Development of the BWAT-CUA Scale to Assess Wounds in Patients with Calciphylaxis
Abstract
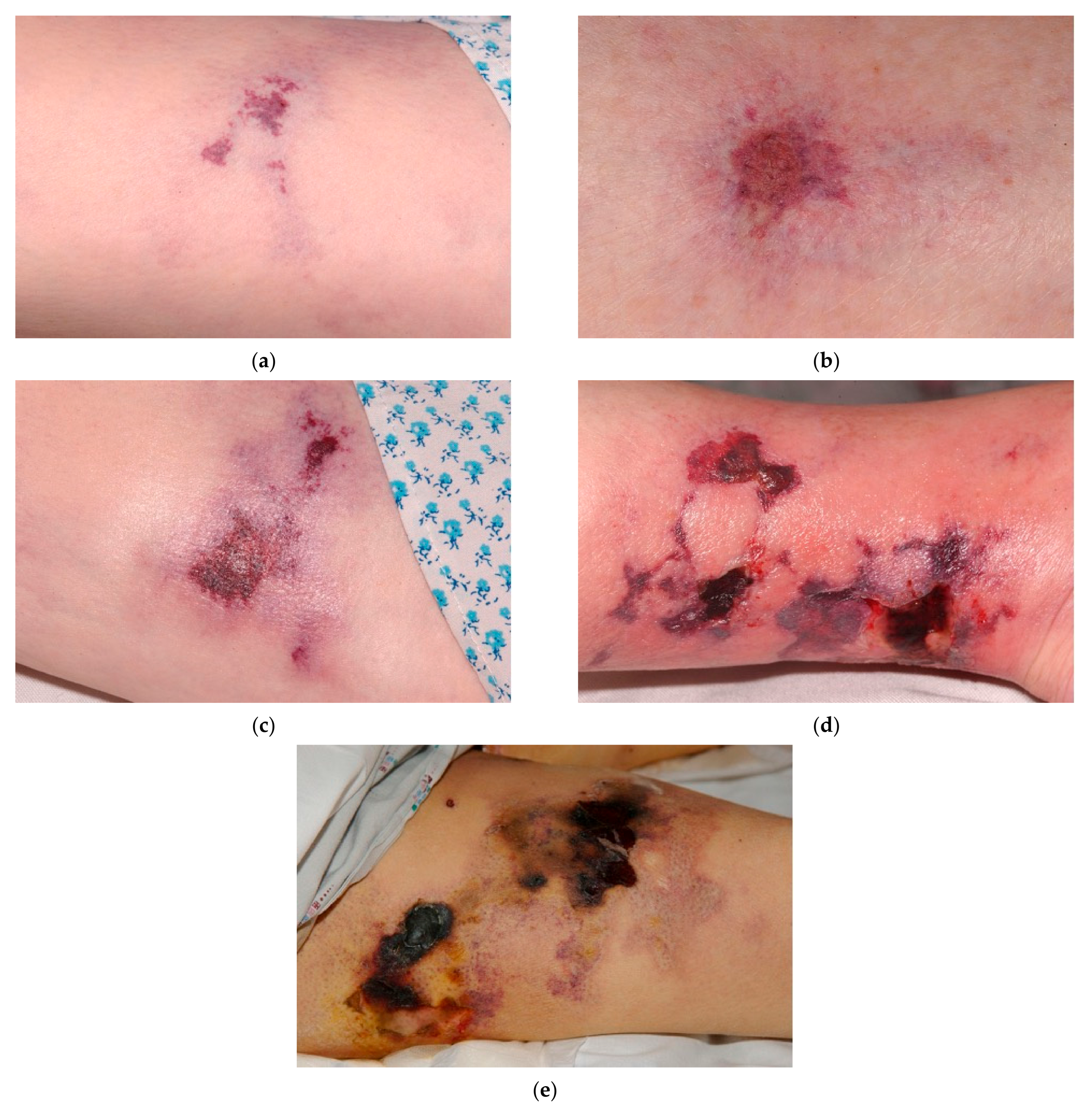
1. Introduction
2. BWAT: A Chronic Wound Assessment Tool
3. Development of the BWAT-CUA Scale
3.1. Necrotic Tissue Type and Necrotic Tissue Amount
3.2. Exudate Type and Exudate Amount
3.3. Skin Color Surrounding Wound, Peripheral Tissue Edema, and Peripheral Tissue Induration
3.4. Granulation Tissue
3.5. Items Excluded
4. Application of the BWAT-CUA Scale
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nigwekar, S.U.; Thadhani, R.; Brandenburg, V.M. Calciphylaxis. N. Engl. J. Med. 2018, 378, 1704–1714. [Google Scholar] [CrossRef]
- Chang, J.J. Calciphylaxis: Diagnosis, pathogenesis, and treatment. Adv. Skin Wound Care 2019, 32, 205–215. [Google Scholar] [CrossRef]
- Goel, S.K.; Bellovich, K.; McCullough, P.A. Treatment of severe metastatic calcification and calciphylaxis in dialysis patients. Int. J. Nephrol. 2011, 2011, 701603. [Google Scholar] [CrossRef]
- Floege, J.; Kubo, Y.; Floege, A.; Chertow, G.M.; Parfrey, P.S. The effect of cinacalcet on calcific uremic arteriolopathy events in patients receiving hemodialysis: The EVOLVE Trial. Clin. J. Am. Soc. Nephrol. 2015, 10, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, A.R.; Johnson, R.J.; Gillen, D.; Stivelman, J.C.; Ryan, M.J.; Davis, C.L.; Stehman-Breen, C.O. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001, 60, 324–332. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Solid, C.A.; Ankers, E.; Malhotra, R.; Eggert, W.; Turchin, A.; Thadhani, R.I.; Herzog, C.A. Quantifying a rare disease in administrative data: The example of calciphylaxis. J. Gen. Intern. Med. 2014, 29 (Suppl. 3), S724–S731. [Google Scholar] [CrossRef] [PubMed]
- Angelis, M.; Wong, L.L.; Myers, S.A.; Wong, L.M. Calciphylaxis in patients on hemodialysis: A prevalence study. Surgery 1997, 122, 1083–1090. [Google Scholar] [CrossRef]
