Field Performances of Rapid Diagnostic Tests Detecting Human Plasmodium Species: A Systematic Review and Meta-Analysis in India, 1990–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Electronic Databases
2.3. Search Strategy
2.4. Objectives
2.5. Eligibility Criteria
2.6. Screening Strategy
2.7. Data of Interest
2.8. Methodological Quality Assessment of Studies
2.9. Data Verification for Consistency
2.10. Outcomes to Appraise Field Performances
2.11. Data Management
3. Results
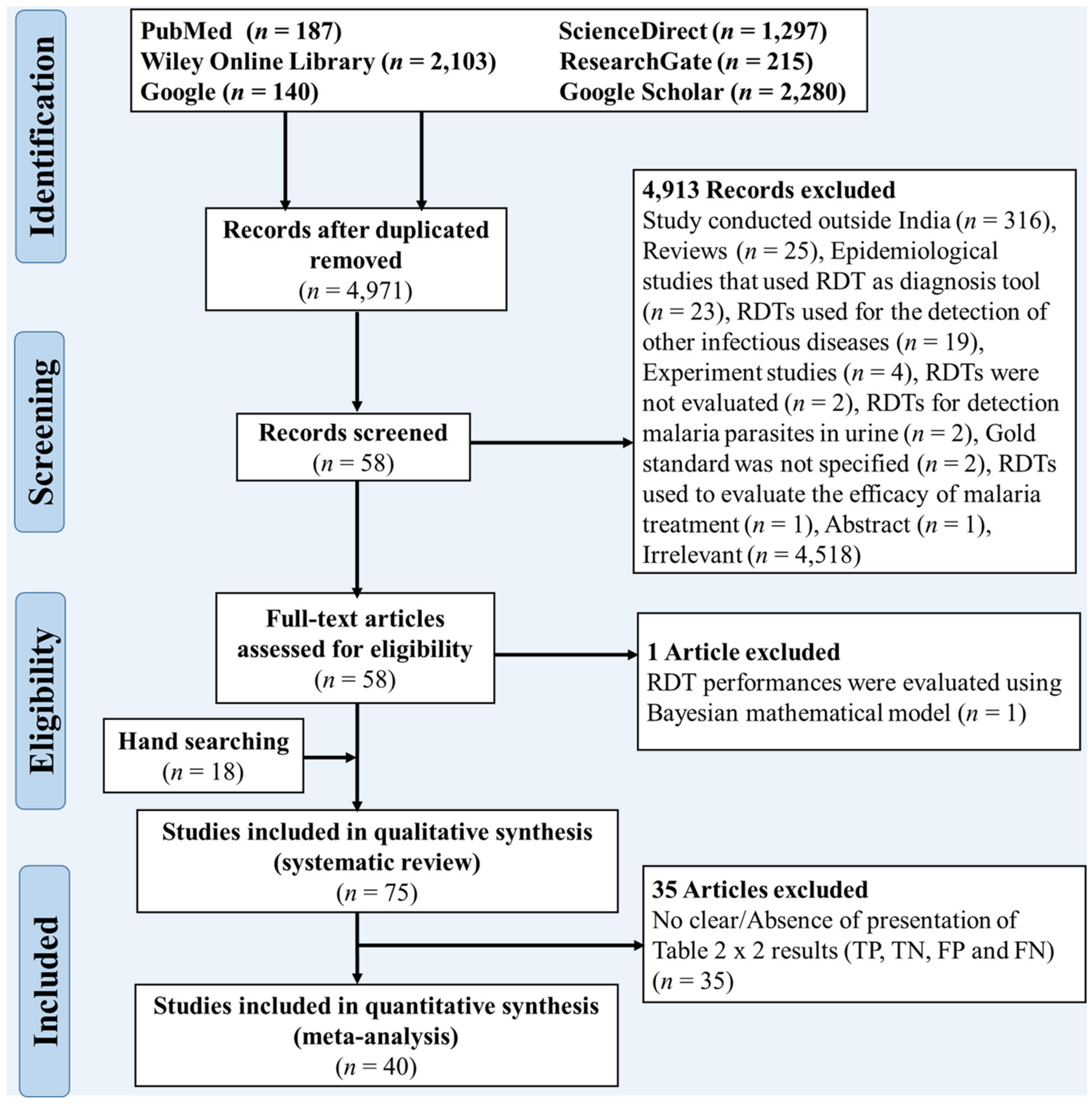
3.1. Selection Process Results
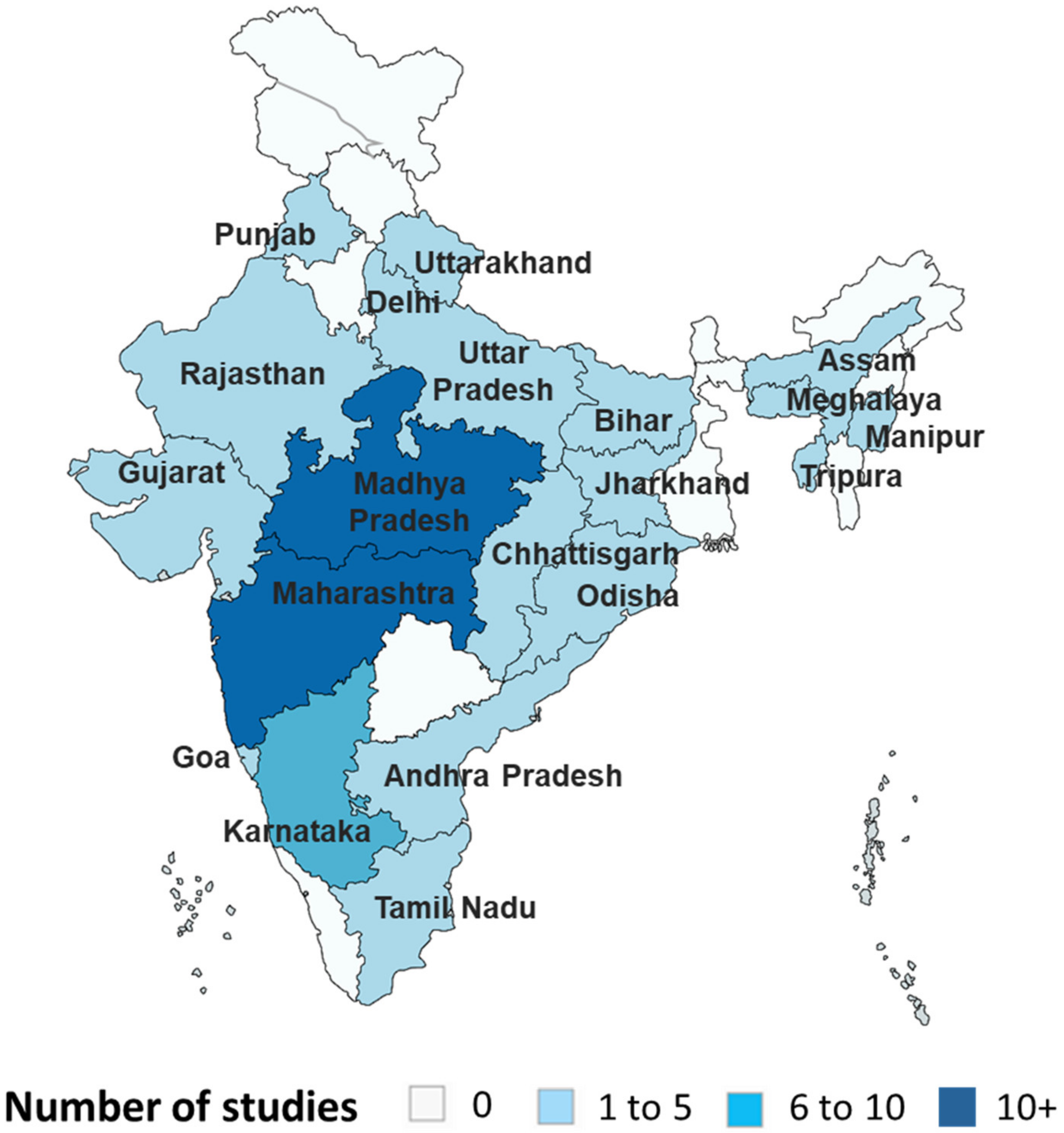
3.2. Geographical Distribution of the Included Studies
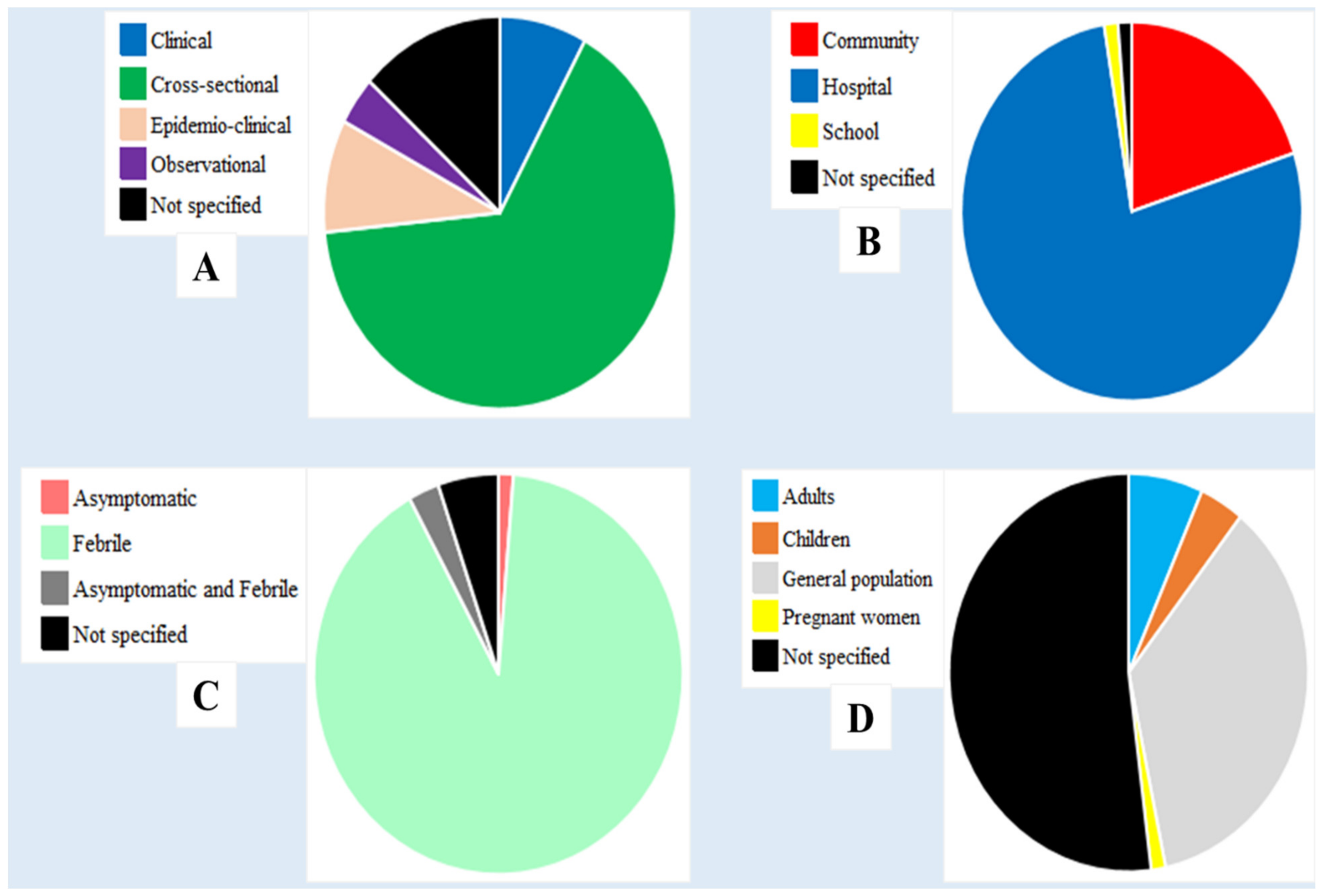
3.3. Characteristics of the Included Studies
3.4. Gold Standard Used to Evaluate the Performances of RDTs
3.5. Characteristics of RDTs Evaluated
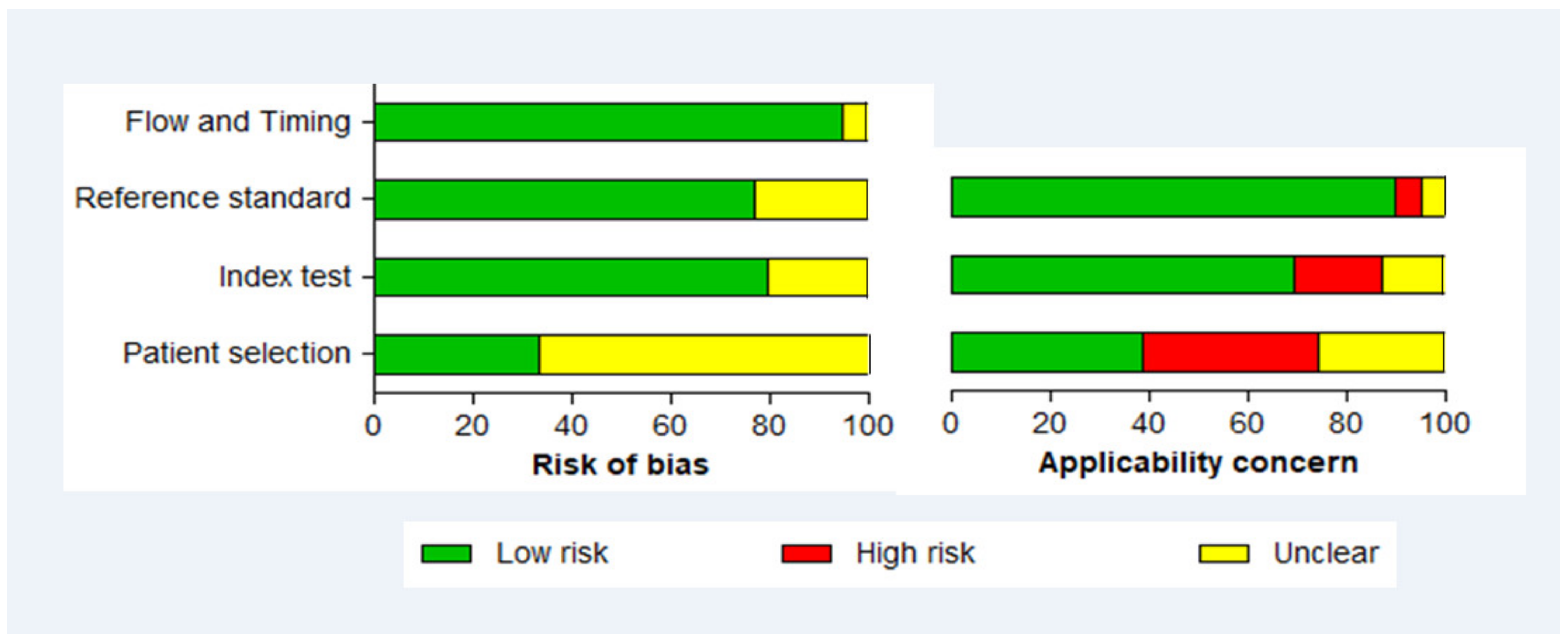
3.6. Methodological Quality of Included Studies
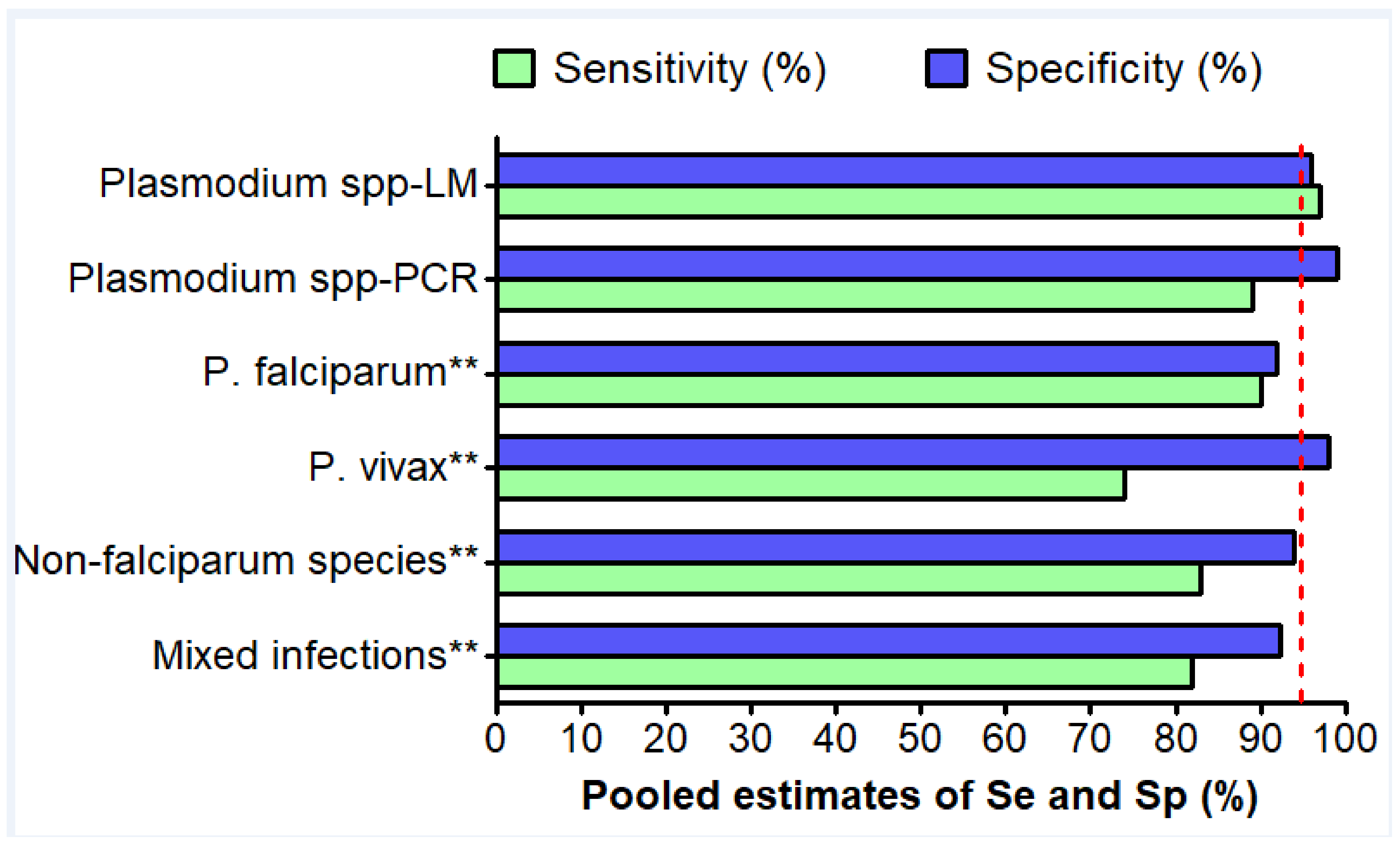
3.7. Detection of Plasmodium spp. Species
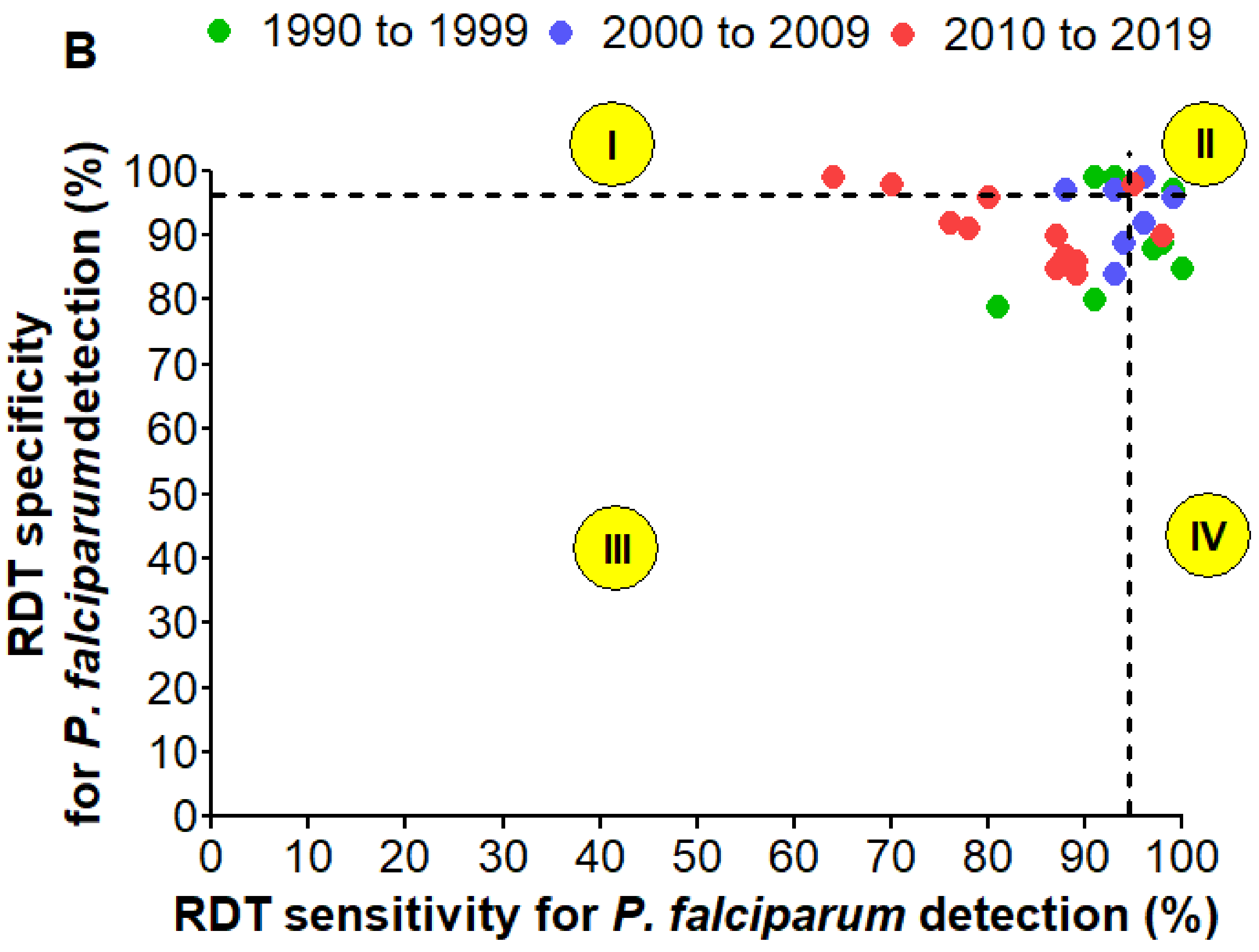
3.8. Detection of P. Falciparum
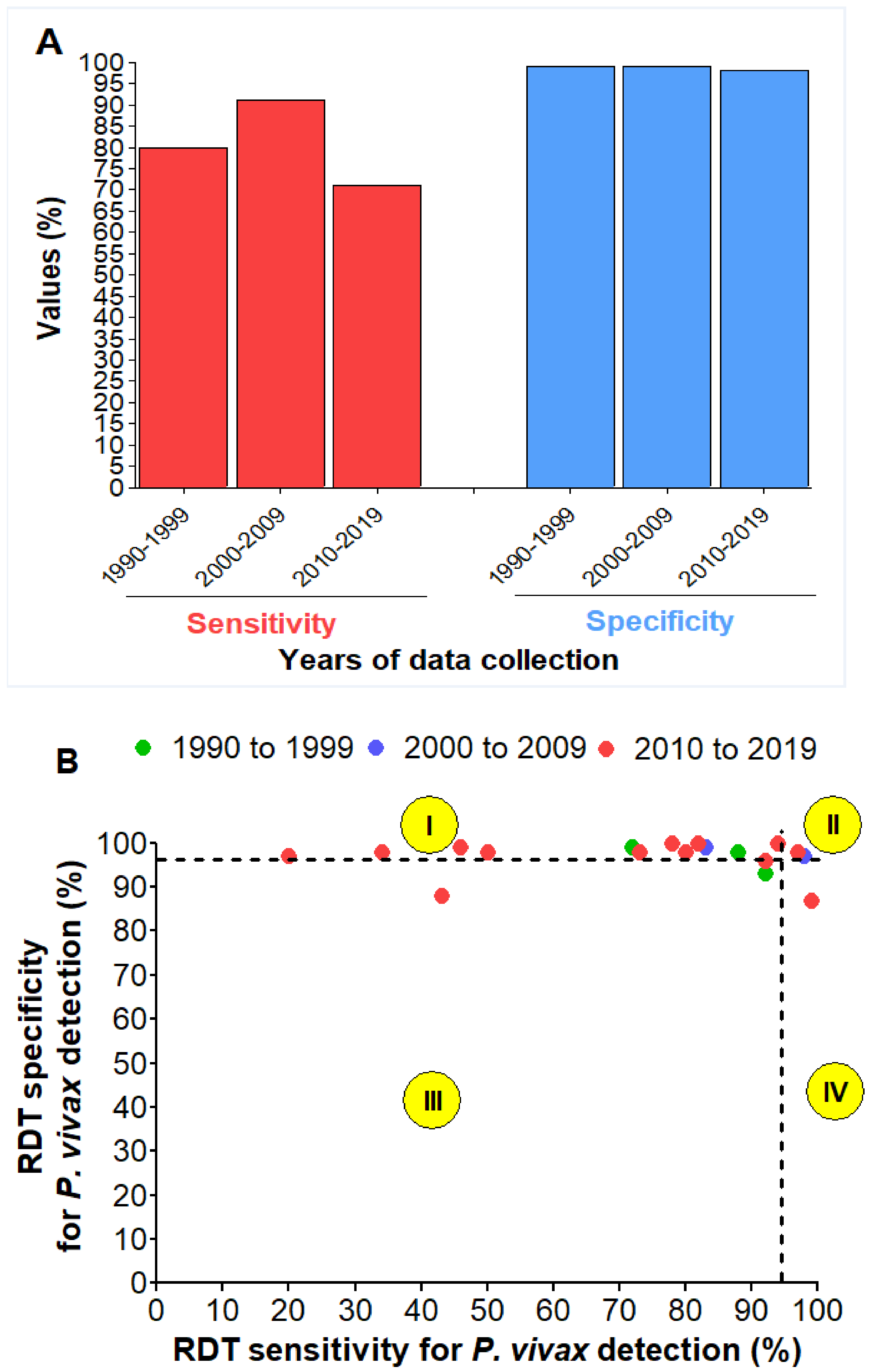
3.9. Detection of P. vivax
3.10. Paired Comparison of Performances of RDT for P. falciparum and P. vivax
3.11. Detection of Non-Falciparum Species
3.12. Detection of Mixed Infections
