of Animal Numbers. Available online: https://research.uci.edu/compliance/animalcare-use/protocol-writing-tips-and-guidance/justify-numbers.html (accessed on 15 March 2021).
- Sorzano, C.; Tabas-Madrid, D.; Núñez, F.; Fernández-Criado, C.; Naranjo, A. Sample Size for Pilot Studies and Precision Driven Experiments. arXiv 2017, arXiv:1707.00222. [Google Scholar]
- Torvinen, S.; Silvennoinen, M.; Piitulainen, H.; Närväinen, J.; Tuunanen, P.; Gröhn, O.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Rats bred for low aerobic capacity become promptly fatigued and have slow metabolic recovery after stimulated, maximal muscle contractions. PLoS ONE 2012, 7, e48345. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, N.M.; Ciapaite, J.; Nicolay, K.; Prompers, J.J. Comparison of in vivo postexercise phosphocreatine recovery and resting ATP synthesis flux for the assessment of skeletal muscle mitochondrial function. Am. J. Physiol. Cell Physiol. 2010, 299, C1136–C1143. [Google Scholar] [CrossRef]
- Jablonski, M.; Starcukova, J.; Starcuk, Z., Jr. Processing tracking in jMRUI software for magnetic resonance spectra quantitation reproducibility assurance. BMC Bioinform. 2017, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Buchli, R.; Meier, D.; Martin, E.; Boesiger, P. Assessment of absolute metabolite concentrations in human tissue by 31P MRS in vivo. Part II: Muscle, liver, kidney. Magn. Reson. Med. 1994, 32, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G.J.; Meyerspeer, M.; Moser, E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: A quantitative review. NMR Biomed. 2007, 20, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Hata, J. 3. Introduction to MRI Image Analysis Using ImageJ. Nihon Hoshasen Gijutsu Gakkai Zasshi 2019, 75, 89–94. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
- Altman, D.G.; Gore, S.M.; Gardner, M.J.; Pocock, S.J. Statistical guidelines for contributors to medical journals. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1489–1493. [Google Scholar] [CrossRef]
- Budsberg, S.C.; Shuler, M.S.; Hansen, M.; Uhl, E.; Freedman, B.A. Comparison of NIRS, serum biomarkers, and muscle damage in a porcine balloon compression model of acute compartment syndrome. J. Trauma Acute Care Surg. 2016, 81, 876–881. [Google Scholar] [CrossRef]
- Tzika, A.A.; Vignerson, D.; Hricak, H.; Kogan, B.A.; Moseley, M.E.; James, T.L. Role of P-31 MR Spectroscopy in Assessing Testicular Torsion: Rat Model; Radiological Society of North America Inc: Oak Brook, IL, USA, 1988. [Google Scholar]
- Cui, W.; Jang, A.; Zhang, P.; Thompson, B.; Townsend, D.; Metzger, J.M.; Zhang, J. Early Detection of Myocardial Bioenergetic Deficits: A 9.4 Tesla Complete Non Invasive 31P MR Spectroscopy Study in Mice with Muscular Dystrophy. PLoS ONE 2015, 10, e0135000. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Labruto, F.; Weiss, R.J.; Jansson, K.-Å. Increasing thigh pain: Acute compartment syndrome! BMJ Case Rep. 2010, 2010, bcr1220092513. [Google Scholar] [CrossRef] [PubMed]
