Signature of Circulating Biomarkers in Recurrent Non-Infectious Anterior Uveitis. Immunomodulatory Effects of DHA-Triglyceride. A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Screening Procedures
2.2.1. Selection of Participants
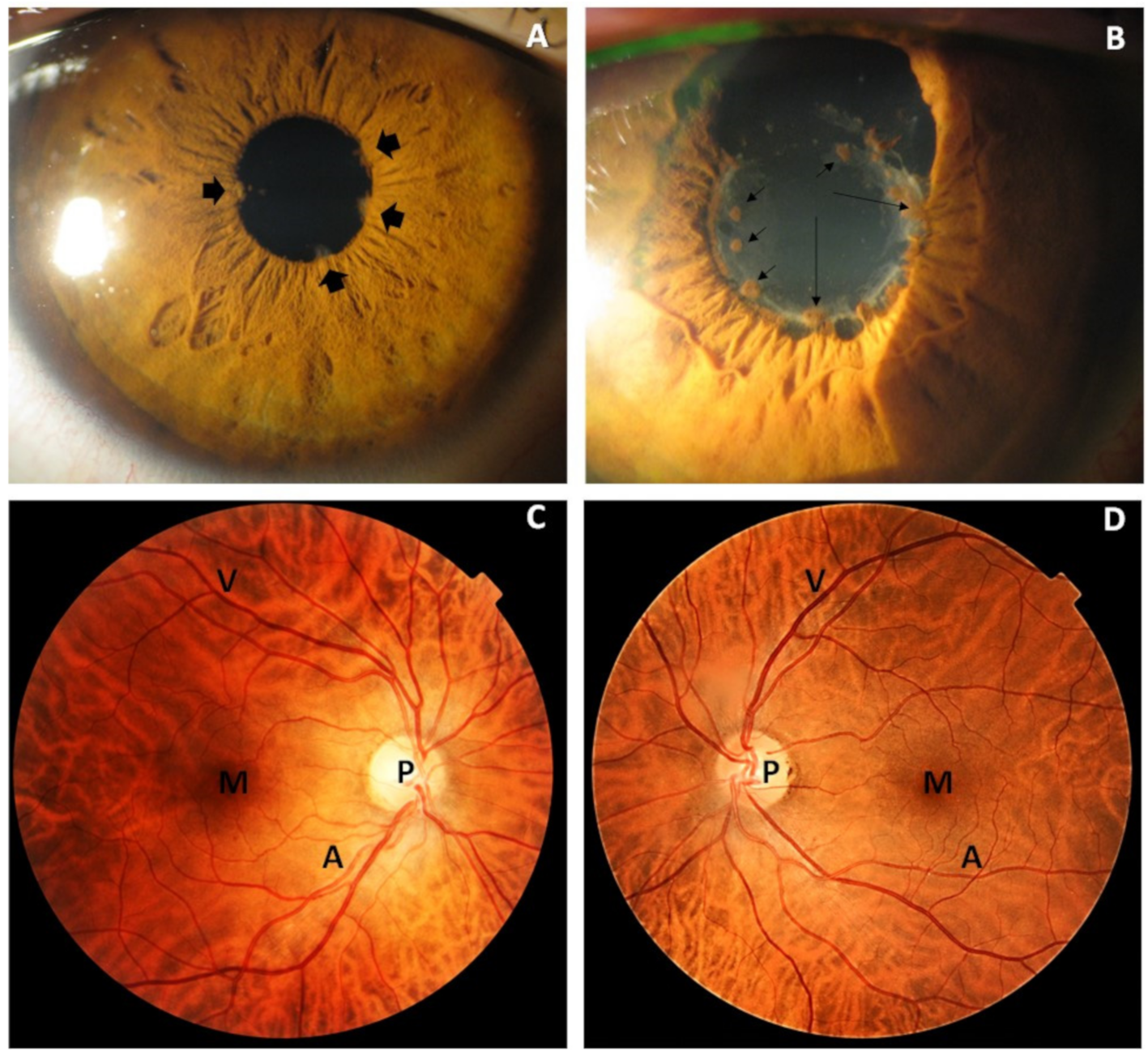
2.2.2. Ophthalmic Examination
2.2.3. DHA-TG Supplementation Study
2.2.4. Sampling Procedures
2.2.5. Multiplex Determination of Plasmatic Cytokines
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| B cells | Bone marrow lymphocytes |
| BMD | Biomicroscopy |
| BV-2 cells | Immortalized murine microglial cell line BV-2 |
| CG | Control group |
| g | Gravitation force of centrifugation |
| GM-CSF | Granulocyte macrophage colony stimulating factor |
| DHA | Docosahexaenoic acid |
| DHA-TG | Enzymatically re-esterified DHA as DHA–triglyceride |
| DNA | Deoxyribonucleic acid |
| DR | Diabetic retinopathy |
| EDTA | Ethylene diamine tetra acetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| IL | Interleukin |
| INFγ | Interferon gamma |
| IOP | Intraocular pressure |
| LPS | Lipopolysaccharide |
| miRNAs | micro RNAs |
| NIU | Non-infectious uveitis |
| NIAU | Non-infectious anterior uveitis |
| OHT | Ocular hypertension |
| OSD | Ocular surface disease/disorder |
| POAG | Primary open-angle glaucoma |
| RNA | Ribonucleic acid |
| RPE | Retinal pigment epithelium |
| PUFA | Polyunsaturated fatty acid |
| PVR | Proliferative vitreoretinopathy |
| SUN | The Standardization of Uveitis Nomenclature working group |
| TG | Triglyceride |
| T cells | Thymic lymphocytes |
| Th cells | T-helper cells/CD4+ cells |
| TNFα | Tumor necrosis factor alpha |
| UG | Uveitis group |
| VEGF | Vascular endothelial growth factor |
| ω3 | Omega 3 |
References
- Miserocchi, E.; Fogliato, G.; Modorati, G.; Bandello, F. Review on the worldwide epidemiology of uveitis. Eur. J. Ophthalmol. 2013, 23, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; for the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar]
- Gallego-Pinazo, R.; Dolz-Marco, R.; Martínez-Castillo, S.; Arévalo, J.F.; Díaz-Llopis, M. Update on the principles and novel local and systemic therapies for the treatment of non-infectious uveitis. Inflamm. Allergy Drug Targets 2013, 12, 38–45. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, P.J.; Towler, H.M.; Lightman, S. Management of chronic uveitis. BMJ 2000, 320, 555–558. [Google Scholar] [CrossRef]
- Agrawal, R.V.; Murthy, S.; Sangwan, V.; Biswas, J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010, 58, 11–19. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Gallego-Pinazo, R.; Díaz-Llopis, M.; Cunningham, E.T., Jr.; Arévalo, J.F. Noninfectious uveitis: Strategies to optimize treatment compliance and adherence. Clin. Ophthalmol. 2015, 9, 1477–1481. [Google Scholar] [PubMed] [Green Version]
- Pan, J.; Kapur, M.; McCallum, R. Noninfectious immune-mediated uveitis and ocular inflammation. Curr Allergy. Asthma Rep. 2014, 14, 409. [Google Scholar] [CrossRef]
- Thorne, J.E.; Skup, M.; Tundia, N.; Macaulay, D.; Revol, C.; Chao, J.; Joshi, A.; Dick, A.D. Direct and indirect resource use, healthcare costs and work force absence in patients with non-infectious intermediate, posterior or panuveitis. Acta Ophthalmologica. 2016, 94, e331–e339. [Google Scholar] [CrossRef] [Green Version]
- Curnow, S.J.; Murray, P.I. Inflammatory mediators of uveitis: Cytokines and chemokines. Curr. Opin. Ophthalmol. 2007, 17, 532–537. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Pepple, K.L. Cytokines in uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Balamurugan, S.; Das, D.; Hasanreisoglu, M.; Toy, B.C.; Akhter, M.; Anuradha, V.K.; Anthony, E.; Gurnani, B.; Kaur, K. Interleukins and cytokine biomarkers in uveitis. Indian J. Ophthalmol. 2020, 68, 1750–1763. [Google Scholar] [CrossRef]
- Diedrichs-Möhring, M.; Kaufmann, U.; Wildner, G. The immunopathogenesis of chronic and relapsing autoimmune uveitis-Lessons from experimental rat models. Prog. Retin. Eye Res. 2018, 65, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Galbis-Estrada, C.; Pinazo-Durán, M.D.; Cantú-Dibildox, J.; Marco-Ramírez, C.; Díaz-Llópis, M.; Benítez-del-Castillo, J. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin. Interv. Aging 2013, 8, 711–719. [Google Scholar] [PubMed] [Green Version]
- Pinazo-Durán, M.D.; Galbis-Estrada, C.; Pons-Vázquez, S.; Cantú-Dibildox, J.; Marco-Ramírez, C.; Benítez-del-Castillo, J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin. Int. Aging 2013, 8, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Ribelles, A.; Galbis-Estrada, C.; Parras, M.A.; Vivar-Llopis, B.; Marco-Ramírez, C.; Diaz-Llopis, M. Ocular Surface and Tear Film Changes in Older Women Working with Computers. BioMed. Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Senni, C.; Bernabei, F.; Cicero, A.F.G.; Vagge, A.; Maestri, A.; Scorcia, V.; Giannaccare, G. The Role of Nutrition and Nutritional Supplements in Ocular Surface Diseases. Nutrients 2020, 12, 952. [Google Scholar] [CrossRef] [Green Version]
- Al Owaifeer, A.M.; Al Taisan, A.A. The Role of Diet in Glaucoma: A Review of the Current Evidence. Ophthalmol. Ther. 2018, 7, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Mangayarkarasi T, Putt D, Gierhart DL. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Sanz-González, S.M.; García-Medina, J.J.; Zanón-Moreno, V.; López-Gálvez, M.I.; Galarreta-Mira, D.; Duarte, L.; Valero-Velló, M.; Ramírez, A.I.; Arévalo, J.F.; Pinazo-Durán, M.D.; et al. Clinical and Molecular-Genetic Insights into the Role of Oxidative Stress in Diabetic Retinopathy: Antioxidant Strategies and Future Avenues. Antioxidants 2020, 9, 1101. [Google Scholar] [CrossRef]
- Goldberg, J.; Flowerdew, G.; Smithe, E.; Brody, J.A.; Tso, M.O.M. Factors associated with age-related macular degeneration. An analysis of data from the first national health and nutrition examination survey (NHANES). Am. J. Epidemiol. 1988, 128, 700–710. [Google Scholar] [CrossRef]
