Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases
Abstract
:1. Introduction
2. Technique and Equipment
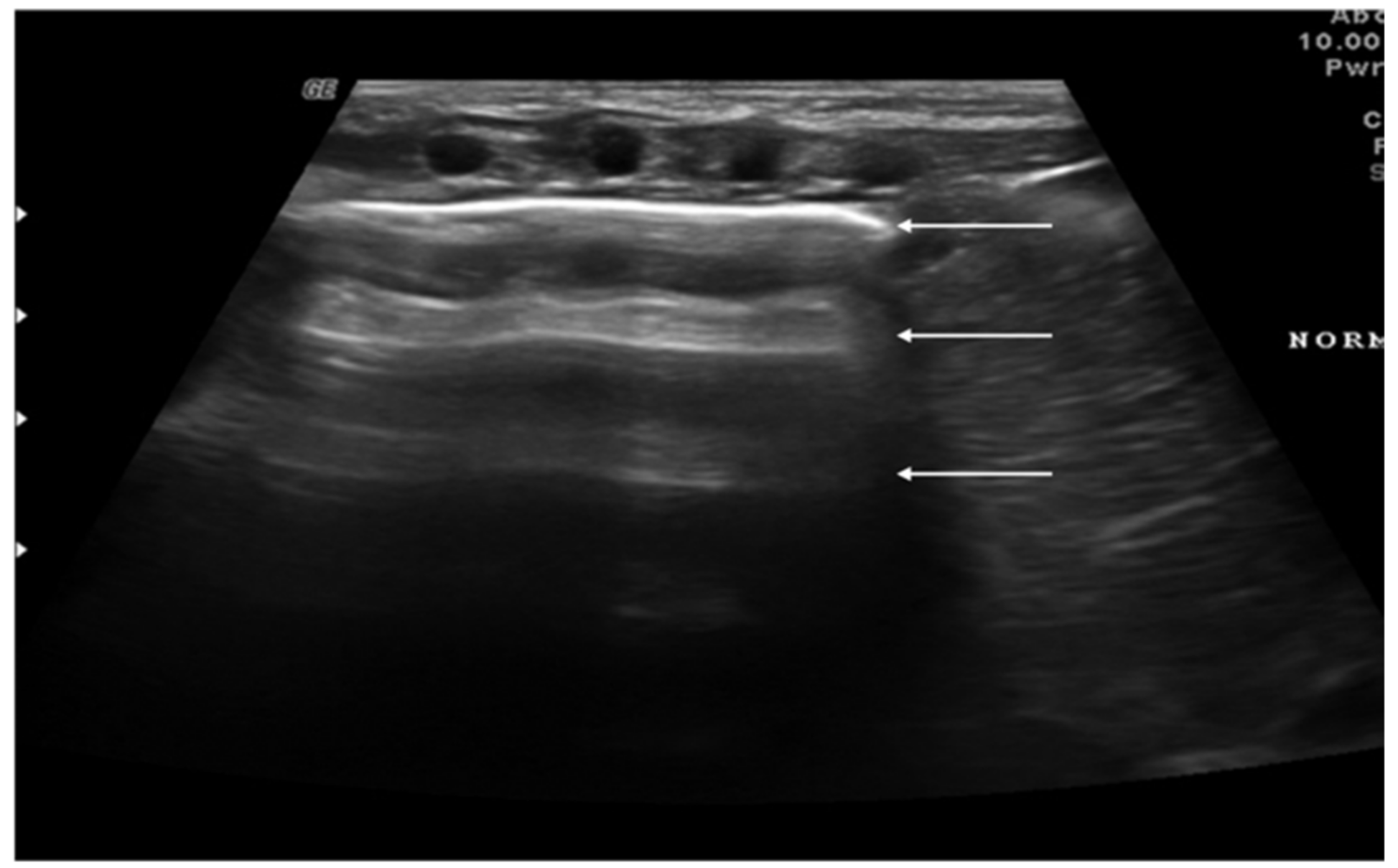
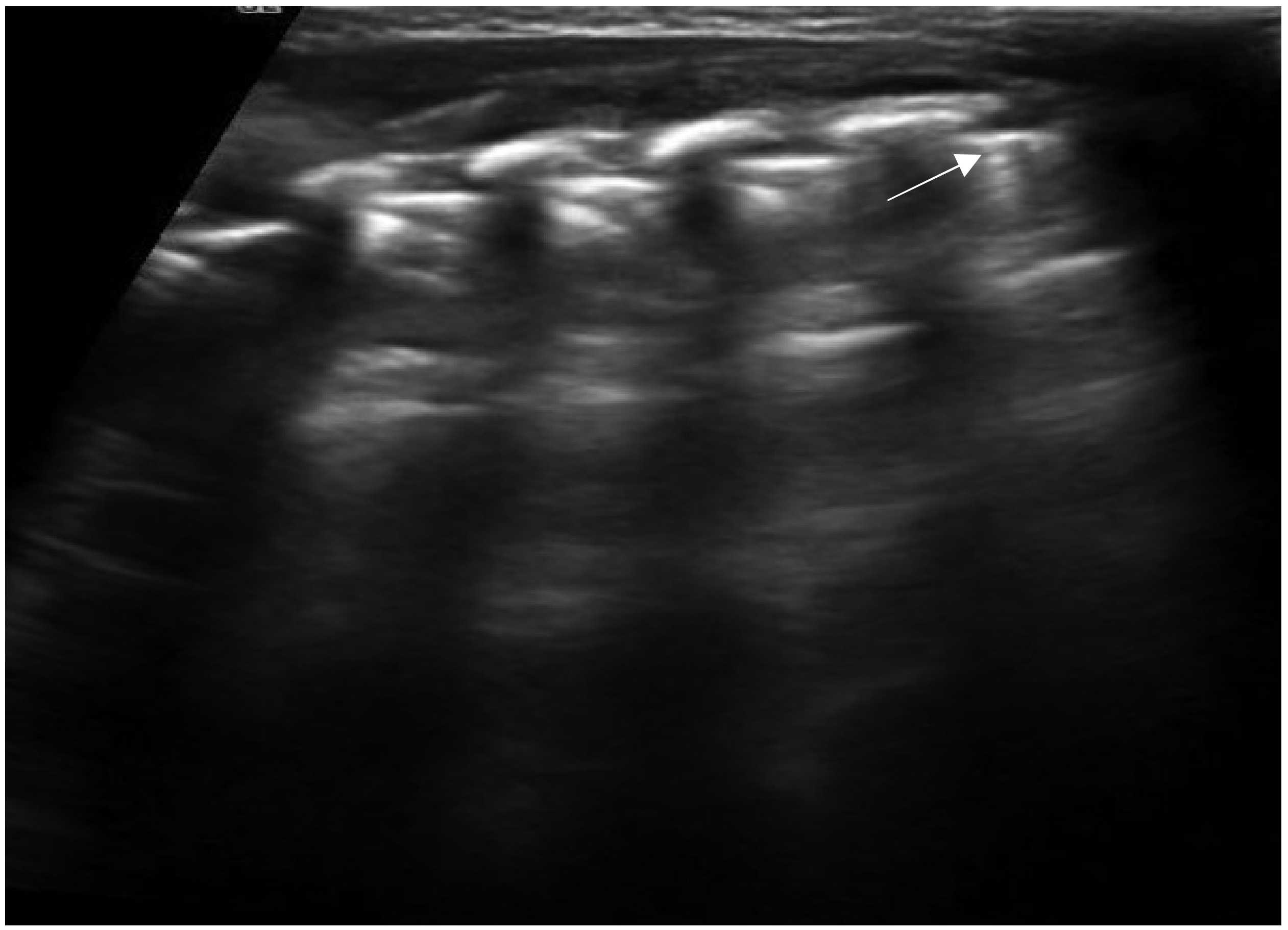
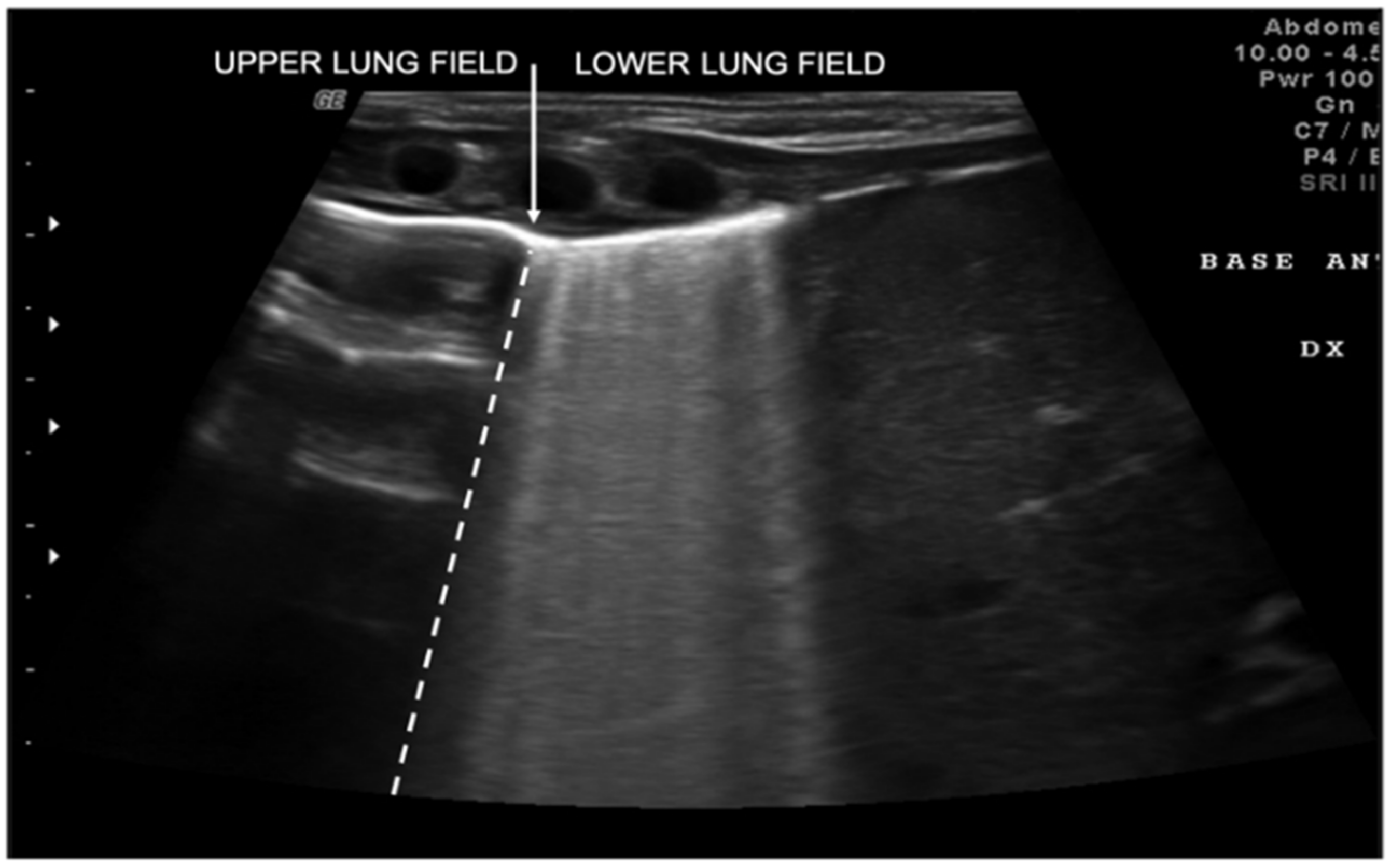
3. Lung Ultrasound Findings in Healthy Subjects
4. Lung and Chest Wall Ultrasound Applications in Children
- Diagnosis and follow-up of neonatal lung diseases;
- Confirm antenatal diagnosis of lung malformations;
- Diagnosis and follow-up of pediatric lung infectious diseases (namely bronchiolitis and pneumonia) and lung complications (such as pneumothorax, pleural effusion and lung abscess);
- Diagnosis and follow-up of pulmonary edema;
- Diagnosis of thoracic trauma and early detection of signs of child abuse;
- Follow-up in children undergoing cardiac surgery;
- Diaphragm ultrasound.
5. Neonatal Lung Diseases
6. Congenital Pulmonary Airway Malformations and Congenital Diaphragmatic Hernia
7. Respiratory Infectious Diseases in Children
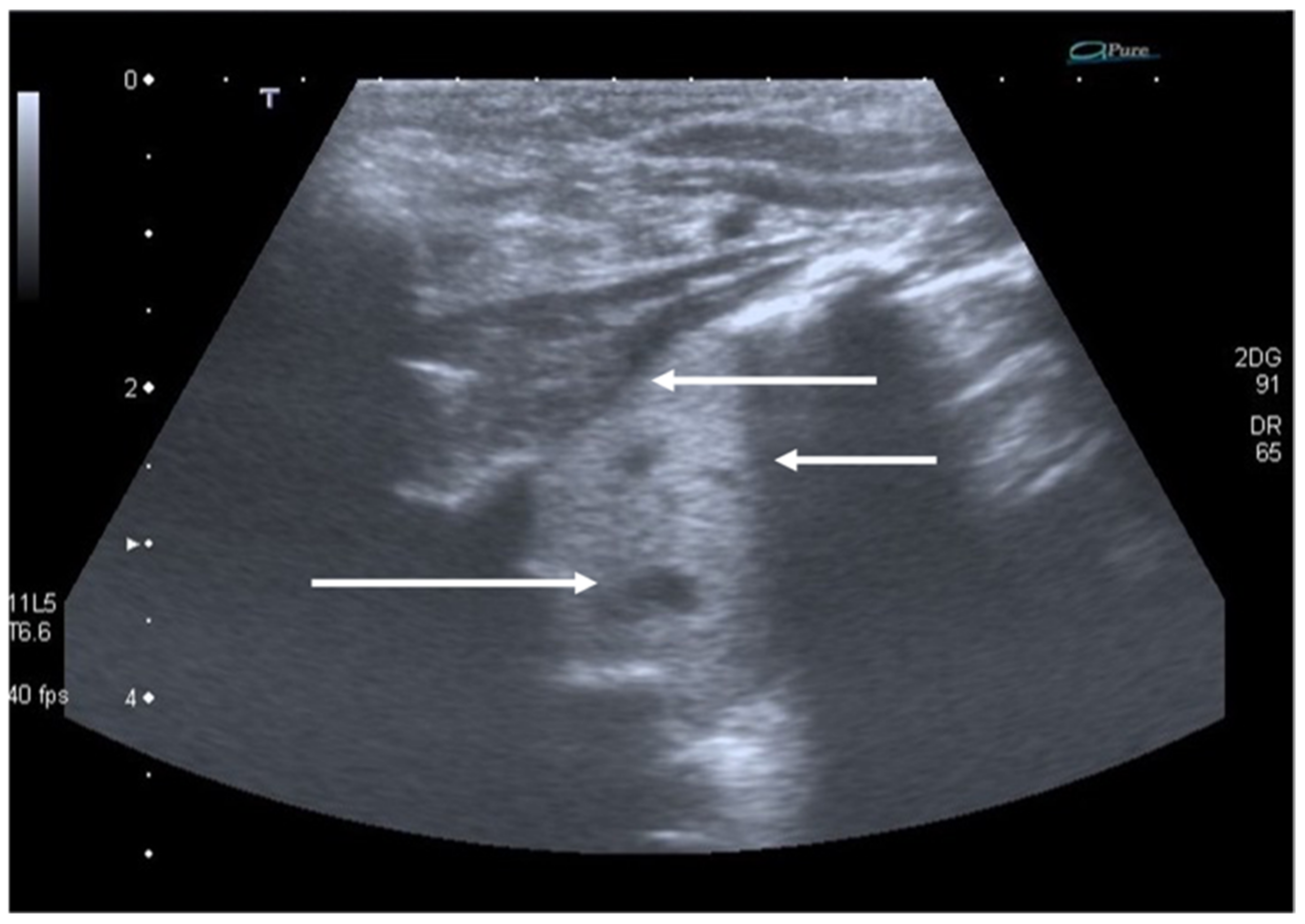
8. Pneumothorax, Pleural Effusion Empyema and Lung Abscess
9. COVID-19 Pneumonia
10. Diagnosis and Follow-Up of Pulmonary Edema
11. Thoracic Trauma and Detection of Signs of Child Abuse
12. Follow-Up in Pediatric Patients Undergoing Cardiac Surgery
13. Diaphragm Ultrasound
14. New Perspectivations of LUS
15. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomas, K.E.; Parnell-Parmley, J.E.; Haidar, S.; Moineddin, R.; Charkot, E.; Bendavid, G.; Krajewski, C. Assessment of radiation dose awareness among pediatricians. Pediatr. Radiol. 2006, 36, 823–832. [Google Scholar] [CrossRef]
- Zechner, P.M.; Seibel, A.; Aichinger, G.; Steigerwald, M.; Dorr, K.; Scheiermann, P.; Schellhaas, S.; Cuca, C.; Breitkreutz, R.; Arbeitsgruppe des Moduls 5 in Anästhesie Fokussierte Sonographie der DGAI. Lung ultrasound in acute and critical care medicine. Anaesthesist 2012, 6, 608–617. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.; Vasishta, A.; Gupta, M. Ultrasound of the pediatric chest. Br. J. Radiol. 2019, 92, 20190058. [Google Scholar] [CrossRef] [PubMed]
- Cattarossi, L. Lung ultrasound: Its role in neonatology and pediatrics. Early Hum. Dev. 2013, 89. [Google Scholar] [CrossRef]
- Copetti, R.; Cattarossi, L. Ultrasound diagnosis of pneumonia in children. Radiol. Med. 2008, 113, 190. [Google Scholar] [CrossRef] [PubMed]
- Denina, M.; Scolfaro, C.; Silvestro, E.; Pruccoli, G.; Mignone, F.; Zoppo, M.; Ramenghi, U.; Garazzino, S. Lung ultrasound in children with COVID-19. Pediatrics 2020, 146, e20201157. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.Y.; Tao, X.W.; Zeng, L.K.; Wang, W.Q.; Li, G. Application of pulmonary ultrasound in the diagnosis of COVID-19 pneumonia in neonates. Zhonghua Er Ke Za Zhi 2020, 58, 347–350. [Google Scholar]
- Reissig, A.; Copetti, R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration 2014, 87, 179–189. [Google Scholar] [CrossRef]
