Comparison of Compressed Sensing and Gradient and Spin-Echo in Breath-Hold 3D MR Cholangiopancreatography: Qualitative and Quantitative Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MR Examination
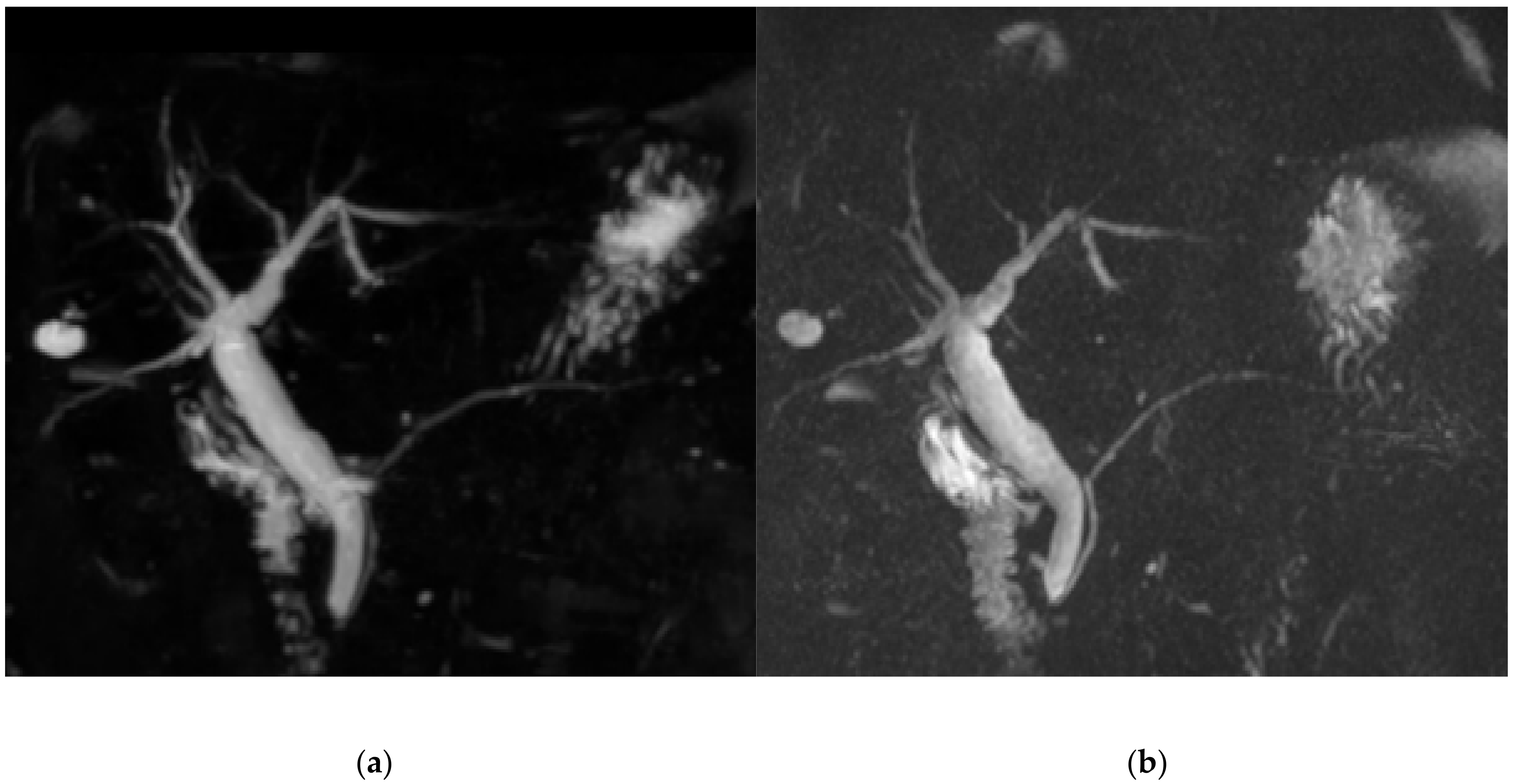
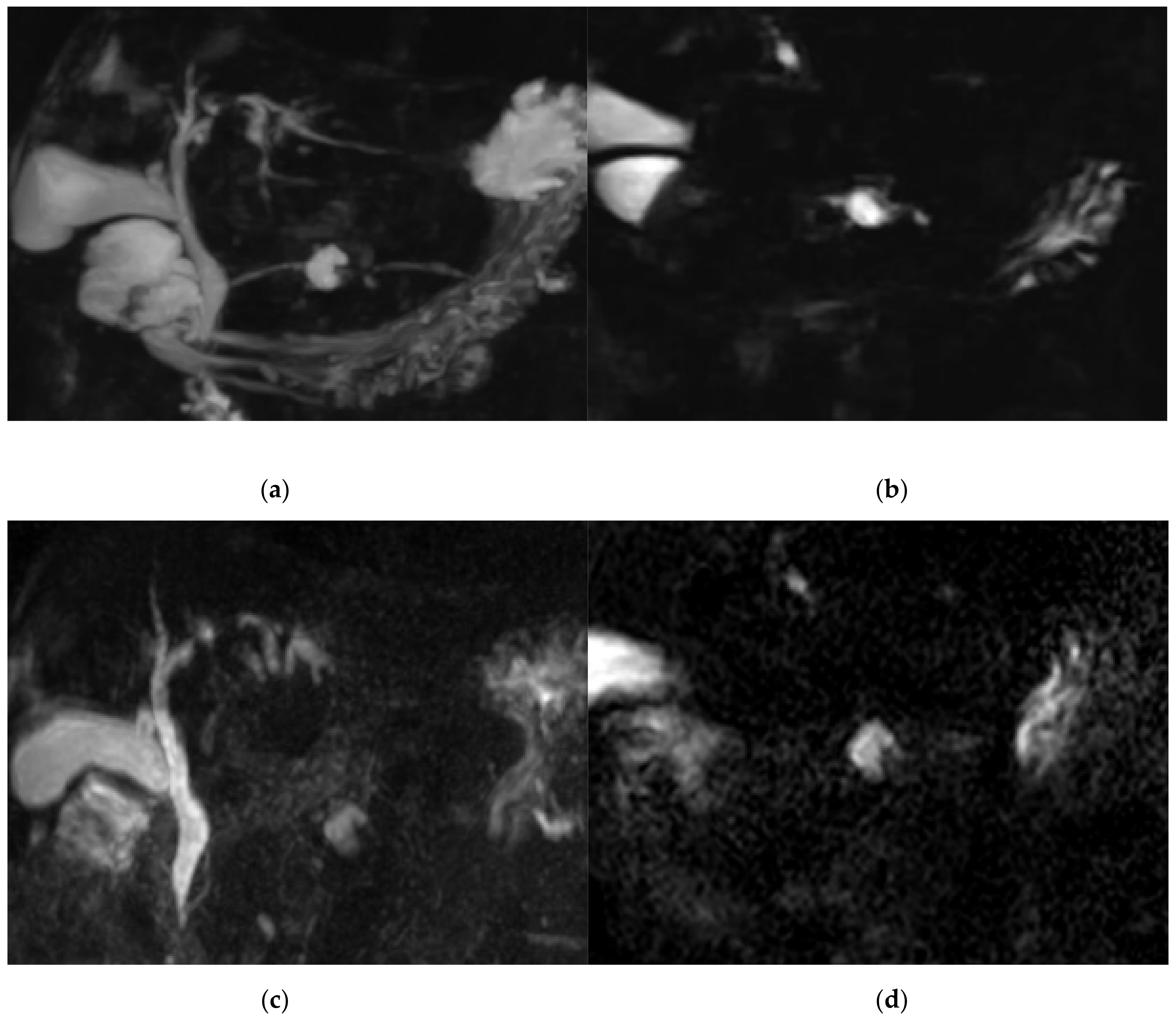
2.3. Compressed Sensing MRCP
2.4. Gradient and Spin-Echo MRCP
2.5. Image Analysis
2.5.1. Qualitative Image Analysis
2.5.2. Quantitative Image Analysis
2.6. Statistical Analyses
3. Results
3.1. Qualitative Image Analysis
3.2. Quantitative Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, S.; Jeon, T.Y.; Yoo, S.-Y.; Hwang, S.M.; Choi, Y.H.; Kim, W.S.; Choe, Y.H.; Kim, J.H. Incremental Value of MR Cholangiopancreatography in Diagnosis of Biliary Atresia. PLoS ONE 2016, 11, e0158132. [Google Scholar] [CrossRef]
- Katabathina, V.S.; Dasyam, A.K.; Dasyam, N.; Hosseinzadeh, K. Adult Bile Duct Strictures: Role of MR Imaging and MR Cholangiopancreatography in Characterization. Radiographics 2014, 34, 565–586. [Google Scholar] [CrossRef]
- Barish, M.; Yucel, E.K.; Ferrucci, J.T. Magnetic Resonance Cholangiopancreatography. N. Engl. J. Med. 1999, 341, 258–264. [Google Scholar] [CrossRef]
- Limanond, P.; Raman, S.S.; Ghobrial, R.M.; Busuttil, R.W.; Lu, D.S.K. The utility of MRCP in preoperative mapping of biliary anatomy in adult-to-adult living related liver transplant donors. J. Magn. Reson. Imaging 2004, 19, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hur, B.Y.; Lee, J.M.; Park, J.Y.; Kim, S.J.; Joo, I.; Shin, C.I.; Baek, J.H.; Kim, J.H.; Han, J.K.; Choi, B.I. Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: Comparison with pancreatic adenocarcinoma. J. Magn. Reson. Imaging 2012, 36, 188–197. [Google Scholar] [CrossRef]
- Shanmugam, V.; Beattie, G.C.; Yule, S.R.; Reid, W.; Loudon, M.A. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br. J. Radiol. 2005, 78, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Saito, N.; Suzuki, K.; Mitsuhashi, N. Biliary anatomy on 3D MRCP: Comparison of volume-rendering and maximum-intensity-projection algorithms. J. Magn. Reson. Imaging 2009, 29, 601–606. [Google Scholar] [CrossRef]
- Cai, L.; Yeh, B.M.; Westphalen, A.C.; Roberts, J.; Wang, Z.J. 3D T2-weighted and Gd-EOB-DTPA-enhanced 3D T1-weighted MR cholangiography for evaluation of biliary anatomy in living liver donors. Abdom. Radiol. 2016, 42, 842–850. [Google Scholar] [CrossRef]