- Chapman, N.A.; Jacobs, R.J.; Braakhuis, A.J. Role of diet and food intake in age-related macular degeneration: A systematic review. Clin. Exp. Ophthalmol. 2019, 47, 106–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance-A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Horman, T.; Fernandes, M.F.; Tache, M.C.; Hucik, B.; Mutch, D.M.; Leri, F. Dietary n-6/n-3 Ratio Influences Brain Fatty Acid Composition in Adult Rats. Nutrients 2020, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Valls-Pedret, C.; Cofán, M.; Sabaté, J.; Serra-Mir, M.; Pérez-Heras, A.M.; Arechiga, A.; Casaroli-Marano, R.P.; Alforja, S.; Sala-Vila, A.; et al. The Walnuts and Healthy Aging Study (WAHA): Protocol for a Nutritional Intervention Trial with Walnuts on Brain Aging. Front. Aging Neurosci. 2017, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Freitas-Simoes, T.M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Corella, D.; Asensio, E.M.; Serra-Mir, M.; Roth, I.; Calvo, C.; et al. The red blood cell proportion of arachidonic acid relates to shorter leukocyte telomeres in Mediterranean elders: A secondary analysis of a randomized controlled trial. Clin. Nutr. 2019, 38, 958–961. [Google Scholar] [CrossRef]
- Mun, J.G.; Legette, L.L.; Ikonte, C.J.; Mitmesser, S.H. Choline and DHA in Maternal and Infant Nutrition: Synergistic Implications in Brain and Eye Health. Nutrients 2019, 11, 1125. [Google Scholar] [CrossRef] [Green Version]
- Rask, D.M.G.; Puntel, M.R.; Patzkowski, J.C.; Patzkowski, M.S. Multivitamin Use in Enhanced Recovery after Surgery Protocols: A Cost Analysis. Mil. Med. 2020. [Google Scholar] [CrossRef]
- Silver, H.J. Oral strategies to supplement older adults’ dietary intakes: Comparing the evidence. Nutr. Rev. 2009, 67, 21–31. [Google Scholar] [CrossRef]
- Díaz-Marsá, M.; Alberdi-Páramo, I.; Niell-Galmés, L. Nutritional supplements in eating disorders. Actas Esp. Psiquiatr. 2017, 45, 26–36. [Google Scholar] [PubMed]
- Park, J.; Yoo, Y.S.; Shin, E.; Han, G.; Shin, K.; Hui Lim, D.; Chung, T.Y. Effects of the re-esterified triglyceride (rTG) form of omega-3 supplements on dry eye following cataract surgery. Br. J. Ophthalmol. 2020, 10, 1–6. [Google Scholar]
- Mancera, P.; Wappenhans, B.; Cordobilla, B.; Virgili, N.; Pugliese, M.; Rueda, F.; Espinosa-Parrilla, J.F. Domingo-Pedrol, J.C. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients 2017, 9, 681. [Google Scholar] [CrossRef]
- Villadoniga, S.; Rodriguez Garcia, E.; Sagastagoia Epelde, O.; Alvarez Diaz, M.D.; Domingo-Pedrol, J.C. Effects of oral supplementation with docosahexaenoic Acid (DHA) plus antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.E.; González-Herrero, M.; Ruiz, M.; López Román, F.J.; Sánchez, J.M.M.; Domingo Pedrol, J.C. Supplementation with a highly concentrated docosahexaenoic acid plus xanthophyll carotenoid multivitamin in nonproliferative diabetic retinopathy: Prospective controlled study of macular function by fundus microperimetry. Clin. Ophthalmol. 2018, 12, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Lafuente, M.; Ortín, L.; Argente, M.; Guindo, J.L.; López-Bernal, M.D.; López-Román, F.J.; Domingo-Pedrol, J.C.; Lajara, J. Three-year outcomes in a randomized single-blind controlled trial of intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema. Retina 2019, 39, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; López-Román, F.J.; Miñarro, J.; Contreras, C.; Soto-Méndez, F.; Domingo Pedrol, J.C.; Luque-Rubia, A.J. Supplementation of Re-Esterified Docosahexaenoic and Eicosapentaenoic Acids Reduce Inflammatory and Muscle Damage Markers after Exercise in Endurance Athletes: A Randomized, Controlled Crossover Trial. Nutrients 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar]