- Platz, E.; Jhund, P.S.; Girerd, N.; Pivetta, E.; McMurray, J.J.V.; Peacock, W.F.; Masip, J.; Martin-Sanchez, F.J.; Miró, Ò.; Price, S.; et al. Study Group on Acute Heart Failure of the Acute Cardiovascular Care Association and the Heart Failure Association of the European Society of Cardiology. Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur. J. Heart Fail. 2019, 21, 844–851. [Google Scholar]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19. A simple, quantitative, reproducible method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Nenna, R.; Iovine, E.; Laudisa, M.; Bloise, S.; La Regina, D.P.; Midulla, F. Comment on Jaworska, J.; et al. Consensus on the Application of Lung Ultrasound in Pneumonia and Bronchiolitis in Children. Diagnostics 2021, 11, 55. [Google Scholar] [CrossRef]
- Jaworska, J.; Komorowska-Piotrowska, A.; Pomiećko, A.; Wiśniewski, J.; Woźniak, M.; Littwin, B.; Kryger, M.; Kwaśniewicz, P.; Szczyrski, J.; Kulińska-Szukalska, K.; et al. Response to Comment on Jaworska, J.; et al. Consensus on the Application of Lung Ultrasound in Pneumonia and Bronchiolitis in Children. Diagnostics 2021, 11, 66. [Google Scholar] [CrossRef]
- Lobo, V.; Weingrow, D.; Perera, P.; Williams, S.R.; Gharahbaghian, L. Thoracic ultrasonography. Crit. Care Clin. 2014, 30, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, D.; Meziere, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact. An ultrasound sign of alveolar interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Copetti, R.; Cattarossi, L. The “double lung point”: An ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology 2007, 91, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Soldati, G.; Curatola, A.; Morello, R.; De Rose, C.; Vacca, M.E.; Lazzareschi, I.; Musolino, A.M.; Valentini, P. Lung Ultrasound Pattern in Healthy Infants during the First 6 Months of Life. J. Ultrasound Med. 2020, 39, 2379–2388. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mauriat, P. Lung ultrasound in the critically ill neonate. Curr. Pediatr. Rev. 2012, 8, 217–223. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N. Role of chest ultrasound in neonatal lung disease: A review of current evidences. J. Matern. Fetal Neonatal Med. 2019, 32, 310–316. [Google Scholar] [CrossRef]
- Copetti, R.; Cattarossi, L.; Macagno, F.; Violino, M.; Furlan, R. Lung ultrasound in respiratory distress syndrome: A useful tool for early diagnosis. Neonatology 2008, 94. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorio-Hernández, R.; Arriaga-Redondo, M.; Pérez-Pérez, A.; Ramos-Navarro, C.; Sánchez-Luna, M. Lung ultrasound in preterm infants with respiratory distress: Experience in a neonatal intensive care unit. Eur. J. Pediatrics 2020, 179, 81–89. [Google Scholar] [CrossRef]