- Paillard, M. Direct intracellular pH measurement in rat and crab muscle. J. Physiol. 1972, 223, 297–319. [Google Scholar] [CrossRef]
- Hassan, Z.A.; Schattner, P.; Mazza, D. Doing A Pilot Study: Why Is It Essential? Malays. Fam. Physician 2006, 1, 70–73. [Google Scholar] [PubMed]
- Ohta, H.; Liu, X.; Maeda, M. Autologous adipose mesenchymal stem cell administration in arteriosclerosis and potential for anti-aging application: A retrospective cohort study. Stem Cell Res. Ther. 2020, 11, 538. [Google Scholar] [CrossRef] [PubMed]









| Pre-Ischemic | Ischemic | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Minute | 0 | 60 | 120 | 5 | 60 | 90 |
| PCr/(Pi + PCr) | ||||||
| Case 1 | 0.85 | 0.15 | 0.02 | 0.29 | 0.46 | 0.46 |
| Case 2 | 0.91 | 0.23 | 0.03 | 0.28 | 0.48 | 0.41 |
| Case 3 | 0.89 | 0.24 | 0.05 | 0.59 | 0.80 | 0.84 |
| Case 4 | 0.66 | 0.07 | 0.05 | 0.37 | 0.85 | 0.87 |
| Case 5 | 0.90 | 0.20 | 0.03 | 0.30 | 0.81 | 0.80 |
| Case 6 | 0.93 | 0.19 | 0.03 | 0.46 | 0.67 | 0.66 |
| 0.86 ± 0.10 | 0.18 ± 0.06 | 0.04 ± 0.01 | 0.38 ± 0.12 | 0.68 ± 0.17 | 0.67 ± 0.20 | |
| (0.85–0.91 *) | (0.15–0.23 *) | (0.03–0.05 *) | (0.29–0.46 *) | (0.48–0.81 *) | (0.46–0.84 *) | |
| p = 0.003 ** | p < 0.0001 ** | p = 0.067 | p > 0.999 | p > 0.999 | ||
| CPK level | ||||||
| Case 1 | 71 | 147 | 334 | 819 | 2222 | 2222 |
| Case 2 | 61 | 102 | 245 | 205 | 1309 | 2222 |
| Case 3 | 65 | 300 | 313 | 381 | 1289 | 1337 |
| Case 4 | 91 | 419 | 91 | 216 | 749 | 919 |
| Case 5 | 87 | 261 | 119 | 539 | 1349 | 2222 |
| Case 6 | 141 | 220 | 88 | 319 | 638 | 1141 |
| 89 ± 29.46 | 241.50 ± 113.28 | 198.33 ± 112.88 | 413.16 ± 233.51 | 1259.33 ± 562.95 | 1677.16 ± 611.31 | |
| (65–87 *) | (147–220 *) | (91–313 *) | (319–539 *) | (749–1349 *) | (919–2222 *) | |
| p = 0.948 ** | p > 0.999 ** | p = 0.126 ** | p = 0.001 ** | p < 0.0001 ** | ||
| Pre-Ischemic | Ischemic | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Minute | 0 | 60 | 120 | 5 | 60 | 90 |
| pH (31P-MRS) | ||||||
| Case 1 | 7.11 | 6.85 | 6.47 | 6.39 | 6.54 | 6.53 |
| Case 2 | 7.08 | 6.84 | 6.41 | 6.35 | 6.44 | 6.57 |
| Case 3 | 7.10 | 6.85 | 6.55 | 6.54 | 7.02 | 7.06 |
| Case 4 | 7.08 | 6.52 | 6.20 | 6.23 | 6.09 | 6.13 |
| Case 5 | 7.08 | 6.83 | 6.46 | 6.37 | 5.84 | 5.84 |
| Case 6 | 7.10 | 6.88 | 6.49 | 6.49 | 7.05 | 7.07 |
| 7.09 ± 0.01 | 6.80 ± 0.14 | 6.430 ± 0.13 | 6.40 ± 0.10 | 6.50 ± 0.49 | 6.53 ± 0.49 | |
| (7.08–7.10 *) | (6.83–6.85 *) | (6.41–6.49 *) | (6.35–6.49 *) | (6.09–7.02 *) | (6.13–7.06 *) | |
| p = 0.614 ** | p = 0.004 ** | p = 0.0008 ** | p = 0.021 ** | p = 0.083 ** | ||
| pH (arterial blood) | ||||||
| Case 1 | 7.50 | 7.38 | 7.35 | 7.35 | 7.36 | 7.36 |
| Case 2 | 7.45 | 7.44 | 7.40 | 7.38 | 7.39 | 7.39 |
| Case 3 | 7.47 | 7.42 | 7.39 | 7.38 | 7.41 | 7.39 |
| Case 4 | 7.50 | 7.43 | 7.45 | 7.45 | 7.48 | 7.46 |