- Nakaura, T.; Kidoh, M.; Maruyama, N.; Kawahara, T.; Namimoto, T.; Sakai, Y.; Harada, K.; Yamashita, Y. Usefulness of the SPACE pulse sequence at 1.5T MR cholangiography: Comparison of image quality and image acquisition time with conventional 3D-TSE sequence. J. Magn. Reson. Imaging 2013, 38, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, F.; Martinelli, M.; Al Ansari, N.; Kagarmanova, A.; De Marco, V.; Zippi, M.; Marini, M. Magnetic resonance cholangiography: Past, present and future: A review. Eur. Rev. Med Pharmacol. Sci. 2010, 14, 721–725. [Google Scholar] [PubMed]
- Bates, D.D.B.; LeBedis, C.A.; Soto, J.A.; Gupta, A. Use of Magnetic Resonance in Pancreaticobiliary Emergencies. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Wielopolski, P.A.; Gaa, J.; Wielopolski, D.R.; Oudkerk, M. Breath-hold MR Cholangiopancreatography with Three-dimensional, Segmented, Echo-planar Imaging and Volume Rendering. Radiology 1999, 210, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Glockner, J.F.; Saranathan, M.; Bayram, E.; Lee, C.U. Breath-held MR Cholangiopancreatography (MRCP) using a 3D Dixon fat–water separated balanced steady state free precession sequence. Magn. Reson. Imaging 2013, 31, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Israel, G.M.; Hecht, E.M.; Krinsky, G.A.; Babb, J.S.; Lee, V.S. Isotropic 3D T2-Weighted MR Cholangiopancreatography with Parallel Imaging: Feasibility Study. Am. J. Roentgenol. 2006, 187, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Sodickson, A.; Mortele, K.J.; Barish, M.A.; Zou, K.H.; Thibodeau, S.; Tempany, C.M.C. Three-dimensional Fast-Recovery Fast Spin-Echo MRCP: Comparison with Two-dimensional Single-Shot Fast Spin-Echo Techniques. Radiology 2006, 238, 549–559. [Google Scholar] [CrossRef]
- Nam, J.G.; Lee, J.M.; Kang, H.-J.; Lee, S.M.; Kim, E.; Peeters, J.M.; Lee, J.M. GRASE Revisited: Breath-hold three-dimensional (3D) magnetic resonance cholangiopancreatography using a Gradient and Spin Echo (GRASE) technique at 3T. Eur. Radiol. 2018, 28, 3721–3728. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef]
- Runge, V.M.; Richter, J.K.; Heverhagen, J.T. Speed in Clinical Magnetic Resonance. Investig. Radiol. 2017, 52, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chandarana, H.; Doshi, A.M.; Shanbhogue, A.; Babb, J.S.; Bruno, M.T.; Zhao, T.; Raithel, E.; Zenge, M.O.; Li, G.; Otazo, R. Three-dimensional MR Cholangiopancreatography in a Breath Hold with Sparsity-based Reconstruction of Highly Undersampled Data. Radiology 2016, 280, 585–594. [Google Scholar] [CrossRef]