- Figueiredo-Braga, M.; Mota-Garcia, F.; O’Connor, J.E.; Garcia, J.R.; Mota-Cardoso, R.; Cardoso, C.S.; de Sousa, M. Cytokines and anxiety in systemic lupus erythematosus (SLE) patients not receiving antidepressant medication: A little-explored frontier and some of its brief history. Ann. N. Y. Acad. Sci. 2009, 1173, 286–291. [Google Scholar] [CrossRef]
- De Jager, W.; Bourcier, K.; Rijkers, G.T.; Prakken, B.J.; Seyfert-Margolis, V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Altara, R.; Manca, M.; Hermans, K.C.M.; Daskalopoulos, E.P.; Rocca, H.-P.B.-L.; Hermans, R.J.J.; Struijker-Boudier, H.A.J.; Blankesteijn, M.W. Diurnal rhythms of serum and plasma cytokine profiles in healthy elderly individuals assessed using membrane based multiplexed immunoassay. J. Transl. Med. 2015, 13, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchtey, J.; Rezaei, K.A.; Jaru-Ampornpan, P.; Sternberg, P., Jr.; Kuchtey, R.W. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Invest Ophthalmol. Vis. Sci. 2010, 51, 6441–6447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: Potentials and pitfalls. Proteom. Clin. Appl. 2015, 9, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Koliha, N.; Wiencek, Y.; Heider, U.; Jüngst, C.; Kladt, N.; Krauthäuser, S.; Johnston, I.C.; Bosio, A.; Schauss, A.; Wild, S. A novel multiplex bead-based platform highlights the diversity of extracellular vesicles. J. Extracell. Ves. 2016, 5, 29975. [Google Scholar] [CrossRef]
- Márquez, A.; Cordero-Coma, M.; Martín-Villa, J.M.; Gorroño-Echebarría, M.B.; Blanco, R.; Díaz Valle, D.; Del Rio, M.J.; Blanco, A.; Olea, J.L.; Cordero, Y.; et al. New insights into the genetic component of non-infectious uveitis through an Immunochip strategy. J. Med. Genet. 2017, 54, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.-L.; Lee, J.S.; Lee, E.Y.; Lee, H.Y.; Yu, H.G. Recurrent anterior uveitis and subsequent incidence of ankylosing spondylitis: A nationwide cohort study from 2002 to 2013. Arthritis Res. Ther. 2018, 20, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Morelle, G.; Gueudry, J.; Uettwiller, F.; Wouters, C.; Bader-Meunier, B.; Robert, M.P.; Monnet, D.; Bodaghi, B.; Grall-lerosey, M.; Quartier, P. Chronic and recurrent non-infectious paediatric-onset uveitis: A French cohort. RMD Open 2019, 5, e000933. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, L.; Newcomb, C.W.; Daniel, E.; Kaçmaz, R.O.; Jabs, D.A.; Levy-Clarke, G.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; Suhler, E.B.; Thorne, J.E.; et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study. Risk of relapse in primary acute anterior uveitis. Ophthalmology 2011, 118, 1911–1915. [Google Scholar] [CrossRef] [Green Version]
- Brydak-Godowska, J.; Moskal, K.; Borkowski, P.K.; Przybyś, M.; Turczyńska, M.; Kęcik, D. A Retrospective Observational Study of Uveitis in a Single Center in Poland with a Review of Findings in Europe. Medical science monitor. Int. Med. J. Exp. Clin. Res. 2018, 24, 8734–8749. [Google Scholar]
- Cimino, L.; Aldigeri, R.; Marchi, S.; Mastrofilippo, V.; Viscogliosi, F.; Coassin, M.; Soldani, A.; Savoldi, L.; De Fanti, A.; Belloni, L.; et al. Changes in patterns of uveitis at a tertiary referral center in Northern Italy: Analysis of 990 consecutive cases. Int. Ophthalmol. 2018, 38, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gritz, D.C.; Wong, I.G. Incidence and prevalence of uveitis in northern california; The Northern california epidemiology of uveitis study. Ophthalmology 2004, 111, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Gritz, D.C.; Schwaber, E.J.; Wong, I.G. Complications of uveitis: The northern california epidemiology of uveitis study. Ocul. Immunol. Inflamm. 2017, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.R.; Tham, V.M.; Esterberg, E.; Borkar, D.S.; Parker, J.V.; Vinoya, A.C.; Uchida, A. Incidence and prevalence of uveitis: Results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013, 131, 1405–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Ben Dhifallah, I.; Borhani-Haghighi, A.; Hamzaoui, A.; Hamzaoui, K. Decreased Level of IL-37 Correlates Negatively with Inflammatory Cytokines in Cerebrospinal Fluid of Patients with Neuro-Behcet’s Disease. Iran J. Immunol. 2019, 16, 299–310. [Google Scholar]
- Foxman, E.F.; Zhang, M.; Hurst, S.D.; Muchamuel, T.; Shen, D.; Wawrousek, E.F.; Chan, C.C.; Gery, I. Inflammatory Mediators in Uveitis: Differential Induction of Cytokines and Chemokines in Th1- Versus Th2-Mediated Ocular Inflammation. J. Immunol. 2002, 168, 2483–2492. [Google Scholar] [CrossRef]
- Takase, H.; Futagami, Y.; Yoshida, T.; Kamoi, K.; Sugita, S.; Imai, Y.; Mochizuki, M. Cytokine Profile in Aqueous Humor and Sera of Patients with Infectious or Noninfectious Uveitis. Inv. Ophthalmol. Vis. Sci. 2006, 47, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Belghith, M.; Bahrini, K.; Kchaou, M.; Maghrebi, O.; Belal, S.; Barbouche, M.R. Cerebrospinal fluid IL-10 as an early stage discriminative marker between multiple sclerosis and neuro-Behçet disease. Cytokine 2018, 108, 160–167. [Google Scholar] [CrossRef]
- Fu, Q.; Man, X.; Wang, X.; Song, N.; Li, Y.; Xue, J.; Sun, Y.; Lin, W. CD83+ CCR7+ NK cells induced by interleukin 18 by dendritic cells promote experimental autoimmune uveitis. J. Cell Mol. Med. 2019, 23, 1827–1839. [Google Scholar] [CrossRef] [Green Version]
- Bonacini, M.; Soriano, A.; Zerbini, A.; Calò, E.; Cimino, L.; Muratore, F.; Fontana, L.; Braglia, L.; Parmeggiani, M.; Salvarani, C.; et al. Higher Frequencies of Lymphocytes Expressing the Natural Killer Group 2D Receptor in Patients With Behçet. Disease Front. Immunol. 2018, 9, 2157. [Google Scholar]
- Dave, N.; Chevour, P.; Mahendradas, P.; Venkatesh, A.; Kawali, A.; Shetty, R.; Ghosh, A.; Sethu, S. Increased Aqueous Humor CD4+/CD8+ Lymphocyte Ratio in Sarcoid Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Marco, E.; Garces, M.; Sempere, A.; Diaz-Llopis, M. CD4/CD8 ratio in aqueous humor in Uveitis. Ocul. Immunol. Inflamm. 2013, 21, 408–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Varki, A. Evolutionary forces shaping the Golgi glycosylation machinery: Why cell surface glycans are universal to living cells. Cold Spring Harb. Perspect Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Kishimoto, T.; Akira, S.; Taga, T. Interleukin-6 and its receptor: A paradigm for cytokines. Science 1992, 258, 593–597. [Google Scholar] [CrossRef]