- Reuter, S.; Moser, C.; Baack, M. Respiratory distress in the newborn. Pediatr. Rev. 2014, 35, 417–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, Y.; Fu, W.; Yang, C.S.; Huang, J.J. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine 2014, 93. [Google Scholar] [CrossRef] [PubMed]
- Piastra, M.; Yousef, N.; Brat, R.; Manzoni, P.; Mokhtari, M.; De Luca, D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum. Dev. 2014, 90. [Google Scholar] [CrossRef]
- David, M.; Lamas-Pinheiro, R.; Henriques-Coelho, T. Prenatal and postnatal management of congenital pulmonary airway malformation. Neonatology 2016, 110, 101–115. [Google Scholar] [CrossRef]
- Farrugia, M.; Raza, S.; Gould, S.; Lakhoo, K. Congenital lung lesions: Classification and concordance of radiological appearance and surgical pathology. Pediatr. Surg. Int. 2008, 24, 987–999. [Google Scholar] [CrossRef]
- Yousef, N.; Mokhtari, M.; Durand, P.; Raimondi, F.; Migliaro, F.; Letourneau, A.; Tissières, P.; De Luca, D. Lung Ultrasound Findings in Congenital Pulmonary Airway Malformation. Am. J. Perinatol. 2018, 35, 1222–1227. [Google Scholar]
- Corsini, I.; Parri, N.; Coviello, C.; Leonardi, V.; Dani, C. Lung ultrasound findings in congenital diaphragmatic hernia. Eur. J. Pediatr. 2019, 178, 491–495. [Google Scholar] [CrossRef]
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; Mckean, M.; Thomson, A. British Thoracic society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 2011, 66, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.; Byington, C.; Shah, S. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, 617–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balk, D.S.; Lee, C.; Schafer, J.; Welwarth, J.; Hardin, J.; Novack, V.; Yarza, S.; Hoffmann, B. Lung ultrasound compared to chest-X-ray for diagnosis of pediatric pneumonia: A meta-analysis. Pediatr. Pulmonol. 2018, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Andronikou, S.; Zar, H. Lung ultrasound for the Diagnosis of Community-Acquired Pneumonia in children. Pediatr. Radiol. 2017, 47, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supino, M.C.; Buonsenso, D.; Scateni, S.; Scialanga, B.; Mesturino, M.A.; Bock, C.; Chiaretti, A.; Giglioni, E.; Reale, A.; Musolino, A.M. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: A prospective study. Eur. J. Pediatr. 2019, 178, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Campaña, M.; Sainz, T.; Alba, M.; Del Rosal, T.; Mendez-Echevarría, A.; Echevarria, R.; Tagarro, A.; Ruperez-Lucas, M.; Herrreros, M.L.; Latorre, L.; et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: A cohort study. Pediatr. Pulmonol. 2019, 54, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Komorowska-Piotrowska, A.; Pomiećko, A.; Wiśniewski, J.; Woźniak, M.; Littwin, B.; Kryger, M.; Kwaśniewicz, P.; Szczyrski, J.; Kulińska-Szukalska, K.; et al. Consensus on the Application of Lung Ultrasound in Pneumonia and Bronchiolitis in Children. Diagnostics 2020, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Møller-Sørensen, H.; Gjedsted, J.; Jørgensen, V.L.; Hansen, K.L. COVID-19 Assessment with Bedside Lung Ultrasound in a Population of Intensive Care Patients Treated with Mechanical Ventilation and ECMO. Diagnostics 2020, 10, 447. [Google Scholar] [CrossRef]
