CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PET/CT Imaging
2.3. Image Analysis
2.4. Immunohistochemistry
2.5. Statistics
3. Results
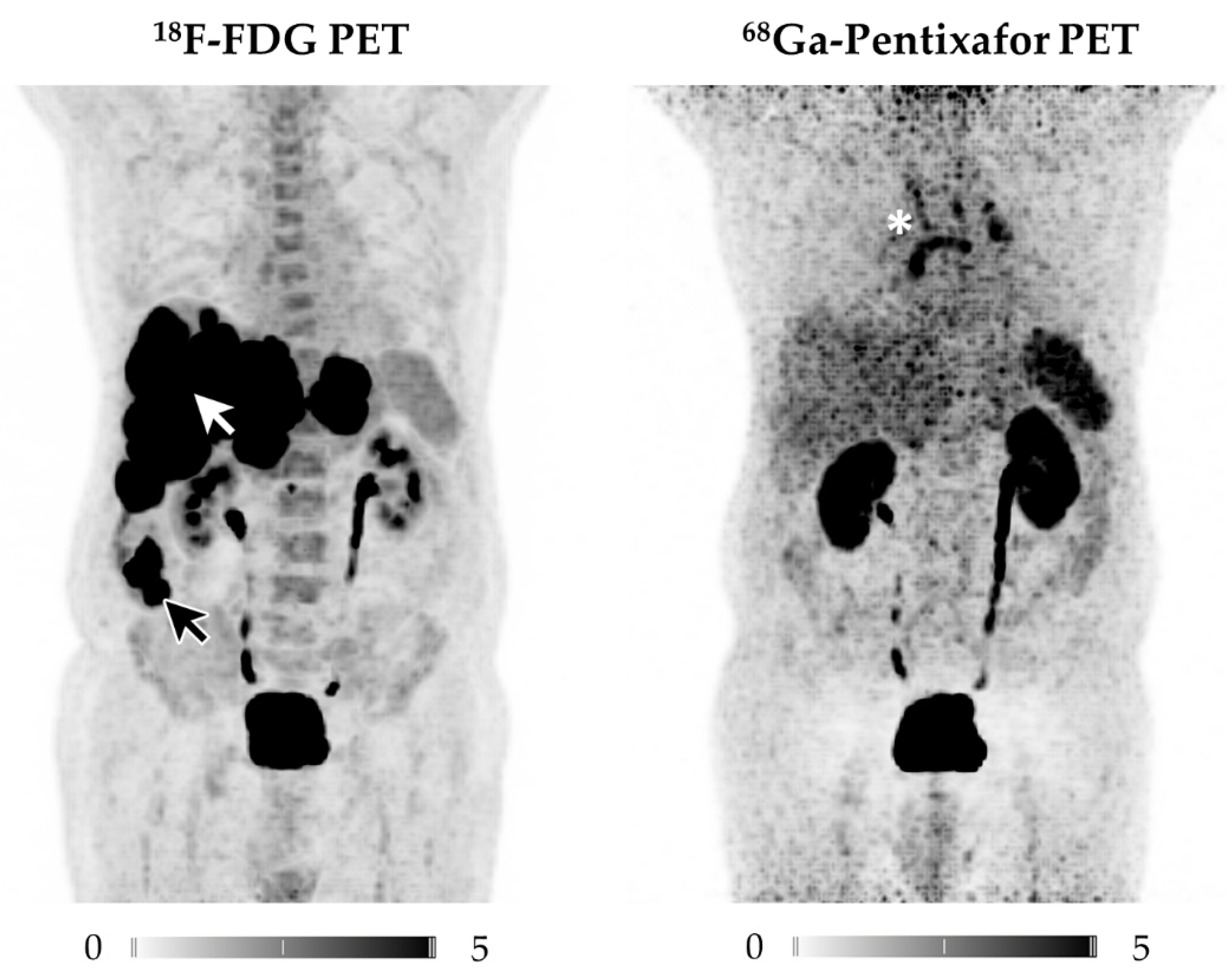
3.1. CXCR4-Directed PET/CT Is Inferior to 18F-FDG PET/CT in NECs
3.1.1. Per-Patient Analysis
3.1.2. Per-Lesion Analysis
3.1.3. Comparison of 18F-FDG and 68Ga-Pentixafor
3.1.4. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klimstra, D.S.; Modlin, I.R.; Coppola, D.; Lloyd, R.V.; Suster, S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas 2010, 39, 707–712. [Google Scholar] [CrossRef]
- Delle Fave, G.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; De Herder, W.W.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kluge, A.W.; Kulkarni, H.; Schorr-Neufing, U.; Niepsch, K.; Bitterlich, N.; Van Echteld, C.J. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-Octreotide ((177)Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics 2016, 6, 501–510. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat. Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Niepsch, K.; Müller, D.; Baum, R.P. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J. Nucl. Med. 2019, 60, 377–385. [Google Scholar] [CrossRef]
- Thang, S.P.; Lung, M.S.; Kong, G.; Hofman, M.S.; Callahan, J.; Michael, M.; Hicks, R.J. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 262–277. [Google Scholar] [CrossRef]
- Rinke, A.; Gress, T.M. Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers. Digestion 2017, 95, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Träger, T.; Hoffmeister, M.; Sipos, B.; Hommann, M.; Sänger, J.; Schulz, S.; Lupp, A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 2015, 6, 27566–27579. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Weich, A.; Higuchi, T.; Schmid, J.S.; Schirbel, A.; Lassmann, M.; Wild, V.; Rudelius, M.; Kudlich, T.; Herrmann, K.; et al. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors—A Triple Tracer Comparative Approach. Theranostics 2017, 7, 1489–1498. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef]
- Lapa, C.; Herrmann, K.; Schirbel, A.; Hänscheid, H.; Lückerath, K.; Schottelius, M.; Kircher, M.; Werner, R.A.; Schreder, M.; Samnick, S.; et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics 2017, 7, 1589–1597. [Google Scholar] [CrossRef]
- Lapa, C.; Hänscheid, H.; Kircher, M.; Schirbel, A.; Wunderlich, G.; Werner, R.A.; Samnick, S.; Kotzerke, J.; Einsele, H.; Buck, A.K.; et al. Feasibility of CXCR4-Directed Radioligand Therapy in Advanced Diffuse Large B-Cell Lymphoma. J. Nucl. Med. 2019, 60, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Jüttler, S.; Müller, M.; Wester, H.J. Cationic eluate pretreatment for automated synthesis of [⁶⁸Ga]CPCR4.2. Nucl. Med. Biol. 2014, 41, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Vag, T.; Gerngross, C.; Herhaus, P.; Eiber, M.; Philipp-Abbrederis, K.; Graner, F.-P.; Ettl, J.; Keller, U.; Wester, H.-J.; Schwaiger, M. First Experience with Chemokine Receptor CXCR4-Targeted PET Imaging of Patients with Solid Cancers. J. Nucl. Med. 2016, 57, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Kircher, S.; Higuchi, T.; Kircher, M.; Schirbel, A.; Wester, H.-J.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; Lapa, C. CXCR4-Directed Imaging in Solid Tumors. Front. Oncol. 2019, 9, 770. [Google Scholar] [CrossRef]
- Maurer, S.; Herhaus, P.; Lippenmeyer, R.; Hänscheid, H.; Kircher, M.; Schirbel, A.; Maurer, H.C.; Buck, A.K.; Wester, H.-J.; Einsele, H.; et al. Side Effects of CXC-Chemokine Receptor 4-Directed Endoradiotherapy with Pentixather Before Hematopoietic Stem Cell Transplantation. J. Nucl. Med. 2019, 60, 1399–1405. [Google Scholar] [CrossRef]
- Linde, P.; Baues, C.; Wegen, S.; Trommer, M.; Quaas, A.; Rosenbrock, J.; Celik, E.; Marnitz, S.; Bruns, C.J.; Fischer, T.; et al. Pentixafor PET/CT for imaging of chemokine receptor 4 expression in esophageal cancer—A first clinical approach. Cancer Imaging 2021, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Breun, M.; Monoranu, C.M.; Kessler, A.F.; Matthies, C.; Löhr, M.; Hagemann, C.; Schirbel, A.; Rowe, S.P.; Pomper, M.G.; Buck, A.K.; et al. [(68)Ga]-Pentixafor PET/CT for CXCR4-Mediated Imaging of Vestibular Schwannomas. Front. Oncol. 2019, 9, 503. [Google Scholar] [CrossRef]
- Osl, T.; Schmidt, A.; Schwaiger, M.; Schottelius, M.; Wester, H.J. A new class of PentixaFor- and PentixaTher-based theranostic agents with enhanced CXCR4-targeting efficiency. Theranostics 2020, 10, 8264–8280. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Thackeray, J.T.; Diekmann, J.; Weiberg, D.; Bauersachs, J.; Bengel, F.M. The Changing Face of Nuclear Cardiology: Guiding Cardiovascular Care Toward Molecular Medicine. J. Nucl. Med. 2020, 61, 951–961. [Google Scholar] [CrossRef]
- Circelli, L.; Sciammarella, C.; Guadagno, E.; Tafuto, S.; de Caro, M.D.B.; Botti, G.; Pezzullo, L.; Aria, M.; Ramundo, V.; Tatangelo, F.; et al. CXCR4/CXCL12/CXCR7 axis is functional in neuroendocrine tumors and signals on mTOR. Oncotarget 2016, 7, 18865–18875. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Lückerath, K.; Kircher, S.; Hänscheid, H.; Grigoleit, G.U.; Rosenwald, A.; Stolzenburg, A.; Kropf, S.; Einsele, H.; Wester, H.J.; et al. Potential influence of concomitant chemotherapy on CXCR4 expression in receptor directed endoradiotherapy. Br. J. Haematol. 2019, 184, 440–443. [Google Scholar] [CrossRef]
- Komek, H.; Gundogan, C.; Can, C. 68Ga-FAPI PET/CT Versus 68Ga-DOTATATE PET/CT in the Evaluation of a Patient With Neuroendocrine Tumor. Clin. Nucl. Med. 2020. [Google Scholar] [CrossRef]

| Location of Primary/Metastases | PET-Positive * | IHC † | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex | Age | ECOG | Primary | N1 | M1 | FDG | CXCR4 | Site of Biopsy | Ki-67 | CXCR4 |
| #1 | M | 56 | 0 | oesophagus | local + distant | liver, lung, bone | yes | yes | oesophagus | 60 | 1 |
| #2 | M | 76 | 0 | ileum | local | liver | yes | no | liver | 70 | 1 |
| #3 | M | 70 | 1 | pancreas | local | liver | yes | yes | pancreas | 90 | 3 |
| #4 | F | 54 | 1 | oesophagus | local | liver, lung | yes | yes | liver | 90 | 2 |
| #5 | F | 44 | 0 | rectum | none | liver | yes | yes | liver | 90 | 1 |
| #6 | M | 78 | 0 | CUP | local | none | yes | yes | axillary LN | 70 | 1 |
| #7 | M | 69 | 1 | stomach | local | liver | yes | yes | stomach | 90 | 1 |
| #8 | M | 77 | 0 | stomach | distant | liver, lung | yes | yes | stomach | 90 | 2 |
| #9 | M | 64 | 1 | pancreas | none | liver, stomach | yes | yes | stomach | 45 | 1 |
| #10 | M | 76 | 0 | stomach | local | liver | yes | yes | stomach | 80 | 1 |
| #11 | M | 45 | 0 | CUP | local + distant | lung, bone | yes | yes | inguinal LN | 50 | N/A |
| 18F-FDG | 68Ga-Pentixafor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SUVmax | SUVmax | |||||||||
| Case | Primary | TBRPrimary * | N1 | M1 | Lesions+ | Primary | TBRPrimary * | N1 | M1 | Lesions+ |
| #1 | 17.2 | 8.9 | 10.1 | 20.7 | 6/6 | 8.4 | 5.2 | 9.3 | 10.5 | 5/6 |
| #2 | 21.3 | 19.7 | 6.9 | 37.1 | 7/9 | N/A | N/A | N/A | N/A | 0/9 |
| #3 | 6.5 | 4.5 | N/A | 3.4 | 8/8 | 10.2 | 5.9 | 9.7 | N/A | 4/8 |
| #4 | 15.4 | 8.3 | 17.5 | N/A | 16/17 | 6.8 | 4.2 | 3.7 | N/A | 5/17 |
| #5 | 6.5 | 3.6 | 2.8 | 5.0 | 5/5 | 3.7 | 2.9 | 4.3 | 3.6 | 3/5 |
| #6 | N/A | N/A | 9.2 | N/A | 2/2 | N/A | N/A | 11.3 | N/A | 2/2 |
| #7 | 9.2 | 4.7 | 4.3 | 11.5 | 16/16 | 8.7 | 5.2 | 8.3 | 15.9 | 3/16 |
| #8 | 6.7 | 3.7 | 6.9 | 40.5 | 13/13 | 5.7 | 3.6 | 8.9 | 14.6 | 6/13 |
| #9 | 31.9 | 22.6 | N/A | 15.7 | 7/7 | 10.6 | 5.7 | N/A | 5.8 | 3/7 |
| #10 | 5.2 | 2.8 | 6.6 | 13.7 | 10/12 | 7.4 | 5.2 | 10.2 | 14.3 | 7/12 |
| #11 | N/A | N/A | 21.3 | 9.6 | 12/12 | N/A | N/A | 7.8 | 9.1 | 4/12 |
| Mean ± SD | 13.3 ± 8.5 | 8.8 ± 6.9 | 9.5 ± 5.8 | 17.5 ± 12.4 | 7.7 ± 2.2 | 4.7 ± 1.0 | 8.2 ± 2.4 | 11.7 ± 3.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weich, A.; Werner, R.A.; Buck, A.K.; Hartrampf, P.E.; Serfling, S.E.; Scheurlen, M.; Wester, H.-J.; Meining, A.; Kircher, S.; Higuchi, T.; et al. CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas. Diagnostics 2021, 11, 605. https://doi.org/10.3390/diagnostics11040605
Weich A, Werner RA, Buck AK, Hartrampf PE, Serfling SE, Scheurlen M, Wester H-J, Meining A, Kircher S, Higuchi T, et al. CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas. Diagnostics. 2021; 11(4):605. https://doi.org/10.3390/diagnostics11040605
Chicago/Turabian StyleWeich, Alexander, Rudolf A. Werner, Andreas K. Buck, Philipp E. Hartrampf, Sebastian E. Serfling, Michael Scheurlen, Hans-Jürgen Wester, Alexander Meining, Stefan Kircher, Takahiro Higuchi, and et al. 2021. "CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas" Diagnostics 11, no. 4: 605. https://doi.org/10.3390/diagnostics11040605
APA StyleWeich, A., Werner, R. A., Buck, A. K., Hartrampf, P. E., Serfling, S. E., Scheurlen, M., Wester, H.-J., Meining, A., Kircher, S., Higuchi, T., Pomper, M. G., Rowe, S. P., Lapa, C., & Kircher, M. (2021). CXCR4-Directed PET/CT in Patients with Newly Diagnosed Neuroendocrine Carcinomas. Diagnostics, 11(4), 605. https://doi.org/10.3390/diagnostics11040605