- Chien, C.-P.; Chiu, F.-M.; Shen, Y.-C.; Chen, Y.-H.; Chung, H.-W. Magnetic resonance cholangiopancreatography at 3T in a single breath-hold: Comparative effectiveness between three-dimensional (3D) gradient- and spin-echo and two-dimensional (2D) thick-slab fast spin-echo acquisitions. Quant. Imaging Med. Surg. 2020, 10, 1265–1274. [Google Scholar] [CrossRef]
- Nagata, S.; Goshima, S.; Noda, Y.; Kawai, N.; Kajita, K.; Kawada, H.; Tanahashi, Y.; Matsuo, M. Magnetic resonance cholangiopancreatography using optimized integrated combination with parallel imaging and compressed sensing technique. Abdom. Radiol. 2019, 44, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xu, J.; Sun, Z.; Wang, S.; Zhu, L.; Wang, X.; Wang, J.; Feng, F.; Xue, H.-D.; Jin, Z. Comparison and evaluation of the efficacy of compressed SENSE (CS) and gradient- and spin-echo (GRASE) in breath-hold (BH) magnetic resonance cholangiopancreatography (MRCP). J. Magn. Reson. Imaging 2020, 51, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zaitsev, M.; Büchert, M.; Raithel, E.; Paul, D.; Korvink, J.G.; Hennig, J. Improving the robustness of 3D turbo spin echo imaging to involuntary motion. Magmat. Magn. Reson. Mater. Phys. Biol. Med. 2014, 28, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Nakaura, T.; Iyama, Y.; Sakamoto, S.; Takemura, A.; Okuaki, T.; Harada, K.; Yamashita, Y. Usefulness of 3D hybrid profile order technique with 3T magnetic resonance cholangiography: Comparison of image quality and acquisition time. J. Magn. Reson. Imaging 2016, 44, 1346–1353. [Google Scholar] [CrossRef]
- Itatani, R.; Namimoto, T.; Kusunoki, S.; Mizuguchi, T.; Ohtsuka, S.; Yamashita, Y. Usefulness of the Short–Echo Time Cube Sequence at 3-T Magnetic Resonance Cholangiopancreatography. J. Comput. Assist. Tomogr. 2016, 40, 551–556. [Google Scholar] [CrossRef]
- McClellan, T.R.; Motosugi, U.; Middleton, M.S.; Allen, B.C.; Jaffe, T.A.; Miller, C.M.; Reeder, S.B.; Sirlin, C.B.; Bashir, M.R. Intravenous Gadoxetate Disodium Administration Reduces Breath-holding Capacity in the Hepatic Arterial Phase: A Multi-Center Randomized Placebo-controlled Trial. Radiology 2017, 282, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xue, H.; Sun, Z.; Qian, T.; Weiland, E.; Kuehn, B.; Asbach, P.; Hamm, B.; Jin, Z. Modified breath-hold compressed-sensing 3D MR cholangiopancreatography with a small field-of-view and high resolution acquisition: Clinical feasibility in biliary and pancreatic disorders. J. Magn. Reson. Imaging 2018, 48, 1389–1399. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, S.-H.; Kuehn, B.; Paek, M.Y. Optimized Breath-Hold Compressed-Sensing 3D MR Cholangiopancreatography at 3T: Image Quality Analysis and Clinical Feasibility Assessment. Diagnostics 2020, 10, 376. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakaura, T.; Inoue, T.; Tanoue, S.; Takada, S.; Utsunomiya, D.; Tsumagari, S.; Harada, K.; Yamashita, Y. Magnetic resonance cholangiopancreatography with GRASE sequence at 3.0T: Does it improve image quality and acquisition time as compared with 3D TSE? Eur. Radiol. 2018, 28, 2436–2443. [Google Scholar] [CrossRef]
- Chu, M.-L.; Chien, C.-P.; Wu, W.-C.; Chung, H.-W. Gradient- and spin-echo (GRASE) MR imaging: A long-existing technology that may find wide applications in modern era. Quant. Imaging Med. Surg. 2019, 9, 1477–1484. [Google Scholar] [CrossRef]
- Mugler, J.P.; Brookeman, J.R. Off-resonance image artifacts in interleaved-EPI and GRASE pulse sequences. Magn. Reson. Med. 1996, 36, 306–313. [Google Scholar] [CrossRef]
- Johnson, G.; Feinberg, D.A.; Venkataraman, V. A comparison of phase encoding ordering schemes inT2-weighted GRASE imaging. Magn. Reson. Med. 1996, 36, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Itatani, R.; Namimoto, T.; Takaoka, H.; Katahira, K.; Noda, S.; Toyonari, N.; Yamashita, Y. Clinical Impact of 3-Dimensional Balanced Turbo-Field-Echo Magnetic Resonance Cholangiopancreatography at 3 T: Prospective Intraindividual Comparison With 3-Dimensional Turbo-Spin-Echo Magnetic Resonance Cholangiopancreatography. J. Comput. Assist. Tomogr. 2015, 39, 19–24. [Google Scholar] [CrossRef]
- Feng, L.; Benkert, T.; Block, K.T.; Sodickson, D.K.; Otazo, R.; Chandarana, H. Compressed sensing for body MRI. J. Magn. Reson. Imaging 2017, 45, 966–987. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.-Y.; Kretzler, M.; Sudarski, S.; Gulani, V.; Seiberlich, N. Sparse Reconstruction Techniques in Magnetic Resonance Imaging. Investig. Radiol. 2016, 51, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, K.G. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction. Phys. Med. Biol. 2015, 60, R297–R322. [Google Scholar] [CrossRef]
- Jaspan, O.N.; Fleysher, R.; Lipton, M.L. Compressed sensing MRI: A review of the clinical literature. Br. J. Radiol. 2015, 88, 20150487. [Google Scholar] [CrossRef]