- Urbankowska, E.; Krenke, K.; Drobczyński, Ł.; Korczyński, P.; Urbankowski, T.; Krawiec, M.; Kraj, G.; Brzewski, M.; Kulus, M. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir. Med. 2015, 109, 1207–1212. [Google Scholar] [CrossRef] [Green Version]
- Reissig, A.; Copetti, R.; Mathis, G.; Mempel, C.; Schuler, A.; Zechner, P.; Aliberti, S.; Neumann, R.; Kroegel, C.; Hoyer, H. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: A prospective, multicenter, diagnostic accuracy study. Chest 2012, 142, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonsenso, D.; Brancato, F.; Valentini, P.; Curatola, A.; Supino, M.; Musolino, A.M. The Use of Lung Ultrasound to Monitor the Antibiotic Response of Community-Acquired Pneumonia in Children: A Preliminary Hypothesis. J. Ultrasound Med. 2020, 39, 817–826. [Google Scholar] [CrossRef]
- Berce, V.; Tomazin, M.; Gorenjak, M. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci. Rep. 2019, 29, 17957. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, S.; Duggan, N.; Cohen, A. Lung Ultrasound in Children with Respiratory Tract Infections: Viral, Bacterial or COVID-19? A Narrative Review. Open Access Emerg. Med. 2020, 12, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Bloise, S.; La Regina, D.; Pepino, D.; Iovine, E.; Laudisa, M.; Di Mattia, G.; Nicolai, A.; Nenna, R.; Petrarca, L.; Mancino, E.; et al. Lung ultrasound compared to chest X-ray for the diagnosis of CAP in children. Pediatr. Int. 2020. [Google Scholar] [CrossRef]
- Claes, A.-S.; Clapuyt, P.; Menten, R.; Michoux, N.; Dumitriu, D. Performance of chest ultrasound in pediatric pneumonia. Eur. J. Radiol. 2017, 88, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E. Lung ultrasound in bronchiolitis: Comparison with chest X-ray. Eur. J. Pediatr. 2011, 170, 1427–1433. [Google Scholar] [CrossRef]
- La Regina, D.P.; Bloise, S.; Pepino, D.; Iovine, E.; Laudisa, M.; Cristiani, L.; Nicolai, A.; Nenna, R.; Mancino, E.; Di Mattia, G.; et al. Lung ultrasound in bronchiolitis. Pediatr. Pulmonol. 2021, 56, 234–239. [Google Scholar] [CrossRef]
- Özkaya, A.; Yilmaz, H.; Kendir, Ö.; Gökay, S.S.; Eyüboğlu, İ. Lung Ultrasound Findings and Bronchiolitis Ultrasound Score for Predicting Hospital Admission in Children with Acute Bronchiolitis. Pediatr. Emerg. Care 2020, 36. [Google Scholar] [CrossRef]
- Dahmarde, H.; Parooie, F.; Salarzaei, M. Accuracy of Ultrasound in Diagnosis of Pneumothorax: A Comparison between Neonates and Adults-A Systematic Review and Meta-Analysis. Can. Respir. J. 2019, 2019, 5271982. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, D.; Mezière, G.; Biderman, P.; Gepner, A. The “lung point”: An ultrasound sign specific to pneumothorax. Intensive Care Med. 2000, 26, 1434–1440. [Google Scholar] [CrossRef]