- Haruta, H.; Ohguro, N.; Fujimoto, M.; Hohki, S.; Terabe, F.; Serada, S.; Nomura, S.; Nishida, K.; Kishimoto, T.; Naka, T. Blockade of interleukin-6 signaling suppresses not only th17 but also interphotoreceptor retinoid binding protein-specific Th1 by promoting regulatory T cells in experimental autoimmune uveoretinitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3264–3271. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Ding, T.; Kuang, D.M.; Zheng, L. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol. Immunol. 2009, 6, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Ogata, A.; Narazaki, M. Tocilizumab: An updated review of its use in the treatment of rheumatoid arthritis and its application for other immune-mediated diseases. Clin. Med. Insights Ther. 2013, 5, 33–52. [Google Scholar] [CrossRef]
- Wong, J.B.; Coates, P.M.; Russell, R.M.; Dwyer, J.T.; Schuttinga, J.A.; Bowman, B.A. Economic Analysis of 710 Nutrition Interventions for Chronic Disease Prevention. Methods Res. Pol. Nutr. Rev. 2011, 69, 533–549. [Google Scholar]
- Webb, D.; Leahy, M.M.; Milner, J.A.; Allison, D.B.; Dodd, K.W.; Gaine, P.C. Strategies to Optimize the 713 Impact of Nutritional Surveys and Epidemiological Studies. Adv. Nutr. 2013, 4, 545–547. [Google Scholar] [CrossRef] [Green Version]
- Boadi-Kusi, S.B.; Asiamah, E.; Ocansey, S.; Abu, S.L. Nutrition knowledge and dietary patterns in 740 ophthalmic patients. Clin. Exp. Optom. 2020. [Google Scholar] [CrossRef]
- Vinetskaia, M.I.; Iomdina, E.N.; Tarutta, E.P.; Kushnarevich, N.I.A.U.; Lazuk, A.V. Significance of lacrimal fluid peroxidation and anti-radical defense parameters for prediction and treatment of complicated myopia. Vestn. Oftalmol. 2000, 116, 54–56. [Google Scholar] [PubMed]
- Lim, J.C.; Caballero Arredondo, M.; Braakhuis, A.J.; Donaldson, P.J. Vitamin C and the Lens: New Insights into Delaying the Onset of Cataract. Nutrients 2020, 12, 3142. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Hong, T.; Bahrami, B.; Flood, V.; Liew, G.; Chang, A.A. Diet and risk of visual impairment: A review of dietary factors and risk of common causes of visual impairment. Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- Kao, Y.W.; Hsu, S.K.; Chen, J.Y.; Lin, I.L.; Chen, K.J.; Lee, P.Y.; Ng, H.S.; Chiu, C.C.; Cheng, K.C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agrón, E.; Mares, J.; Clemons, T.E.; van Asten, F.; Swaroop, A.; Chew, E.Y. Age-Related Eye Disease Studies (AREDS) 1 and 2 Research Groups. Adherence to the Mediterranean Diet and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020, 127, 1515–1528. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Du, X. The therapeutic use of quercetin in ophthalmology: Recent applications. Biomed. Pharmacother. 2021, 137, 111371. [Google Scholar] [CrossRef] [PubMed]