- McCullough, K.P.; Morgenstern, H.; Saran, R.; Herman, W.H.; Robinson, B.M. Projecting ESRD incidence and prevalence in the United States through 2030. J. Am. Soc. Nephrol. 2019, 30, 127–135. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Zhao, S.; Wenger, J.; Hymes, J.L.; Maddux, F.W.; Thadhani, R.I.; Chan, K.E. A nationally representative study of calcific uremic arteriolopathy risk factors. J. Am. Soc. Nephrol. 2016, 27, 3421–3429. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Sinha, S.; Lowney, A.C.; Miller, M. Pain management in patients with end-stage renal disease and calciphylaxis- a survey of clinical practices among physicians. BMC Nephrol. 2020, 21, 403. [Google Scholar] [CrossRef]
- Weenig, R.H.; Sewell, L.D.; Davis, M.D.; McCarthy, J.T.; Pittelkow, M.R. Calciphylaxis: Natural history, risk factor analysis, and outcome. J. Am. Acad. Dermatol. 2007, 56, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Wolf, M.; Sterns, R.H.; Hix, J.K. Calciphylaxis from nonuremic causes: A systematic review. Clin. J. Am. Soc. Nephrol. 2008, 3, 1139–1143. [Google Scholar] [CrossRef]
- McCarthy, J.T.; El-Azhary, R.A.; Patzelt, M.T.; Weaver, A.L.; Albright, R.C.; Bridges, A.D.; Claus, P.L.; Davis, M.D.; Dillon, J.J.; El-Zoghby, Z.M.; et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin. Proc. 2016, 91, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Huckle, A.; Hegarty, J.; Kalra, P.; Sinha, S. Calciphylaxis in end-stage kidney disease: Outcome data from the United Kingdom Calciphylaxis Study. J. Nephrol. 2021. [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, I.; Toussaint, N.D.; Hawley, C.M.; Krishnasamy, R.; Pedagogos, E.; Lioufas, N.; Elder, G.J. The Australian Calciphylaxis Registry: Reporting clinical features and outcomes of patients with calciphylaxis. Nephrol. Dial. Transplant. 2019. [CrossRef]
- Gaisne, R.; Pere, M.; Menoyo, V.; Hourmant, M.; Larmet-Burgeot, D. Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: A case-control study. BMC Nephrol. 2020, 21, 63. [Google Scholar] [CrossRef]
- Robinson, M.R.; Augustine, J.J.; Korman, N.J. Cinacalcet for the treatment of calciphylaxis. Arch. Dermatol 2007, 143, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.; Fontaine, B. Calciphylaxis: The beginning of the end? Perit. Dial. Int. 2008, 28, 268–270. [Google Scholar] [CrossRef]
- Seethapathy, H.; Brandenburg, V.M.; