Abstract
This study aimed to investigate the performance of innovative and traditional cardiometabolic indices, including body mass index (BMI), waist circumference (WC), Chinese visceral adiposity index (CVAI), visceral adiposity index, lipid accumulation product, a body shape index (ABSI), body roundness index, conicity index (CI), triglyceride-glucose (TyG) index, TyG-BMI, and TyG-WC, in estimating atherosclerotic cardiovascular disease (ASCVD) risk in 3143 Taiwanese adults aged 20–79 years. Elevated 10-year ASCVD risk was defined as ≥7.5% using the Pooled Cohort Equations. The performance of different indices in estimating elevated ASCVD risk was assessed by receiver operating characteristic (ROC) curves. In multivariate-adjusted logistic regression analyses, all cardiometabolic indices (p-value < 0.001) were significantly associated with elevated ASCVD risk in both genders, except for ABSI and CI in women. In particular, CVAI had the largest area under the curve (AUC) in men (0.721) and women (0.883) in the ROC analyses. BMI had the lowest AUC in men (0.617), while ABSI had the lowest AUC in women (0.613). The optimal cut-off value for CVAI was 83.7 in men and 70.8 in women. CVAI performed best among various cardiometabolic indices in estimating elevated ASCVD risk. CVAI may be a reliable index for identifying people at increased risk of ASCVD.
1. Introduction
Although therapeutic techniques and prevention strategies advance in recent decades, atherosclerotic cardiovascular disease (ASCVD) remains the major leading cause of death worldwide [1,2]. The increasing prevalence of diabetes, hypertension, dyslipidemia, overweight, and sedentary lifestyle as the global economy grows is believed to be the key elements contributed to ASCVD [3,4]. Among these factors, central obesity increases the cardiovascular risk through insulin resistance, secretion of adipokines, and pro-inflammatory proteins, leading to atherogenic endothelial dysfunction [5,6,7]. Central obesity and fat mass are modifiable factors and potentially treatment targets for reducing the burden of ASCVD [8].
Body mass index (BMI) has been widely used as a surrogate for overweight and obesity and was associated with lifetime cardiovascular disease across diverse populations [9,10]. Since BMI has its limitation of describing the distribution of the body fat [11], waist circumference (WC) can reflect visceral and central obesity better than BMI [12]. The pattern of fat distribution was recently recognized a stronger predictor than traditional anthropometric parameters for ASCVD [13]. Various novel anthropometric and cardiometabolic indices have been increasingly developed in recent years to better describe visceral obesity and body composition.
Chinese visceral adiposity index (CVAI) is a novel surrogate for assessment of metabolic health and prediction of diabetes [14,15]. Visceral adiposity index (VAI) is a marker of adipose function and distribution, and independently associated with the 10-year ASCVD incidence [16,17]. Lipid accumulation product (LAP) is associated with insulin resistance and cardiovascular disease, reflecting the central fat accumulation [18,19]. A body shape index (ABSI) and body roundness index (BRI) as well as conicity index (CI) were developed for better discriminative capacity of abdominal adipose tissue and body fat percentage, and were practical cardiometabolic indicators in previous studies [20,21,22]. Triglyceride-glucose (TyG) index, TyG-BMI, and TyG-WC are emerging indicators for insulin resistance, diabetes, and ischemic stroke [23,24,25,26]. Taken together, these traditional and novel cardiometabolic indices have their important roles linking with cardiovascular disease and risk. However, the comparison of performance of these indices in estimating elevated 10-year ASCVD risk remains uncertain. Therefore, this study aimed to investigate the ability in estimation of elevated ASCVD risk and the optimal cut-off value among the cardiometabolic indices in adults.
2. Materials and Methods
2.1. Study Design and Participants
This study enrolled 3171 individuals undergoing health examination from January 2014 to April 2019 at a regional hospital in Taiwan. Thirteen participants aged <20 or >79 years were excluded from the study because ASCVD risk estimation is applicable for individuals with the age between 20 and 79 years [27,28]. In addition, fifteen participants with missing anthropometry or blood biochemical measurements were also excluded. Finally, a total number of 3143 adults were included in this study (Figure 1). Written informed consent has been received from each of the individuals. The study protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital. The methods were carried out in accordance with the approved guidelines.
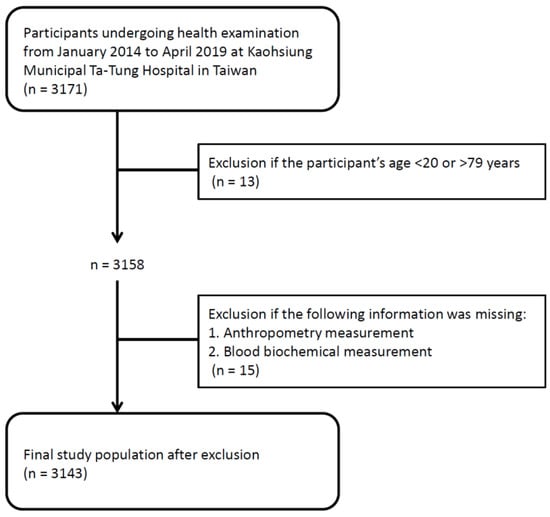
Figure 1.
Study population and flowchart.
2.2. Demographic Information and Biochemical Data
Demographic information, including age, gender, smoking habits, and comorbid conditions, was obtained by the interview of study participants with the physicians. Diabetes mellitus, hypertension, and dyslipidemia were defined by self-reported history and medical history of taking anti-diabetic, anti-hypertensive, and lipid-lowering drugs from the study participants. Overnight fasting blood samples were obtained from each participant for biochemical measurements, including fasting glucose, total cholesterol, triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), uric acid, high sensitivity C-reactive protein (hs-CRP), and serum creatinine. Study participants’ kidney function was assessed by the estimated glomerular filtration rate (eGFR) using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [29]. Proteinuria of ≥1+ detected by a dipstick urinalysis was defined as a positive result.
2.3. Anthropometry Measurement, Anthropometric and Cardiometabolic Indices
Blood pressure was obtained using standard sphygmomanometers in sitting positions after taking rest at least 3 min. Body height in meter and weight in kilogram were measured according to standard methods. BMI was defined as body weight divided by the square of height. The WC was measured at the meddle point between the bottom of the rib cage and the uppermost border of the iliac crests at the end of exhalation in standing positions using an inelastic tape.
The anthropometric and cardiometabolic indices used in this study were listed as follows:
CVAI was calculated as [14]:
CVAI in men: −267.93 + 0.68 × age + 0.03 × BMI + 4.00 × WC + 22.00 × Log10TG − 16.32 × HDL-C
CVAI in women: −187.32 + 1.71 × age + 4.23 × BMI + 1.12 × WC + 39.76 × Log10TG − 11.66 × HDL-C
VAI was calculated using the following formula [16]:
VAI in men = (WC (cm)/39.68 + 1.88 × BMI (kg/m2)) × (TG (mmol/L)/1.03) × (1.31/HDL-C (mmol/L)); and (WC (cm)/36.58 + 1.89 × BMI (kg/m2)) × (TG (mmol/L)/0.81) × (1.52/HDL-C (mmol/L)) in women.
LAP was calculated as (WC (cm) − 65) × (TG (mmol/L)) in men, and (WC (cm) − 58) × (TG (mmol/L)) in women [30].
ABSI was calculated as WC/(BMI2/3 × height1/2) [31]
BRI was calculated as 364.2 − 365.5 [1 − π−2 WC2(m) Height−2 (m)]1/2 [32].
CI was calculated as 0.109−1 WC (m) [Weight (kg)/Height (m)]−1/2 [33].
TyG index was calculated as Ln (TG (mg/dL) × fasting glucose (mg/dL)/2) [34].
TyG-BMI was calculated as TyG index × BMI, while TyG-WC was calculated as TyG index × WC [35].
2.4. Definition of 10-Year ASCVD Risk
Each participant’s 10-year ASCVD risk was assessed using the Pool Cohort Equations, which include age, gender, race, total cholesterol, HDL-C, systolic blood pressure, diabetes mellitus, and current smoking status as variables [36]. A 10-year risk ≥7.5% was defined as an elevated risk for ASCVD, defined as nonfatal myocardial infarction or coronary heart disease death, nonfatal, or fatal stroke [37].
2.5. Statistical Analyses
All statistical analyses were performed using SPSS v.20.0 (SPSS Inc., Chicago, IL, USA) for Windows. Data are expressed as percentages for categorical variables, mean ± standard deviation for continuous variables with approximately normal distribution, or median (25th–75th percentile) for continuous variables with skewed distribution, such as TG and hs-CRP levels. The study participants were stratified into 10-year ASCVD risk <7.5% and ≥7.5%. We analyzed the differences between these two groups by using the chi-square test for categorical variables and the independent t-test for continuous variables. The univariate and multivariate-adjusted logistic regression analyses were performed to investigate the associations among the anthropometric and cardiometabolic indices with elevated 10-year ASCVD risk. Receiver operating characteristic (ROC) curves analyses were carried out to assess the performance of different anthropometric and cardiometabolic indices in estimating elevated ASCVD risk. Comparison of area under curves (AUCs) was performed using the DeLong method. The optimal cut-off values of these anthropometric and cardiometabolic indices were determined by the highest Youden index value (sensitivity + specificity − 1). A p-value < 0.05 was considered statistically significant.
3. Results
A total number of 3143 adults (1348 men and 1795 women, mean age 48.4 ± 9.2 years) were included in this study. There were 1052 (33.5%) adults had 10-year ASCVD risk ≥7.5%. The characteristics of study participants are showed in Table 1. Men with an ASCVD risk ≥7.5% were more likely to be older, have a higher prevalence of diabetes, hypertension, current smoker and proteinuria, higher blood pressure levels, BMI, WC, CVAI, VAI, LAP, BRI, CI, TyG index, TyG-BMI, and TyG-WC, higher levels of fasting glucose, total cholesterol, LDL-C, TG, hs-CRP, and lower levels of HDL-C and eGFR compared to men with an ASCVD risk <7.5%. The comparisons of characteristics between women with an ASCVD risk ≥7.5% and <7.5% were similar in men, except for the lack of significant differences in the prevalence of current smoker and proteinuria, and higher level of uric acid in women with an ASCVD risk ≥7.5%.

Table 1.
Demographic information of study participants stratified by 10-year atherosclerotic cardiovascular disease (ASCVD) risk <7.5% and ≥7.5%.
Table 2 displays the associations among cardiometabolic indices with elevated 10-year ASCVD risk. In the unadjusted logistic regression, all 11 cardiometabolic indices (all p-value < 0.001) were significantly associated with elevated 10-year ASCVD risk in both genders. In multivariate analyses adjusted for age, diabetes, hypertension, dyslipidemia, current smoking, LDL-C, uric acid, hs-CRP, eGFR and proteinuria, all 11 cardiometabolic indices (all p-value < 0.001) remained significantly associated with elevated 10-year ASCVD risk in men and women, except for ABSI (p-value = 0.924) and CI (p-value = 0.061) in women.

Table 2.
Unadjusted and multivariate-adjusted odds ratios for 10-year ASCVD risk ≥7.5% among cardiometabolic indices.
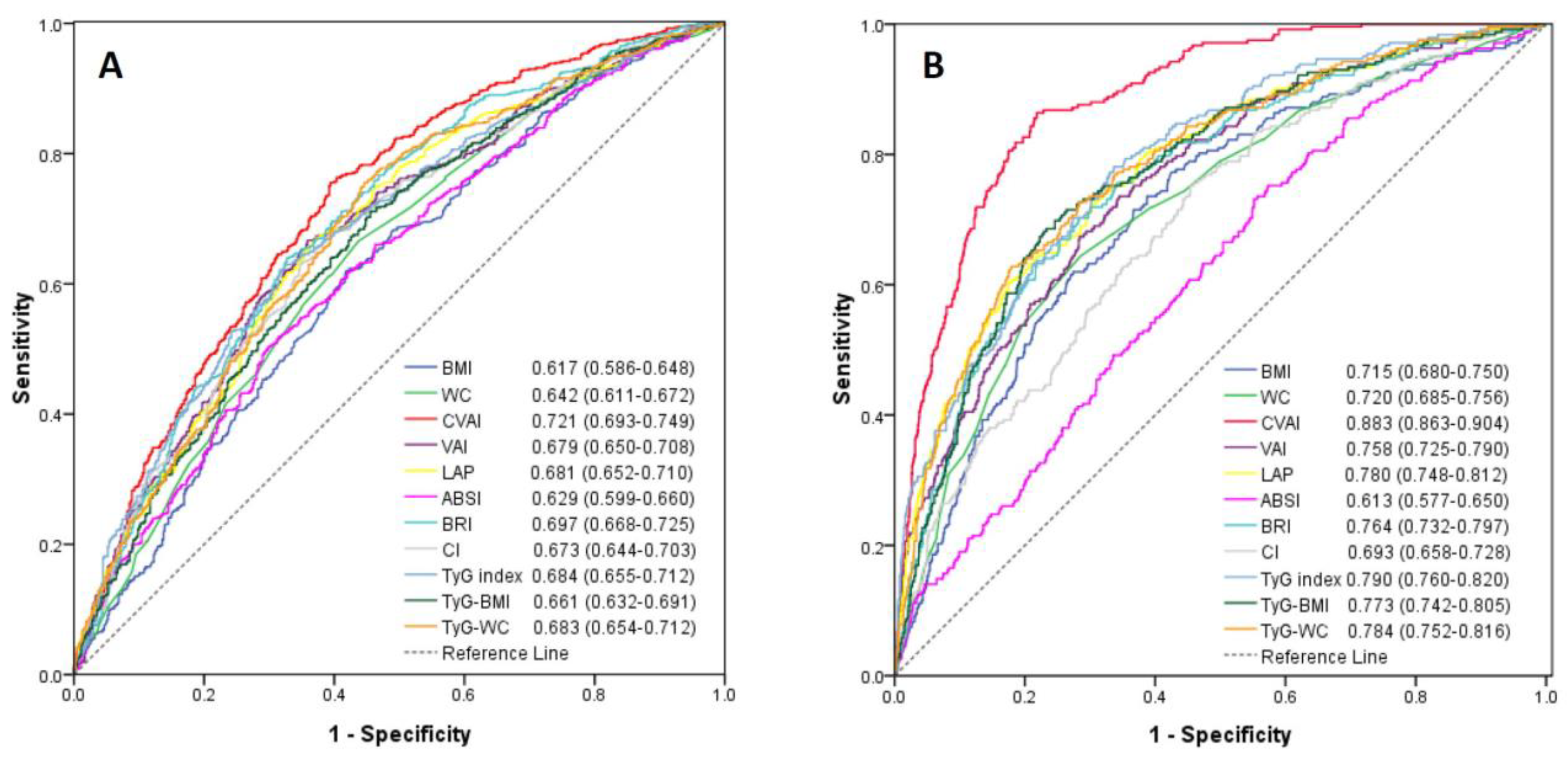
Among the 11 cardiometabolic indices, CVAI had the highest AUC value in estimating an elevated 10-year ASCVD risk in men (AUC = 0.721, 95% confidence interval, 0.693–0.749) and in women (AUC = 0.883; 95% confidence interval, 0.863–0.904). The lowest AUC among these cardiometabolic indices was BMI (AUC = 0.617; 95% confidence interval, 0.586–0.648) in men and ABSI (AUC = 0.613; 95% confidence interval, 0.577–0.650) in women (Figure 2). The predictive performance of CVAI for 10-year ASCVD risk ≥7.5% was significantly better than the other 10 cardiometabolic indices (all p-value < 0.05). Since the menopause may affect the occurrence of ASCVD, the performance of these cardiometabolic indices were compared in women with the age ≤50 and >50 years, respectively. CVAI also had the highest AUC value in women ≤50 and >50 years of age (Figure S1).
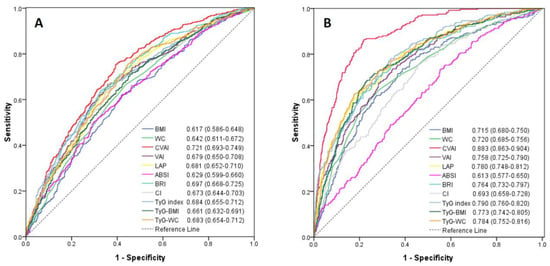
Figure 2.
Receiver-operating characteristic curves of anthropometric and cardiometabolic indices for estimating 10-year ASCVD risk ≥7.5% in men (A) and women (B). There was significant difference of the area under curve for each anthropometric and cardiometabolic index (all p-value < 0.05) when compared with CVAI using the DeLong method.
The sensitivity, specificity, Youden index and corresponding optimal cut-off values of these cardiometabolic indices to predict an elevated ASCVD risk are demonstrated in Table 3. The optimal cut-off value of CVAI was 83.7 for men and 70.8 for women.

Table 3.
Sensitivity, specificity, and Youden index using cut-off values for cardiometabolic indices to predict 10-year ASCVD risk ≥7.5%.
4. Discussion
Our study investigated and compared the performance of estimating elevated ASCVD risk among traditional and novel cardiometabolic indices as well as their optimal cut-off values in adults. We found that these 11 cardiometabolic indices were significantly associated with elevated ASCVD risk in both genders after adjusted for potential confounders, except for ABSI and CI in women. CVAI had the best performance in predicting elevated ASCVD risk in both men and women among traditional and novel cardiometabolic indices.
Obesity is a major challenge of public health and a common nutritional disorder worldwide. In particular, obesity has been well recognized a key factor for the development of ASCVD [38]. Emerging evidence supports that the primary culprit behind the obesity in association with cardiovascular and metabolic burden might be the pattern of fat distribution [39,40]. Although computed tomography (CT) and magnetic resonance imaging (MRI) are able to measure regional and whole body fat accurately, the lack of economic feasibility and the risk of exposure to radiation during CT scanning remain the problems for large-scale epidemiological studies. Thus, various anthropometric and cardiometabolic indices have been widely applied. In our study, the performance of estimating elevated ASCVD risk for BMI and WC were unsatisfactory compared to certain novel indices, such as CVAI, VAI, LAP, TyG index, TyG-BMI, and TyG-WC. Furthermore, gender difference was not observed with regard to the weakness for BMI and WC in prediction of 10-year ASCVD risk ≥7.5%. It indicates that BMI and WC might not be the reliable surrogate markers for identifying people with elevated ASCVD risk. Traditional anthropometric markers, BMI and WC, might marginally reflect the distribution of body fat and visceral fat mass [41].
Accumulating evidence supports that innovative indices outperform conventional anthropometric markers in relation to the incidence of ASCVD and type 2 diabetes. A high VAI is associated with elevated risk of coronary heart disease in both male and female Chinese adults [42]. VAI is independently associated with cardiovascular events among Greek adults in a 10-year follow-up study [17]. Furthermore, CVAI and VAI have better predictive ability than BMI and WC for development of diabetes in Chinese and Caucasian adults, respectively [43,44,45]. In the present study, we found that CVAI exhibited the best performance in relation to 10-year ASCVD risk ≥7.5% in both genders among anthropometric and cardiometabolic indices. Xia et al. and Wu et al. reported that CVAI is superior to VAI, BMI and WC in correlation with visceral fat area as well as the prediction of diabetes [14,15]. While visceral fat tissue has been recognized as a potential biomarker to replace the role of BMI in ASCVD risk stratification [46], excessive accumulation of visceral adiposity is supposed to have more harmful and atherogenic effects than obesity itself on the vessels [13]. Although the prospective study for investigating the link between CVAI and incidence of ASCVD has not yet published, our findings may offer a reasonable niche to advanced studies in the future.
Apart from CVAI, some novel cardiometabolic indices including LAP, TyG index, TyG-BMI, and TyG-WC, demonstrate modest performance in predicting elevated 10-year ASCVD risk. Kyrou et al. showed that LAP is a better predictor of 10-year cardiovascular events than BMI and WC [19]. Moreover, TyG index could predict the development of cardiovascular events in Caucasian and Chinese populations [47,48]. Our findings were in line with these studies, suggesting these innovative cardiometabolic indices have their pivotal roles in association with ASCVD risk. Not all the novel anthropometric markers, however, performed well in estimating elevated ASCVD risk. In Asian populations, ABSI failed to predict metabolic syndrome [49,50]. For identifying cardiovascular disease, ABSI seemed not to be a suitable predictor [20]. This might be because ABSI was generated from the US National Health and Nutrition Examination Survey 1999−2004, with three largest ethnic groups (whites, blacks, and Mexicans) included [31]. In our study, ABSI demonstrated the worst performance among the cardiometabolic indices. Taken together, ABSI may have certain weakness in relation to cardiometabolic risk in Asian adults.
The limitations of this study included an observational and cross-sectional study design. Therefore, we can only investigate the associations of various anthropometric and cardiometabolic indices with elevated ASCVD risk, but we cannot elucidate the causal relationship. Further prospective studies are needed to investigate the link between CVAI and the incidence of ASCVD. The hip circumference of each study participant was not measured in this study; therefore, the performance of estimating ASCVD risk using waist-to-hip or waist-to-height ratio cannot be compared with the other anthropometric and cardiometabolic indices. The information of the age at menopause, surgery of bilateral oophorectomy, and hormone replacement therapy was not obtained for female participants, as these factors may affect the occurrence of ASCVD. Moreover, the 10-year ASCVD risk was determined from the Pool Cohort Equations, which might not be perfectly applicable to Asian populations.
5. Conclusions
In summary, this study indicated that all the cardiometabolic indices were significantly associated with increased ASCVD risk in both sexes, except for ABSI and CI in women. Among the traditional and innovative cardiometabolic markers, CVAI had the best performance for estimating elevated ASCVD risk in adults. For screening and identifying people at increased risk of ASCVD, CVAI might be the relatively relevant and reliable index.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/article/10.3390/diagnostics11040603/s1. Figure S1: Receiver-operating characteristic curves of anthropometric and cardiometabolic indices for estimating 10-year ASCVD risk ≥7.5% in women with the age ≤50 years (A) and the age >50 years (B).
Author Contributions
Conceptualization, Y.-C.H. and J.-C.H.; methodology, Y.-C.H., J.-C.H., F.-W.L., and C.-Y.D.; software, C.-I.L., H.-H.C., and Y.-Y.L.; validation, Y.-C.H., J.-C.H., F.-W.L., and C.-Y.D.; formal analysis, Y.-C.H., J.-C.H., F.-W.L., and C.-Y.D.; investigation, F.-W.L. and C.-Y.D.; data curation, Y.-C.H.; writing—original draft preparation, Y.-C.H.; writing—review and editing, J.-C.H., C.-L.W., F.-W.L., H.-Y.C., and C.-Y.D.; visualization, Y.-C.H. and J.-C.H.; supervision, F.-W.L., and C.-Y.D.; funding acquisition, Y.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, grant number: kmtth-109-014.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(II)-20200025) on 30 March 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Greenland, P.; Knoll, M.D.; Stamler, J.; Neaton, J.D.; Dyer, A.R.; Garside, D.B.; Wilson, P.W. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003, 290, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Canto, J.G.; Kiefe, C.I.; Rogers, W.J.; Peterson, E.D.; Frederick, P.D.; French, W.J.; Gibson, C.M.; Pollack, C.V., Jr.; Ornato, J.P.; Zalenski, R.J.; et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA 2011, 306, 2120–2127. [Google Scholar] [CrossRef]
- Reho, J.J.; Rahmouni, K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin. Sci. 2017, 131, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Van de Voorde, J.; Pauwels, B.; Boydens, C.; Decaluwe, K. Adipocytokines in relation to cardiovascular disease. Metabolism 2013, 62, 1513–1521. [Google Scholar] [CrossRef]
- Schinzari, F.; Tesauro, M.; Cardillo, C. Endothelial and Perivascular Adipose Tissue Abnormalities in Obesity-Related Vascular Dysfunction: Novel Targets for Treatment. J. Cardiovasc. Pharmacol. 2017, 69, 360–368. [Google Scholar] [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.L.; Peters, S.A.E.; Huxley, R.R.; Woodward, M. The sex-specific association between BMI and coronary heart disease: A systematic review and meta-analysis of 95 cohorts with 1·2 million participants. Lancet Diabetes Endocrinol. 2015, 3, 437–449. [Google Scholar] [CrossRef]
- Goossens, G.H. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Nazare, J.A.; Smith, J.; Borel, A.L.; Aschner, P.; Barter, P.; Van Gaal, L.; Tan, C.E.; Wittchen, H.U.; Matsuzawa, Y.; Kadowaki, T.; et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am. J. Cardiol. 2015, 115, 307–315. [Google Scholar] [CrossRef]
- Blüher, M.; Laufs, U. New concepts for body shape-related cardiovascular risk: Role of fat distribution and adipose tissue function. Eur. Heart J. 2019, 40, 2856–2858. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, Y.; Lin, H.D.; Ma, H.; Li, X.M.; Aleteng, Q.; Li, Q.; Wang, D.; Hu, Y.; Pan, B.S.; et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci. Rep. 2016, 6, 38214. [Google Scholar] [CrossRef]
- Wu, J.; Gong, L.; Li, Q.; Hu, J.; Zhang, S.; Wang, Y.; Zhou, H.; Yang, S.; Wang, Z. A Novel Visceral Adiposity Index for Prediction of Type 2 Diabetes and Pre-diabetes in Chinese adults: A 5-year prospective study. Sci. Rep. 2017, 7, 13784. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Kouli, G.M.; Panagiotakos, D.B.; Kyrou, I.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J. Diabetes Complicat. 2018, 32, 266–270. [Google Scholar] [CrossRef]
- Kyrou, I.; Panagiotakos, D.B.; Kouli, G.M.; Georgousopoulou, E.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Lipid accumulation product in relation to 10-year cardiovascular disease incidence in Caucasian adults: The ATTICA study. Atherosclerosis 2018, 279, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Maessen, M.F.; Eijsvogels, T.M.; Verheggen, R.J.; Hopman, M.T.; Verbeek, A.L.; de Vegt, F. Entering a new era of body indices: The feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS ONE 2014, 9, e107212. [Google Scholar] [CrossRef]
- Bertoli, S.; Leone, A.; Krakauer, N.Y.; Bedogni, G.; Vanzulli, A.; Redaelli, V.I.; De Amicis, R.; Vignati, L.; Krakauer, J.C.; Battezzati, A. Association of Body Shape Index (ABSI) with cardio-metabolic risk factors: A cross-sectional study of 6081 Caucasian adults. PLoS ONE 2017, 12, e0185013. [Google Scholar] [CrossRef]
- Andrade, M.D.; Freitas, M.C.; Sakumoto, A.M.; Pappiani, C.; Andrade, S.C.; Vieira, V.L.; Damasceno, N.R. Association of the conicity index with diabetes and hypertension in Brazilian women. Arch. Endocrinol. Metab. 2016, 60, 436–442. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.; Koo, S.H.; Kwon, G.C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2019, 14, e0212963. [Google Scholar] [CrossRef] [PubMed]
- Ramdas Nayak, V.K.; Nayak, K.R.; Vidyasagar, S.; Rekha, P. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab. Syndr. 2020, 14, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shi, S.; Ren, X.; Han, T.; Li, Y.; Chen, Y.; Liu, W.; Hou, P.C.; Hu, Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 2016, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xing, L.; Jing, L.; Tian, Y.; Yan, H.; Sun, Q.; Dai, D.; Shi, L.; Liu, S. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 245–253. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef]
- Valdez, R. A simple model-based index of abdominal adiposity. J. Clin. Epidemiol. 1991, 44, 955–956. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Er, L.K.; Wu, S.; Chou, H.H.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE 2016, 11, e0149731. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 921–925. [Google Scholar] [CrossRef]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.O.; Li, H.; Yang, G.; Xiang, Y.B.; Cai, Q.; Ji, B.T.; Gao, Y.T.; Zheng, W. Visceral adiposity and risk of coronary heart disease in relatively lean Chinese adults. Int. J. Cardiol. 2013, 168, 2141–2145. [Google Scholar] [CrossRef]
- Wei, J.; Liu, X.; Xue, H.; Wang, Y.; Shi, Z. Comparisons of Visceral Adiposity Index, Body Shape Index, Body Mass Index and Waist Circumference and Their Associations with Diabetes Mellitus in Adults. Nutrients 2019, 11, 1580. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Kyrou, I.; Stefanadis, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Skoumas, I.; Tousoulis, D.; Pitsavos, C.; The ATTICA Study Group. Visceral adiposity index outperforms common anthropometric indices in predicting 10-year diabetes risk: Results from the ATTICA study. Diabetes Metab. Res. Rev. 2019, 35, e3161. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Qin, P.; Li, Q.; Qie, R.; Liu, L.; Zhao, Y.; Liu, D.; Zhang, D.; Guo, C.; Zhou, Q.; et al. Chinese visceral adiposity index: A reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab. Res. Rev. 2021, 37, e3370. [Google Scholar] [CrossRef]
- Lee, J.J.; Pedley, A.; Hoffmann, U.; Massaro, J.M.; Fox, C.S. Association of Changes in Abdominal Fat Quantity and Quality with Incident Cardiovascular Disease Risk Factors. J. Am. Coll. Cardiol. 2016, 68, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Inigo, L.; Navarro-Gonzalez, D.; Fernandez-Montero, A.; Pastrana-Delgado, J.; Martinez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, B.; Chen, H.; Shi, Z.; Li, Y.; Tian, Q.; Shi, S. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: A retrospective cohort analysis. Sci. Rep. 2019, 9, 7320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, A.; Zhao, T.; Gong, X.; Pang, T.; Zhou, Y.; Xiao, Y.; Yan, Y.; Fan, C.; Teng, W.; et al. Comparison of anthropometric indices for predicting the risk of metabolic syndrome and its components in Chinese adults: A prospective, longitudinal study. BMJ Open 2017, 7, e016062. [Google Scholar] [CrossRef]
- Baveicy, K.; Mostafaei, S.; Darbandi, M.; Hamzeh, B.; Najafi, F.; Pasdar, Y. Predicting Metabolic Syndrome by Visceral Adiposity Index, Body Roundness Index and a Body Shape Index in Adults: A Cross-Sectional Study from the Iranian RaNCD Cohort Data. Diabetes Metab. Syndr. Obes. 2020, 13, 879–887. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).