- Boo, N.; Cheah, I. Malaysian National Neonatal Registry. Risk factors associated with pneumothorax in Malaysian neonatal intensive care units. J. Paediatr. Child Health 2011, 47, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Shigueoka, D.; Atallah, A.N.; Ajzen, S.; Iared, W. Diagnostic accuracy of sonography for pleural effusion: Systematic review. Sao Paulo Med. J. 2010, 128, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Soni, N.J.; Franco, R.; Velez, M.I.; Schnobrich, D.; Dancel, R.; Restrepo, M.I.; Mayo, P.H. Ultrasound in the diagnosis and management of pleural effusions. J. Hosp. Med. 2015, 10, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prina, E.; Torres, A.; Carvalho, C. Lung ultrasound in the evaluation of pleural effusion. J. Bras. Pneumol. 2014, 40, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kraft, C.; Lasure, B.; Sharon, M.; Patel, P.; Minardi, J. Pediatric Lung Abscess Immediate Diagnosis by Point-of-Care Ultrasound. Pediatr. Emerg. Care 2018, 34, 447–449. [Google Scholar] [CrossRef]
- Lin, F.; Chou, C.; Chang, S. Differentiating pyopneumothorax and peripheral lung abscess: Chest ultrasonography. Am. J. Med. Sci. 2004, 327, 330–335. [Google Scholar] [CrossRef]
- Calder, A.; Owens, C.M. Imaging of parapneumonic pleural effusions and empyema in children. Pediatr. Radiol. 2009, 39, 527–537. [Google Scholar] [CrossRef]
- Svigals, P.; Chopra, A.; Ravenel, J.; Nietert, P.J.; Huggins, J.T. The accuracy of pleural ultrasonography in diagnosing complicated parapneumonic pleural effusions. Thorax 2017, 72, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Yu, Y.H.; Tu, C.Y.; Chen, C.H.; Hsia, T.C.; Tsai, K.D.; Shih, C.M.; Hsu, W.H. Ultrasound in peripheral pulmonary air-fluid lesions. Color doppler imaging as an aid in differentiating empyema and abscess. Chest 2009, 135, 1426–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedictis, F.; Kerem, E.; Chang, A.B.; Colin, A.A.; Zar, H.J.; Bush, A. Complicated pneumonia in children. Lancet 2020, 396, 786–798. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M.; Abrahamson, E.; Cohen, G.; Hartley, J.; King, S.; Parikh, D.; Spencer, D.; Thomson, A.H.; Urquhart, D. Paediatric Pleural Diseases Subcommittee of the BTS Standards of Care Committee. BTS guidelines for the management of pleural infection in children. Thorax 2005, 60, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M.; Micic, T.; Doull, I.J.M.; Evans, A. Real-time ultrasound-guided pigtail catheter chest drain for complicated parapneumonic effusion and empyema in children—16-year, single-centre experience of radiologically placed drains. Pediatr. Radiol. 