- Goldfinger, M.H.; Ridgway, G.R.; Ferreira, C.; Langford, C.R.; Cheng, L.; Kazimianec, A.; Borghetto, A.; Wright, T.G.; Woodward, G.; Hassanali, N.; et al. Quantitative MRCP Imaging: Accuracy, Repeatability, Reproducibility, and Cohort-Derived Normative Ranges. J. Magn. Reson. Imaging 2020, 52, 807–820. [Google Scholar] [CrossRef] [Green Version]
- Ouahabi, A. A review of wavelet denoising in medical imaging. In 2013 8th International Workshop on Systems, Signal Processing and Their Applications (WoSSPA); Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 2013; pp. 19–26. [Google Scholar]


| Parameter | Score | Scoring System |
|---|---|---|
| Image quality degradation by artifacts | 1 | Nondiagnostic image due to severe artifacts |
| 2 | Major artifacts causing significant problems in diagnosis | |
| 3 | Moderate artifacts with some uncertainty in diagnosis | |
| 4 | Minor artifacts without problems in diagnosis | |
| 5 | Excellent image quality without any detectable artifacts | |
| Background suppression | 1 | Significant background signal that rendered image interpretation impossible |
| 2 | Remarkable background signal that rendered image interpretation difficult | |
| 3 | Noticeable background signal that is distracting in image interpretation | |
| 4 | Minimal background signal without problems in observation of pancreaticobiliary tree | |
| 5 | Excellent background suppression | |
| Overall image quality | 1 | Nondiagnostic image |
| 2 | Below average image quality | |
| 3 | Average image quality | |
| 4 | Good image quality | |
| 5 | Excellent image quality | |
| Ductal visualization | 1 | Ductal structure not visible |
| 2 | Ductal structure vaguely identified | |
| 3 | Ductal structure partially visible | |
| 4 | Most of the ductal structure visible, with some blurring | |
| 5 | Entire ductal structure visible with excellent details |
| BH-CS-MRCP | BH-GRASE-MRCP | p-Value * | |
|---|---|---|---|
| Artifact | 4.71 ± 0.42 (3.0–5.0) | 4.86 ± 0.26 (4.0–5.0) | 0.025 |
| Background suppression | 4.46 ± 0.39 (3.7–5.0) | 4.02 ± 0.28 (3.0–4.7) | <0.001 |
| Overall image quality | 4.23 ± 0.61 (2.3–5.0) | 4.21 ± 0.66 (2.3–5.0) | 0.797 |
| Duct visualization | |||
| CBD | 4.78 ± 0.36 (3.7–5.0) | 4.67 ± 0.54 (3.0–5.0) | 0.242 |
| Right 1st IHD | 4.62 ± 0.43 (3.3–5.0) | 4.64 ± 0.62 (3.3–5.0) | 0.589 |
| Left 1st IHD | 4.66 ± 0.39 (3.0–5.0) | 3.42 ± 0.77 (3.0–5.0) | 0.238 |
| Right 2nd IHD | 3.85 ± 0.70 (2.0–5.0) | 3.48 ± 0.97 (2.0–5.0) | 0.004 |
| Left 2nd IHD | 3.81 ± 0.64 (2.3–5.0) | 3.42 ± 0.77 (2.3–5.0) | <0.001 |
| Cystic duct | 4.02 ± 0.73 (2.3–5.0) | 4.22 ± 0.65 (2.3–5.0) | 0.089 |