3.13. Gametocyte Carriage and RDT Positivity
3.14. Intensity of RDT Test Line and Parasitemia
3.15. RDT Performances and Level of Parasitemia
3.16. RDT Performances against P. falciparum Isolates with Pfhrp2 Deletions
4. Discussion
5. Limitations of this Review
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | Confidence interval at 95% |
| DOR | Diagnostic odd ratio |
| FP | False positive |
| FN | False negative |
| JBI | Joanna Briggs Institute |
| LDH | Lactate dehydrogenase |
| LM | Light microscopy |
| NLR | Negative likelihood ratio |
| PDS | Panel detection score |
| PfHRP2 | P. falciparum histidine rich protein 2 |
| PLR | Positive likelihood ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| RDTs | Rapid diagnostic tests |
| Se | Sensitivity |
| SEA | South East Asia |
| sSA | sub-Saharan Africa |
| SR-MA | Systematic review and meta-analysis |
| Sp | Specificity |
| TP | True positive |
| TN | True negative |
| WHO | World Health Organization |
References
- White, N.J.; Pukrittayakamee, S.; Tinh Hien, T.; Abul Faiz, M.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 391, 1608–1621. [Google Scholar] [CrossRef]
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2020; Volume WHO/H™/GM, ISBN 978 92 4 1564403. [Google Scholar]
