Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Image Acquisition
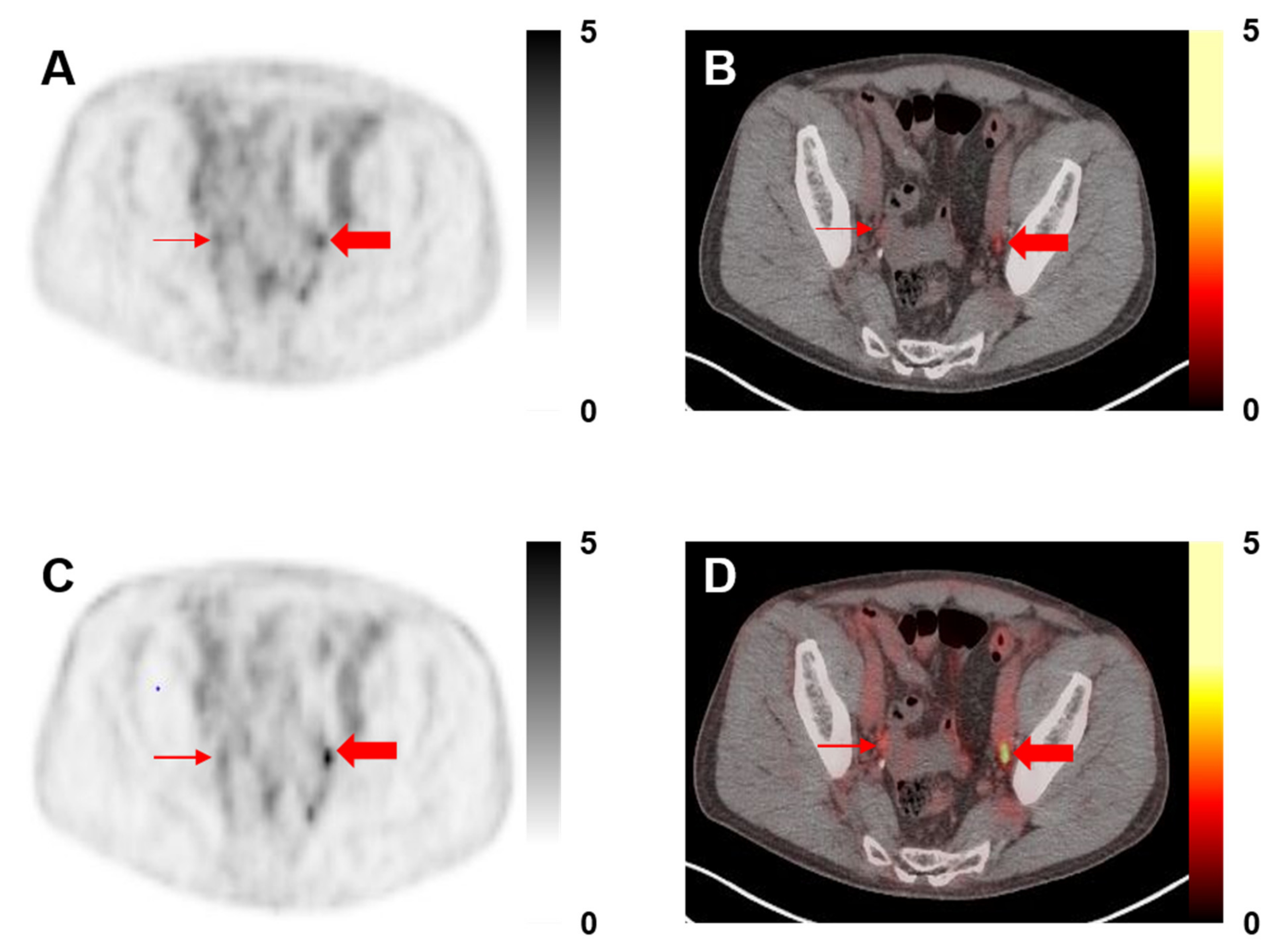
2.3. Image Analysis
- Follow-up PET/CT imaging with 18F-DCFPyL with significantly increasing or decreasing uptake (defined as a change of 30% from baseline) after therapy OR significantly increased uptake during observation. If the follow-up PET/CT was performed with 18F-fluciclovine, focal uptake in a lesion in concordance with the read paradigm [14] for that agent was taken as evidence of true positivity.
- Anatomic imaging with CT or MRI demonstrating a ≥2 mm increase in the long axis diameter of the lesion during a period of observation or a decrease in the lesion long axis diameter of ≥2 mm after beginning PCa treatment.