| Case 5 | 7.43 | 7.38 | 7.35 | 7.36 | 7.38 | 7.38 |
| Case 6 | 7.46 | 7.36 | 7.38 | 7.37 | 7.36 | 7.36 |
| 7.47 ± 0.03 | 7.41 ± 0.03 | 7.39 ± 0.04 | 7.39 ± 0.04 | 7.40 ± 0.04 | 7.39 ± 0.034 | |
| (7.45–7.50 *) | (7.38–7.43 *) | (7.35–7.40 *) | (7.36–7.38 *) | (7.47–7.41 *) | (7.36–7.39 *) | |
| p = 0.103 ** | p = 0.021 ** | p = 0.0008 ** | p = 0.043 ** | p = 0.103 ** | ||
| Pre-Ischemic | Ischemic | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Minute | 0 | 60 | 120 | 5 | 60 | 90 |
| TA | ||||||
| Case 1 | 14.58 | 76.99 | 88.92 | 119.68 | 148.83 | 127.75 |
| Case 2 | 13.88 | 75.03 | 84.21 | 71.18 | 74.22 | 68.17 |
| Case 3 | 10.58 | 72.20 | 77.73 | 82.73 | 76.57 | 80.06 |
| Case 4 | 11.61 | 83.04 | 88.80 | 108.35 | 108.29 | 92.60 |
| Case 5 | 18.08 | 76.87 | 70.70 | 103.82 | 66.78 | 79.96 |
| Case 6 | 12.36 | 84.52 | 96.29 | 86.58 | 109.18 | 103.53 |
| 13.52 ± 2.67 (11.61–14.58 *) | 78.11 ± 4.75 (75.03–83.04 *) | 84.44 ± 9.09 (77.73–88.92 *) | 95.39 ± 18.18 (82.73–108.35 *) | 97.31 ± 31.02 (74.22–109.18 *) | 92.01 ± 21.29 (79.96–103.53 *) | |
| GA | ||||||
| Case 1 | 6.08 | 130.85 | 153.34 | 238.48 | 213.38 | 151.92 |
| Case 2 | 11.78 | 77.82 | 86.81 | 88.18 | 67.87 | 64.29 |
| Case 3 | 8.05 | 87.20 | 85.93 | 103.56 | 135.08 | 141.16 |
| Case 4 | 10.99 | 70.50 | 94.75 | 97.95 | 109.43 | 92.68 |
| Case 5 | 16.43 | 78.55 | 74.09 | 103.50 | 68.74 | 78.24 |
| Case 6 | 8.22 | 74.15 | 80.41 | 77.50 | 116.45 | 102.29 |
| 10.26 ± 3.67 (8.05–11.78 *) | 86.51 ± 22.42 (74.15–78.55 *) | 95.89 ± 28.98 (85.93–94.75 *) | 118.20 ± 59.78 (88.18–103.56 *) | 118.49 ± 53.67 (67.87–116.45 *) | 105.10 ± 34.76 (78.24–102.29 *) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, H.; Vo, N.-M.V.; Hata, J.; Terawaki, K.; Shirakawa, T.; Okano, H.J. Utilizing Dynamic Phosphorous-31 Magnetic Resonance Spectroscopy for the Early Detection of Acute Compartment Syndrome: A Pilot Study on Rats. Diagnostics 2021, 11, 586. https://doi.org/10.3390/diagnostics11040586
Ohta H, Vo N-MV, Hata J, Terawaki K, Shirakawa T, Okano HJ. Utilizing Dynamic Phosphorous-31 Magnetic Resonance Spectroscopy for the Early Detection of Acute Compartment Syndrome: A Pilot Study on Rats. Diagnostics. 2021; 11(4):586. https://doi.org/10.3390/diagnostics11040586
Chicago/Turabian StyleOhta, Hiroki, Nhat-Minh Van Vo, Junichi Hata, Koshiro Terawaki, Takako Shirakawa, and Hirotaka James Okano. 2021. "Utilizing Dynamic Phosphorous-31 Magnetic Resonance Spectroscopy for the Early Detection of Acute Compartment Syndrome: A Pilot Study on Rats" Diagnostics 11, no. 4: 586. https://doi.org/10.3390/diagnostics11040586
APA StyleOhta, H., Vo, N.-M. V., Hata, J., Terawaki, K., Shirakawa, T., & Okano, H. J. (2021). Utilizing Dynamic Phosphorous-31 Magnetic Resonance Spectroscopy for the Early Detection of Acute Compartment Syndrome: A Pilot Study on Rats. Diagnostics, 11(4), 586. https://doi.org/10.3390/diagnostics11040586