- The Global Fund. List of Rapid Diagnostic Test. (RDT) Kits for Malaria Classified According to the Global Fund Quality Assurance Policy; The Global Fund: Geneva, Switzerland, 2015. [Google Scholar]
- Moody, A. Rapid Diagnostic Tests for Malaria Parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries, Part 2, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Kamgain, L.; Assam-Assam, J.-P.; Kojom Foko, L.P.; Fouamno, H. Prevalence of Malaria Infection and Reliability of ACCUCARE One Step Malaria Test® for Diagnosing Malaria in People Living with Human Immunodeficiency Virus Infection in Cameroon. Int. J. Trop. Dis. Health 2017, 21, 30277. [Google Scholar] [CrossRef]
- Visser, T.; Daily, J.; Hotte, N.; Dolkart, C.; Cunningham, J.; Yadav, P. Rapid Diagnostic Tests for Malaria. Bull. World Health Organ. 2015, 93, 862–866. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Anvikar, A.R.; Shah, N.; Dhariwal, A.C.; Sonal, G.S.; Pradhan, M.M.; Ghosh, S.K.; Valecha, N. Epidemiology of Plasmodium vivax Malaria in India. Am. J. Trop. Med. Hyg. 2016, 95 (Suppl. S6), 108–120. [Google Scholar] [CrossRef] [PubMed]
- Siwal, N.; Singh, U.S.; Dash, M.; Kar, S.; Rani, S.; Rawal, C.; Singh, R.; Anvikar, A.R.; Pande, V.; Das, A. Malaria Diagnosis by PCR Revealed Differential Distribution of Mono and Mixed Species Infections by Plasmodium falciparum and P. vivax in India. PLoS ONE 2018, 13, e0193046. [Google Scholar] [CrossRef] [PubMed]
- Kojom, L.P.; Singh, V. Prevalence of Plasmodium falciparum Field Isolates with Deletions in Histidine-rich Protein 2 and 3 Genes in Context with Sub-Saharan Africa and India: A Systematic Review and Meta-analysis. Malar. J. 2020, 19, 46. [Google Scholar] [CrossRef]
- Kattenberg, J.H.; Ochodo, E.A.; Boer, K.R.; Schallig, H.D.; Mens, P.F.; Leeflang, M.M. Systematic Review and Meta-Analysis: Rapid Diagnostic Tests versus Placental Histology, Microscopy and PCR for Malaria in Pregnant Women. Malar. J. 2011, 10, 321. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Beadle, C.; Long, G.W.; McElroy, P.D.; Hoffman, S.L.; Long, G.W.; Weiss, W.R.; Maret, S.M.; Oloo, A.J. Diagnosis of Malaria by Detection of Plasmodium falciparum HRP-2 Antigen with a Rapid Dipstick Antigen-Capture Assay. Lancet 1994, 343, 564–568. [Google Scholar] [CrossRef]
- Shiff, C.J.; Premji, Z.; Minjas, J.N. The Rapid Manual Parasight®-f Test. a New Diagnostic Tool for Plasmodium falciparum Infection. Trans. R. Soc Trop. Med. Hyg. 1993, 87, 646–648. [Google Scholar] [CrossRef]
- Kar, S.S.; Ramalingam, A. Is 30 the Magic Number? Issues in Sample Size. Natl. J. Community Med. 2013, 4, 175–179. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. Group, and the Q.-2. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 530–538. [Google Scholar] [CrossRef]
- Campbell, J.M.; Klugar, M.; Ding, S.; Carmody, D.P.; Hakonsen, S.J.; Jadotte, Y.T.; White, S.; Munn, Z. Diagnostic Test Accuracy: Methods for Systematic Review and Meta-Analysis. Int. J. Evid. Based Healthc. 2015, 13, 154–162. [Google Scholar] [CrossRef]
- Leeflang, M.M.G. Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy. Clin. Microbiol. Infect. 2014, 20, 105–113. [Google Scholar] [CrossRef]
- Parikh, R.; Mathai, A.; Parikh, S.; Sekhar, G.C.; Thomas, R. Understanding and Using Sensitivity, Specificity and Predictive Values. Indian J. Ophthalmol. 2008, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Pewsner, D.; Egger, M.; Nüesch, R.; Bucher, H.C.; Genton, B. Review Meta-Analysis: Accuracy of Rapid Tests for Malaria in Travelers. Ann. Intern. Med. 2005, 142, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M.M. The Diagnostic Odds Ratio: A Single Indicator of Test Performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- Meta-Analyst, O.; Meta-Analyst, O. Open Meta-Analyst—The Tool | Evidence Synthesis in Health. 2018. Available online: http://www.cebm.brown.edu/openmeta/doc/openMA_help.html (accessed on 12 October 2020).
- Wallace, B.C.; Schmid, C.H.; Lau, J.; Trikalinos, T.A. Meta-Analyst: Software for Meta-Analysis of Binary, Continuous and Diagnostic Data. BMC Med. Res. Methodol. 2009, 9, 80. [Google Scholar] [CrossRef]
- Valentine, J.C.; Pigott, T.D.; Rothstein, H.R. How Many Studies Do You Need? A Primer on Statistical Power for Meta-Analysis. J. Educ. Behav. Stat. 2010, 35, 215–247. [Google Scholar] [CrossRef]
- Reid, K. Interpreting and Understanding Meta-Analysis Graphs: A Practical Guide. Aust. Fam. Phys. 2006, 35, 635–638. [Google Scholar]
- Sedgwick, P. Meta-Analyses: Heterogeneity and Subgroup Analysis. BMJ 2013, 346, f4040. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the Statistical Validity of Summary Meta-Analysis and Meta-Regression Results for Use in Clinical Practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- WHO. Malaria Rapid Diagnostic Test. Performance: Results of WHO Product Testing of Malaria RDTs: Round 8 (2016–2018); World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Dalrymple, U.; Arambepola, R.; Gething, P.W.; Cameron, E. How Long Do Rapid Diagnostic Tests Remain Positive after Anti-Malarial Treatment? Malar. J. 2018, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Gillet, P.; Scheirlinck, A.; Stokx, J.; De Weggheleire, A.; Chaúque, H.S.; Canhanga, O.D.; Tadeu, B.T.; Mosse, C.D.; Tiago, A.; Mabunda, S.; et al. Prozone in Malaria Rapid Diagnostics Tests: How Many Cases Are Missed? Malar. J. 2011, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Mccarthy, J.; Gatton, M.; Kyle, D.E.; Belizario, V.; Luchavez, J.; Bell, D.; Cheng, Q. Genetic Diversity of Plasmodium falciparum Histidine-Rich Protein 2 (PfHRP2) and Its Effect on the Performance of PfHRP2-Based Rapid Diagnostic Tests. J. Infect. Dis 2005, 192, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Saunders, D.L.; Meshnick, S.R. The Role of Submicroscopic Malaria in Malaria Transmission: What Is the Evidence? Trends Parasitol. 2014, 30, 183–190. [Google Scholar] [CrossRef]
- Pava, Z.; Burdam, F.H.; Handayuni, I.; Trianty, L.; Utami, R.A.S.; Tirta, Y.K.; Kenangalem, E.; Lampah, D.; Kusuma, A.; Wirjanata, G.; et al. Submicroscopic and Asymptomatic Plasmodium Parasitaemia Associated with Significant Risk of Anaemia in Papua, Indonesia. PLoS ONE 2016, 11, e0165340. [Google Scholar] [CrossRef]
- Rovira-Vallbona, E.; Contreras-Mancilla, J.J.; Ramirez, R.; Guzmán-Guzmán, M.; Carrasco-Escobar, G.; Llanos-Cuentas, A.; Vinetz, J.M.; Gamboa, D.; Rosanas-Urgell, A. Predominance of Asymptomatic and Sub-Microscopic Infections Characterizes the Plasmodium Gametocyte Reservoir in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2017, 11, e0005674. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Deora, N.; Bhandari, D.; Parvez, S.; Sinha, A.; Sharma, A. Trends of Neglected Plasmodium Species Infection in Humans over the Past Century in India. One Health 2021, 11, 100190. [Google Scholar] [CrossRef]
- Haanshuus, C.G.; Chandy, S.; Manoharan, A.; Vivek, R. A High Malaria Prevalence Identified by PCR among Patients with Acute Undifferentiated Fever in India. PLoS ONE 2016, 11, e0158816. [Google Scholar] [CrossRef]
- Bharti, P.K.; Chand, S.K.; Singh, M.P.; Mishra, S.; Shukla, M.M.; Singh, R. Emergence of a New Focus of Plasmodium malariae in Forest Villages of District Balaghat, Central India: Implications for the Diagnosis of Malaria and Its Control. Trop. Med. Int. Health 2013, 18, 12–17. [Google Scholar] [CrossRef]
- Das, A.; Anvikar, A.R.; Cator, L.J.; Dhiman, R.C.; Eapen, A.; Mishra, N.; Nagpal, B.N. Malaria in India: The Center for the Study of Complex Malaria in India. Acta Trop. 2013, 121, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, S.; Campillo, A.; Gonzalez, I.J. A Systematic Review: Performance of Rapid Diagnostic Tests for the Detection of Plasmodium knowlesi, Plasmodium malariae, and Plasmodium ovale Monoinfections in Human Blood. J. Infect. Dis. 2018, 218, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Severity and Mortality of Severe Plasmodium ovale Infection: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235014. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.L.; Lim, H.T.; Chin, P.W.; Lim, S.Y.; Hoo, F.K. A Case of Severe Plasmodium knowlesi in a Splenectomized Patient. Parasitol. Int. 2016, 65, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Langford, S.; Douglas, N.M.; Lampah, D.A.; Simpson, J.A.; Kenangalem, E.; Sugiarto, P.; Anstey, N.M. Plasmodium malariae Infection Associated with a High Burden of Anemia: A Hospital-Based Surveillance Study. PLoS Negl. Trop. Dis. 2015, 9, e0004195. [Google Scholar] [CrossRef]
- Poti, K.E.; Sullivan, D.J.; Dondorp, A.M.; Woodrow, C.J. HRP2: Transforming Malaria Diagnosis, but with Caveats. Trends Parasitol. 2020, 36, 112–126. [Google Scholar] [CrossRef]
- WHO. Protocol for Estimating the Prevalence of Pfhrp2/Pfhrp3 Gene Deletions among Symptomatic Falciparum Patients with False-Negative RDT Results; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kumar, N.; Pande, V.; Bhatt, R.M.; Shah, N.K.; Mishra, N.; Srivastava, B.; Valecha, N.; Anvikar, A.R. Genetic Deletion of HRP2 and HRP3 in Indian Plasmodium falciparum Population and False Negative Malaria Rapid Diagnostic Test. Acta Trop. 2013, 125, 119–121. [Google Scholar] [CrossRef]
- Bharti, P.K.; Chandel, H.S.; Ahmad, A.; Krishna, S.; Udhayakumar, V.; Singh, N. Prevalence of Pfhrp2 and/or Pfhrp3 Gene Deletion in Plasmodium falciparum Population in Eight Highly Endemic States in India. PLoS ONE 2016, 11, e0157949. [Google Scholar] [CrossRef] [PubMed]
- Pati, P.; Dhangadamajhi, G.; Bal, M.; Ranjit, M. High Proportions of Pfhrp2 Gene Deletion and Performance of HRP2-Based Rapid Diagnostic Test in Plasmodium falciparum Field Isolates of Odisha. Malar. J. 2018, 17, 394. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kojom, L.P. Deletions in the Plasmodium falciparum Histidine-Rich Protein 2 Gene: An Emerging Threat to the Elimination of Malaria in India. J. Vector Borne Dis. 2019, 56, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Kori, L.D.; Valecha, N.; Anvikar, A.R. Glutamate Dehydrogenase: A Novel Candidate to Diagnose Plasmodium falciparum through Rapid Diagnostic Test in Blood Specimen from Fever Patients. Sci. Rep. 2020, 10, 6307. [Google Scholar] [CrossRef] [PubMed]

| Types of Information | Nature of the Information Retrieved |
|---|---|
| Information on the Publication | Name of the first author or of the two authors Year of publication |
| Information on study design, population, area | Study design Indian areas and Level of endemicity of area Year of data collection Population (adult, children and pregnant women) Clinical status of patients (febrile, asymptomatic) Type of malaria |
| Diagnostic | Diagnostic methods used (LM, RDT, molecular methods) Staining molecule used in LM (Giemsa, Leishman, Jaswant Singh–Bhattacharji) Type, antigen, brand and manufacturer’s country of the RDTs Number of RDTs evaluated |
| Performance parameters | Total number of individuals tested with different methods Total number of patients positive with gold standard and RDT Total number of P. vivax infections Number of mixed infections Number of false negative (FN), false positive (FP), true negative (TN) and true positive (TP) for diagnosis of P. falciparum, P. vivax and non-falciparum species. |
| Brand | n | Manufacturer | Antigen Targeted | Categorisation as per WHO | PDS (%) at 200 Parasites/µL (Rounds 5–8) for Pfa and Pvb | FP and Invalid Result Rates | WHO Performance Criteria |
|---|---|---|---|---|---|---|---|
| ParaCheck Pf® | 12 | Orchid, Biomedical Systems, Goa, India | HRP2 | Pf only | 94.0a and NAb | NA and 0% |  |
| ParaSight F® | 10 | Becton Dickonson, Cockeys ville, MD, USA | HRP2 | Pf only | - | - | - |
| ICT Malaria Pf™ | 10 | ICT Diagnostics, Brooksvale, NSW, USA | HRP2 | Pf only | - | - | - |
| ParaHIT®-f | 9 | ARKRAY Healthcare Pvt. Ltd., Surat, India | HRP2 | Pf only | 77.0a and NAb | NA |  |
| DiaMed OptiMAL IT® | 6 | DiaMed AG, Cressier, Switzerland | PfLDH + Pan-LDH | Pf/Pan | - | - | - |
| ICT Malaria Pf/Pv™ | 4 | ICT Diagnostics, Brooksvale, NSW, USA | Panmalarial Ag + HRP2 | Pf and Pv | 94.0a and NAb | NA |  |
| ParaHIT® Total | 4 | ARKRAY Healthcare Pvt. Ltd., Mumbai, India | Aldolase + Pan-LDH + HRP2 | Pf/Pan | - | - | - |
| SD Bioline Pf/Pan® | 4 | SD Standard Diagnostics, Inc., South Korea | PfLDH + Pan-LDH | Pf/Pan | 94.0a and 91.4b | - |  |
| Determine™ Malaria Pf | 3 | Abbot Laboratories, Tokyo, Japan | HRP2 | Pf only | - | - | - |
| Parascreen Device Pan/Pf® | 3 | Zephyr Biomedical, Verna, Goa, India | Pan-LDH + HRP2 | Pf/Pan | 91.0a and 91.4b | 0% and 0% |  |
| FalciVax™ (Pf/Pv) | 3 | Zephyr Biomedical, Verna, Goa, India | PvLDH + HRP2 | Pf and Pv/Pvom | 95.0a and 100b | 0.5% and 0% |  |
| First Response Combo Malaria Ag® | 3 | Premier medical corporation Ltd., India | Pan-LDH + HRP2 | Pf/Pan | 91.0-95.0a and NAb | 0% and 0% |  |
| SD Bioline Pf/Pv® | 3 | SD Standard Diagnostics, Inc., South Korea | PfLDH + PvLDH | Pf and Pv | 99.0a and 97.1b | 0% and 0% |  |
| Advantage Mal Card™ | 2 | J. Mitra & Co. Pvt. Ltd., Rajasthan, India | PfLDH + Pan-LDH | Pf/Pan | 30.0a and 94.3b | 0.4% and 0% |  |
| Alere™ Trueline Malaria Ag Pf/Pan | 1 | Alere Medical Pvt. Ltd., India | Pan-LDH + HRP2 | Pf/Pan | 85.0a and 91.4b | 0% and 0% |  |
| DiaMed OptiMAL® | 2 | Flow Inc., Portland, OR, USA | Pan-LDH | Pan only | - | - | - |