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Sweeney, C.J.; Tombal, B. “Gotta catch ’em all”, or do we? Pokemet approach to metastatic prostate cancer. Eur. Urol. 2017, 72, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Gorin, M.A.; Allaf, M.E.; Pienta, K.J.; Tran, P.T.; Pomper, M.G.; Ross, A.E.; Cho, S.Y. PET imaging of prostate-specific membrane antigen in prostate cancer: Current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016, 19, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; de Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET imaging: PSMA-RADS version 1.0. J. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. PSMA-RADS version 1.0: A step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur. Urol. 2018, 73, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Werner, R.A.; Higuchi, T.; Lapa, C.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Rowe, S.P. Follow-up of lesions with equivocal radiotracer uptake on PSMA-targeted PET in patients with prostate cancer: Predictive values of the PSMA-RADS-3A and PSMA-RADS-3B categories. J. Nucl. Med. 2019, 60, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Bundschuh, R.A.; Bundschuh, L.; Javadi, M.S.; Leal, J.P.; Higuchi, T.; Pienta, K.J.; Buck, A.K.; Pomper, M.G.; Gorin, M.A.; et al. Interobserver agreement for the standardized reporting system PSMA-RADS 1.0 on 18F-DCFPyL PET/CT imaging. J. Nucl. Med. 2018, 59, 1857–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, F.L.; Klausen, T.L.; Loft, A.; Beyer, T.; Holm, S. Clinical evaluation of PET image reconstruction using a spatial resolution model. Eur. J. Radiol. 2013, 82, 862–869. [Google Scholar]
- Ravert, H.T.; Holt, D.P.; Chen, Y.; Mease, R.C.; Fan, H.; Pomper, M.G.; Dannals, R.F. An improved synthesis of the radiolabeled prostate-specific membrane antigen inhibitor, [18F]DCFPyL. J. Labelled Comp. Radiopharm. 2016, 59, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, S.P.; Gorin, M.A.; Hammers, H.J.; Javadi, M.S.; Hawasli, H.; Szabo, Z.; Cho, S.Y.; Pomper, M.G.; Allaf, M.E. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2015, 29, 877–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, D.M. Axumin™ (Fluciclovine F 18) Image Interpretation Training. Available online: https://www.snmmilearningcenter.org/lms/activity?@curriculum.id=-1&@activity.id=4521746&@activity.bundleActivityId=-1 (accessed on 10 February 2021).
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Hofman, M.; Lawrentschuk, N.; Maurer, T. Bringing clarity or confusion? The role of prostate-specific membrane antigen positron-emission/computed tomography for primary staging in prostate cancer. BJU Int. 2017, 119, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of observation vs. stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazemi, S.; Khurshid, Z.; Erle, A.; Lütje, S.; Essler, M.; Schultz, T.; Bundschuh, R.A. Machine learning facilitates hotspot classificiation in PSMA-PET/CT with nuclear medicine specialist accuracy. Diagnostics 2020, 10, 622. [Google Scholar] [CrossRef] [PubMed]


| Therapy | Percent |
|---|---|
| Pre-Scan Therapy | |
| - Prostatectomy | 70% |
| - Radiation | 36% |
| - Salvage Radiation | 30% |
| - Brachytherapy | 3% |
| - Cryoablation | 3% |
| - ADT | 30% |
| - Salvage PLND | 3% |
| - None | 13% |
| Post-Scan Therapy | |
| - Prostatectomy | 7% |
| - Cryoablation | 3% |
| - Radiation | 23% |
| - ADT | 60% |
| - Salvage PLND | 7% |
| - Chemotherapy | 20% |
| - Observation | 13% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatri, W.; Chung, H.W.; Werner, R.A.; Leal, J.P.; Pienta, K.J.; Lodge, M.A.; Gorin, M.A.; Pomper, M.G.; Rowe, S.P. Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer. Diagnostics 2021, 11, 665. https://doi.org/10.3390/diagnostics11040665
Khatri W, Chung HW, Werner RA, Leal JP, Pienta KJ, Lodge MA, Gorin MA, Pomper MG, Rowe SP. Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer. Diagnostics. 2021; 11(4):665. https://doi.org/10.3390/diagnostics11040665
Chicago/Turabian StyleKhatri, Wajahat, Hyun Woo Chung, Rudolf A. Werner, Jeffrey P. Leal, Kenneth J. Pienta, Martin A. Lodge, Michael A. Gorin, Martin G. Pomper, and Steven P. Rowe. 2021. "Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer" Diagnostics 11, no. 4: 665. https://doi.org/10.3390/diagnostics11040665
APA StyleKhatri, W., Chung, H. W., Werner, R. A., Leal, J. P., Pienta, K. J., Lodge, M. A., Gorin, M. A., Pomper, M. G., & Rowe, S. P. (2021). Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer. Diagnostics, 11(4), 665. https://doi.org/10.3390/diagnostics11040665