- Christakis, P.G.; Agrón, E.; Klein, M.L.; Clemons, T.E.; Campbell, J.P.; Ferris, F.L.; Chew, E.Y.; Keenan, T.D. Age-Related Eye Diseases Study Research Group. Incidence of Macular Atrophy after Untreated Neovascular Age-Related Macular Degeneration: Age-Related Eye Disease Study Report 40. Ophthalmology 2020, 127, 784–792. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. AREDS and AREDS2 Research Groups. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2012, 75, 645–662. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.L.; Metherel, A.H.; Fiabane, L.; Buddenbaum, N.; Bazinet, R.P.; Shaikh, S.R. Do Eicosapentaenoic Acid and Do-cosahexaenoic Acid Have the Potential to Compete against Each Other? Nutrients 2020, 12, 3718. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, J.E.; Thomas, J.; Bramley, A.L.; Kouris-Blazosa, A.; Radford, B.E.; Scholey, A.B.; Pipingas, A.; Thomas, C.J.; Itsiopoulos, C. Controversies in omega-3 efficacy and novel concepts for application. J. Nutr. Interm. Metab. 2016, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, D.; Verri, M.; Giolitto, A.; Doria, E.; Ghitti, M.; Dossena, M. Effect of 8-week n-3 fatty-acid supplementation on oxidative stress and inflammation in middle- and long-distance running athletes: A pilot study. J. Int. Soc. Sports Nutr. 2020, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, R.; Kroening, P.R.; Parambil, J.; Kita, H. Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses. Mol. Immunol. 2008, 45, 3321–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.S.; Tripathi, V. Smoking under hypoxic conditions: A potent environmental risk factor for inflammatory and autoimmune diseases. Mil. Med. Res. 2018, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Silva, T.; Napoleão, P.; Costa, M.C.; Gabriel, A.F.; Selas, M.; Silva, F.; Enguita, F.J.; Ferreira, R.C.; Carmo, M.M. Cigarette Smoking, miR-27b Downregulation, and Peripheral Artery Disease: Insights into the Mechanisms of Smoking Toxicity. J. Clin. Med. 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Lee, J.H.; Lee, S.Y.; Lee, J.H.; Yu, D.S.; Han, K.D.; Park, Y.G. Association between smoking and Behçet’s disease: A nationwide population-based study in Korea. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2114–2122. [Google Scholar] [CrossRef]
- Costa, E.; Almeida, D.; Cerqueira, M.; Costa, J.R.; Ribeiro, A.R.; Sousa-Neves, J. Smoking as associated factor for spondyloarthritis related uveitis: Results from a single centre cross-sectional study. Acta Reumatol. Port. 2020, 45, 265–269. [Google Scholar]
- Zhao, S.S.; Goodson, N.J.; Robertson, S.; Gaffney, K. Smoking in spondyloarthritis: Unravelling the complexities. Rheumatology 2020, 59, 1472–1481. [Google Scholar] [CrossRef]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Zanon-Viguer, V.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol. Vis. 2009, 15, 2953–2959. [Google Scholar]
- Benito, M.J.; González-García, M.J.; Tesón, M.; García, N.; Fernandez, I.; Calonge, M.; Enríquez-De-Salamanca, A. Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Exp. Eye Res. 2014, 120, 43–49. [Google Scholar] [CrossRef] [PubMed]





| Inclusion | Exclusion |
|---|---|
| Caucasian individuals aged older than 20. | Caucasian individuals over 70 years of age Non-Caucasian individuals. |
| Accurate diagnosis of recurrent NIAU (quiescent stage) for the corresponding uveitis group (UG). | Any other ocular disease than recurrent anterior non-inflammatory uveitis. Patients receiving local treatment that may interfere with the study (including topic nutraceutics). Eye laser/surgery in the previous 12 months. |
| Healthy individuals for the control group of participants (CG). | Patients experiencing any systemic disease, receiving local or systemic treatment that may interfere with the study (including oral nutraceutics). Surgery in the previous 12 months. |
| Individuals without disorders of substance use. | Smoking and/or drinking habits. Drug addiction. |
| Precise and complete data of the medical history. Those who could participate in the study and were able to do so. | History including diagnoses that do not fit with the study purpose. Unfeasibility of having a thorough and complete clinical history. Unable to participate. |
| Cytokine | Assay Sensitivity (pg/mL) | Assay Range (pg/mL) |
|---|---|---|
| IL-1 β | 0.10 | 0.32–1330 |
| IL-2 | 0.19 | 0.44–1820 |
| IL-6 | 0.038 | 1.03–4230 |
| IL-8 | 0.30 | 0.217–890 |
| IL-10 | 0.038 | 0.18–750 |
| IL-12 | 0.04 | 6.84–28,000 |
| GM-CSF | 1.30 | 1.79–7330 |
| IFNγ | 0.075 | 0.31–1250 |
| TNFα | 0.40 | 8.54–35,000 |
| VEGF | 0.88 | 5.86–24,000 |
| Demographics and Characteristics | CG | UG |
|---|---|---|
| Age (years) | 47 (9) | 53 (11) |
| Gender (% male/female) | 44/56 | 47/53 |
| Laterality (% one/two) | - | 63/37 |
| Ethnicity | Caucasians | Caucasians |
| Idiopathic uveitis (%) | - | 60 |
| Duration of disease (months) | - | 43 (20) |
| Number of episodes | - | 5 (2) |
| Familial uveitis background (%) | - | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinazo-Durán, M.D.; García-Medina, J.J.; Sanz-González, S.M.; O’Connor, J.E.; Casaroli-Marano, R.P.; Valero-Velló, M.; López-Gálvez, M.; Peris-Martínez, C.; Zanón-Moreno, V.; Diaz-Llopis, M. Signature of Circulating Biomarkers in Recurrent Non-Infectious Anterior Uveitis. Immunomodulatory Effects of DHA-Triglyceride. A Pilot Study. Diagnostics 2021, 11, 724. https://doi.org/10.3390/diagnostics11040724
Pinazo-Durán MD, García-Medina JJ, Sanz-González SM, O’Connor JE, Casaroli-Marano RP, Valero-Velló M, López-Gálvez M, Peris-Martínez C, Zanón-Moreno V, Diaz-Llopis M. Signature of Circulating Biomarkers in Recurrent Non-Infectious Anterior Uveitis. Immunomodulatory Effects of DHA-Triglyceride. A Pilot Study. Diagnostics. 2021; 11(4):724. https://doi.org/10.3390/diagnostics11040724
Chicago/Turabian StylePinazo-Durán, Maria D., Jose J. García-Medina, Silvia M. Sanz-González, Jose E. O’Connor, Ricardo P. Casaroli-Marano, Mar Valero-Velló, Maribel López-Gálvez, Cristina Peris-Martínez, Vicente Zanón-Moreno, and Manuel Diaz-Llopis. 2021. "Signature of Circulating Biomarkers in Recurrent Non-Infectious Anterior Uveitis. Immunomodulatory Effects of DHA-Triglyceride. A Pilot Study" Diagnostics 11, no. 4: 724. https://doi.org/10.3390/diagnostics11040724
APA StylePinazo-Durán, M. D., García-Medina, J. J., Sanz-González, S. M., O’Connor, J. E., Casaroli-Marano, R. P., Valero-Velló, M., López-Gálvez, M., Peris-Martínez, C., Zanón-Moreno, V., & Diaz-Llopis, M. (2021). Signature of Circulating Biomarkers in Recurrent Non-Infectious Anterior Uveitis. Immunomodulatory Effects of DHA-Triglyceride. A Pilot Study. Diagnostics, 11(4), 724. https://doi.org/10.3390/diagnostics11040724