| SD Bioline Pf® | 2 | SD Standard Diagnostics, Inc., South Korea | HRP2 | Pf only | 94.0a and NAb | NA and 0% |  |
| Malaria Pf (HRPII)/PV (PLDH) Antigen Detection Test Device™ | 1 | GENOMIX Molecular Diagnostics Pvt. Ltd., Hyderabad, Andhra Pradesh, India | PvLDH + HRP2 | Pf and Pv/Pvom | 85.0a and 74.3b | NA |  |
| Malarigen™ Pf/Pv test | 1 | Aspen Laboratories, India | Aldolase + Pan-LDH | NA | - | - | - |
| Malarigen™ Pf/Pv test | 1 | Aspen Laboratories, India | Pan-LDH + HRP2 | NA | - | - | - |
| Malarigen™ Pan test | 1 | Aspen Laboratories, India | Pan-LDH | NA | - | - | - |
| Malascan Device Pf/Pan® | 1 | TulipScan Diagnostics, India | Aldolase + HRP2 | Pf/Pan | - | - | - |
| NecVIPARUM™ One step Pf/Pv | 1 | Nectar Life Science Ltd., Chandigarh, India | PvLDH + HRP2 | Pf and Pv/Pvom | 88.0a and 91.4b | 0% and 4.5% |  |
| New™ Pf-1 mini | 1 | Monozyme India Ltd., Secundradad, India | HRP2 | Pf only | - | - | - |
| DiaMed OptiMAL® 48 | 1 | DiaMed AG, Cressier, Switzerland | Pan-LDH | Pan only | - | - | - |
| Standard Q™ Pf/Pv | 1 | SD Biosensor healthcare Pvt. Ltd., India | PvLDH + HRP2 | Pf and Pv/Pvom | 85.0a and 100b | 0% and 0.5 % |  |
| Not specified | 11 | - | - | - | - | - | - |
| Total | 101§ |
| RDT | Authors’ Findings | References as Seen in Table S4 | |
|---|---|---|---|
| Gametocyte detection versus RDT positivity | ICT Malaria Pf™ | Presence or absence of gametocytes was not related to RDT positivity | Valecha et al._1998 |
| ICT Malaria Pf™ | Presence or absence of gametocytes was not related to RDT positivity | Ghosh et al._2000 | |
| ParaCheck Pf® | No correlation between presence of falciparum gametocytes and RDT positivity | Ghosh et al._2002 | |
| ParaCheck Pf® | Three individuals infected only with falciparum gametocytes were all RDT positive | Singh et al._2002 | |
| ParaCheck Pf® | Two individuals infected only with falciparum gametocytes were all RDT negative | Arora et al._2003a | |
| ParaSight F® | RDT failed to detect one individual with falciparum gametocytes only | Arora et al._2003b | |
| DiaMed OptiMAL® | Two individuals infected only with falciparum gametocytes were all positive with the RDT | Singh et al._2003 | |
| ParaCheck Pf® | P. falciparum gametocytes not detected or weakly detected by the RDT in all carriers | Gokhale et al._2004 | |
| ParaHit®-f | 14 individuals infected with falciparum gametocytes were RDT positive | Singh et al._2005a | |
| ParaCheck Pf® | Three only falciparum gametocytes -infected individuals were all RDT positive | Bhat Sandhya et al._2012 | |
| Advantage mal Card™ | On day 8, falciparum gametocytes were detected by the RDT in all 9 patients | Kocharekar et al._2014 | |
| Parasitemia versus Intensity band of RDT | ICT Malaria Pf™ | No correlation found | Valecha et al._1998 |
| ICT Malaria Pf™ | No correlation found | Ghosh et al._2000 | |
| ParaCheck Pf® | Positive correlation for development stage (Ring only, and Ring + gametocyte) | Ghosh et al._2002 |
| WHO Performance Indicators& | ||||||
|---|---|---|---|---|---|---|
| RDTs | Antigen Targeted | PDS (%) | Positivity Rate (%) | FP Rate (%) | Invalid Rate (%) | Overall Evaluation |
| SD Bioline Pf/Pan | PfLDH + Pan-LDH | 32.5 | 68.1 | NA | 0.0 |  |
| ParaCheck® F Pf | HRP2 | 15.0 | 40.5 | NA | 0.0 |  |
| Malarigen Pf/Pv | HRP2 + Pan-LDH | 32.5 | 43.1 | 0.0 | 0.0 |  |
| FalciVax (Pf/Pv) | HRP2 + Pv-LDH | 0.0 | 3.1 | 0.0 | 0.0 |  |
| First Response® Combo Malaria Ag | HRP2 + Pan-LDH | 12.5 | 23.1 | 0.0 | 0.0 |  |
| NecVIPARUM One step Pf/Pv | HRP2 + Pv-LDH | 32.5 | 45.9 | 0.6 | 0.6 |  |
| Parascreen Device Pan/Pf | HRP2 + Pan-LDH | 0.0 | 0.6 | 50.6 | 0.0 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojom Foko, L.P.; Pande, V.; Singh, V. Field Performances of Rapid Diagnostic Tests Detecting Human Plasmodium Species: A Systematic Review and Meta-Analysis in India, 1990–2020. Diagnostics 2021, 11, 590. https://doi.org/10.3390/diagnostics11040590
Kojom Foko LP, Pande V, Singh V. Field Performances of Rapid Diagnostic Tests Detecting Human Plasmodium Species: A Systematic Review and Meta-Analysis in India, 1990–2020. Diagnostics. 2021; 11(4):590. https://doi.org/10.3390/diagnostics11040590
Chicago/Turabian StyleKojom Foko, Loick Pradel, Veena Pande, and Vineeta Singh. 2021. "Field Performances of Rapid Diagnostic Tests Detecting Human Plasmodium Species: A Systematic Review and Meta-Analysis in India, 1990–2020" Diagnostics 11, no. 4: 590. https://doi.org/10.3390/diagnostics11040590
APA StyleKojom Foko, L. P., Pande, V., & Singh, V. (2021). Field Performances of Rapid Diagnostic Tests Detecting Human Plasmodium Species: A Systematic Review and Meta-Analysis in India, 1990–2020. Diagnostics, 11(4), 590. https://doi.org/10.3390/diagnostics11040590