2018, 48, 1410–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT imaging features of 2019 novel coronavirus (2019 nCoV). Radiology 2020, 4, 200–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isoldi, S.; Mallardo, S.; Marcellino, A.; Bloise, S.; Dilillo, A.; Iorfida, D.; Testa, A.; Del Giudice, E.; Martucci, V.; Sanseviero, M.; et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: A 6-months prospective study. J. Med. Virol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among children in China. Pediatrics 2020, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonsenso, D.; Pata, D.; Chiaretti, A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir. Med. 2020, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Li, Q.; Du, H.; Kang, W.; Lian, J.; Yuan, L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit. Care 2020, 24, 174. [Google Scholar] [CrossRef]
- Musolino, A.M.; Supino, M.C.; Buonsenso, D.; Ferro, V.; Valentini, P.; Magistrelli, A.; Lombardi, M.H.; Romani, L.; D’Argenio, P.; Campana, A. Roman Lung Ultrasound Study Team for Pediatric COVID-19 (ROMULUS COVID Team). Lung Ultrasound in Children with COVID-19: Preliminary Findings. Ultrasound Med. Biol. 2020, 46, 2094–2098. [Google Scholar] [CrossRef]
- Musolino, A.M.; Supino, M.C.; Buonsenso, D.; Papa, R.E.; Chiurchiù, S.; Magistrelli, A.; Barbieri, M.A.; Raponi, M.; D’Argenio, P.; Villani, A.; et al. Lung ultrasound in the diagnosis and monitoring of 30 children with coronavirus disease 2019. Pediatric Pulmonol. 2021, 6. [Google Scholar] [CrossRef]
- Norbedo, S.; Blaivas, M.; Raffaldi, I.; Caroselli, C. Lung Ultrasound Point-of-View in Pediatric and Adult COVID-19 Infection. J. Ultrasound Med. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Frassi, F.; Soldati, G.; Tesorio, P.; Gheorghiade, M.; Picano, E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: A comparison with natriuretic peptides. Eur. J. Heart Fail. 2008, 10, 70–77. [Google Scholar] [CrossRef]
- Vitturi, N.; Soattin, M.; Allemand, E.; Simoni, F.; Realdi, G. Thoracic ultrasonography: A new method for the work-up of patients with dyspnea. J. Ultrasound 2011, 14, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef]
- Picano, E.; Pellikka, P. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur. Heart J. 2016, 37, 2097–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongwaisayawan, S.; Suwannanon, R.; Prachanukool, T.; Sricharoen, P.; Saksobhavivat, N.; Kaewlai, R. Trauma Ultrasound. Ultrasound Med. Biol. 2015, 41, 2543–2561. [Google Scholar] [CrossRef]
- Flato, U.; Guimarães, H.; Lopes, R.D.; Valiatti, J.L.; Flato, E.M.; Lorenzo, R.G. Usefulness of Extended-FAST (EFAST-Extended Focused Assessment with Sonography for Trauma) in critical care setting. Rev. Bras. Ter. Intensiva 2010, 22, 291–299. [Google Scholar] [CrossRef]
- Staub, L.; Biscaro, R.; Kaszubowski, E.; Maurici, R. Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and haemothorax: A systematic review and meta-analysis. Injury 2018, 49, 457–466. [Google Scholar] [CrossRef]
- Yousefifard, M.; Baikpour, M.; Ghelichkhani, P.; Asady, H.; Darafarin, A.; Esfahani, M.R.A.; Hosseini, M.; Yaseri, M.; Safari, S. Comparison of ultrasonography and radiography in detection of thoracic bone fractures; a systematic review and meta-analysis. Emergency 2016, 4, 55–64. [Google Scholar]
- Bloise, S.; Martucci, V.; Marcellino, A.; Mallardo, S.; Lubrano, R. Possible Role of Thoracic Ultrasound in the Diagnostic Pathway of Infant Abuse in the Pediatric Emergency Department. J. Ultrasound Med. 2020, 24. [Google Scholar] [CrossRef]
- Battle, C.; Hayward, S.; Eggert, S.; Evans, P.A. Comparison of the use of lung ultrasound and chest radiography in the diagnosis of rib fracture: A systematic review. Emerg. Med. J. 2019, 36, 185–190. [Google Scholar] [CrossRef]
- Song, I.; Kim, E.; Lee, J.; Kang, P.; Kim, H.S.; Kim, J.T. Utility of perioperative lung ultrasound in pediatric cardiac surgery: A randomized controlled trial. Anesthesiology 2018, 128, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Townsley, M. Lung Ultrasound in Pediatric Cardiac Surgery: A Complementary Tool for Predicting and Identifying Postoperative Pulmonary Complications. J. Cardiothorac. Vasc. Anesth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Migliaro, F.; Sodano, A.; Umbaldo, A.; Romano, A.; Vallone, G.; Capasso, L. Can neonatal ultrasound monitor fluid clearence and preditc the need of respiratory support? Crit. Care 2012, 16, 220. [Google Scholar] [CrossRef] [Green Version]
- Vitale, V.; Ricci, Z.; Gaddi, S.; Testa, G.; Toma, P.; Cogo, P. Lung ultrasound profile after cardiopulmonary bypass in paediatric cardiac surgery: First experience in a simple cohort. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Cantinotti, M.; Giordano, R.; Volpicelli, G.; Kutty, S.; Murzi, B.; Assanta, N.; Gargani, L. Lung ultrasound in adult and paediatric cardiac surgery: Is it time for routine use? Interact. Cardiovasc. Thorac. Surg. 2016, 22, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Vitale, V.; Ricci, Z.; Cogo, P. Lung ultrasonography and pediatric cardiac surgery: First experience with a new tool for postoperative lung complications. Ann. Thorac. Surg. 2014, 97, e121–e124. [Google Scholar] [CrossRef]
- Polito, A.; Biasucci, D.; Cogo, P. Point-of-care pleural and lung ultrasound in a newborn suffering from cardiac arrest due to tension pneumothorax after cardiac surgery. Cardiol. Young 2016, 26, 400–402. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Assanta, N.; Murzi, B.; Gargani, L. Chest Ultrasound: A New, Easy, and Radiation-Free Tool to Detect Retrosternal Clot after Pediatric Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, 59–60. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wu, L.; Zhang, K.; Tan, R.; Bai, J.; Zhang, M.; Zheng, J. Lung ultrasound evaluation of incremental PEEP recruitment maneuver in children undergoing cardiac surgery. Pediatr. Pulmonol. 2020, 55, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Sferrazza Papa, G.; Pellegrino, G.; Di Marco, F.; Imeri, G.; Brochard, L.; Goligher, E.; Centanni, S. A Review of the Ultrasound Assessment of Diaphragmatic Function in Clinical Practice. Respiration 2016, 91, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, Z.; Sheng, C.-Q.; Li, Y.-M.; Jia, F.-Y. The predictive value of diaphragm ultrasound for weaning outcomes in critically ill children. BMC Pulm. Med. 2019, 19, 270. [Google Scholar] [CrossRef] [Green Version]
- Buonsenso, D.; Supino, M.C.; Giglioni, E. Point of care diaphragm ultrasound in infants with bronchiolitis: A prospective study. Pediatr. Pulmonol. 2018, 53, 778–786. [Google Scholar] [CrossRef]
- Moshavegh, R.; Hansen, K.L.; Moller-Sorensen, H.; Nielsen, M.B.; Jensen, J.A. Automatic Detection of B-Lines in In Vivo Lung Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019, 66, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, X.; Zhang, X.; Curran, W.J.; Liu, T. Ultrasound Elastography for Lung Disease Assessment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2020, 67, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Osborn, T.; Zhou, B.; Brian Bartholmai, B.; Greenleaf, J.F.; Kalra, S. An ultrasound surface wave elastography technique for noninvasive measurement of surface lung tissue. J. Acoust. Soc. Am. 2017, 141, 3721. [Google Scholar] [CrossRef]
- Zhang, X.; Osborn, T.; Zhou, B.; Meixner, D.; Kinnick, R.R.; Bartholmai, B.; Greenleaf, J.F.; Kalra, S. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1298–1304. [Google Scholar] [CrossRef]
- Clay, R.; Bartholmai, B.; Zhou, B.; Karwoski, R.; Peikert, T.; Osborn, T.; Rajagopalan, S.; Kalra, S.; Zhang, X. Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography: A Novel Technique with Clinicoradiologic Correlates. J. Thorac. Imaging 2019, 34, 313–319. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovine, E.; Nenna, R.; Bloise, S.; La Regina, D.P.; Pepino, D.; Petrarca, L.; Frassanito, A.; Lubrano, R.; Midulla, F. Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases. Diagnostics 2021, 11, 652. https://doi.org/10.3390/diagnostics11040652
Iovine E, Nenna R, Bloise S, La Regina DP, Pepino D, Petrarca L, Frassanito A, Lubrano R, Midulla F. Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases. Diagnostics. 2021; 11(4):652. https://doi.org/10.3390/diagnostics11040652
Chicago/Turabian StyleIovine, Elio, Raffaella Nenna, Silvia Bloise, Domenico Paolo La Regina, Daniela Pepino, Laura Petrarca, Antonella Frassanito, Riccardo Lubrano, and Fabio Midulla. 2021. "Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases" Diagnostics 11, no. 4: 652. https://doi.org/10.3390/diagnostics11040652
APA StyleIovine, E., Nenna, R., Bloise, S., La Regina, D. P., Pepino, D., Petrarca, L., Frassanito, A., Lubrano, R., & Midulla, F. (2021). Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases. Diagnostics, 11(4), 652. https://doi.org/10.3390/diagnostics11040652