| Proximal PD | 4.05 ± 1.07 (1.0–5.0) | 3.89 ± 1.32 (1.0–5.0) | 0.643 |
| Mid PD | 3.99 ± 1.17 (1.0–5.0) | 3.55 ± 1.41 (1.0–5.0) | 0.003 |
| Distal PD | 3.71 ± 12.5 (1.0–5.0) | 3.38 ± 1.42 (1.0–5.0) | 0.041 |
| Communication between PD and cyst | 21/24 † (87.5%) | 15/24 † (62.5%) | 0.070 |
| BH-CS-MRCP | BH-GRASE-MRCP | |
|---|---|---|
| Artifact | 0.74 (0.60, 0.84) | 0.52 (0.26, 0.70) |
| Background suppression | 0.35 (0.00, 0.59) | 0.31 (−0.07, 0.57) |
| Overall image quality | 0.72 (0.57, 0.82) | 0.79 (0.67, 0.87) |
| Duct visualization | ||
| CBD | 0.71 (0.55, 0.82) | 0.88 (0.82, 0.93) |
| Right 1st IHD | 0.64 (0.44, 0.77) | 0.86 (0.78, 0.91) |
| Left 1st IHD | 0.47 (0.18, 0.67) | 0.70 (0.54, 0.81) |
| Right 2nd IHD | 0.72 (0.57, 0.83) | 0.84 (0.75, 0.90) |
| Left 2nd IHD | 0.67 (0.49, 0.79) | 0.71 (0.55, 0.82) |
| Cystic duct | 0.81 (0.69, 0.88) | 0.73 (0.56, 0.83) |
| Proximal PD | 0.92 (0.88, 0.95) | 0.94 (0.91, 0.96) |
| Mid PD | 0.93 (0.89, 0.96) | 0.95 (0.93, 0.97) |
| Distal PD | 0.94 (0.90, 0.96) | 0.95 (0.92, 0.97) |
| BH-CS-MCRP | BH-GRASE-MRCP | p-Value * | |
|---|---|---|---|
| T2 signal intensity | |||
| CBD | 251.49 ± 71.99 | 821.95 ± 202.03 | <0.001 |
| Periductal tissue | 4.04 ± 2.22 | 124.69 ± 43.48 | <0.001 |
| Liver | 1.69 ± 2.51 | 71.83 ± 19.40 | <0.001 |
| Noise | |||
| CBD | 11.01 ± 4.12 | 75.66 ± 187.53 | 0.01 |
| Periductal tissue | 2.93 ± 1.45 | 39.66 ± 9.17 | <0.001 |
| Liver | 0.73 ± 1.20 | 28.52 ± 8.29 | <0.001 |
| Signal-to-noise ratio | 26.86 ± 14.58 | 17.16 ± 6.38 | <0.001 |
| Contrast ratio | 96.70 ± 0.02 | 73.30 ± 0.07 | <0.001 |
| Contrast-to-noise ratio | 37.48 ± 20.27 | 18.30 ± 6.21 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Song, J.S.; Kim, S.H.; Yang, J.D. Comparison of Compressed Sensing and Gradient and Spin-Echo in Breath-Hold 3D MR Cholangiopancreatography: Qualitative and Quantitative Analysis. Diagnostics 2021, 11, 634. https://doi.org/10.3390/diagnostics11040634
Jang W, Song JS, Kim SH, Yang JD. Comparison of Compressed Sensing and Gradient and Spin-Echo in Breath-Hold 3D MR Cholangiopancreatography: Qualitative and Quantitative Analysis. Diagnostics. 2021; 11(4):634. https://doi.org/10.3390/diagnostics11040634
Chicago/Turabian StyleJang, Weon, Ji Soo Song, Sang Heon Kim, and Jae Do Yang. 2021. "Comparison of Compressed Sensing and Gradient and Spin-Echo in Breath-Hold 3D MR Cholangiopancreatography: Qualitative and Quantitative Analysis" Diagnostics 11, no. 4: 634. https://doi.org/10.3390/diagnostics11040634





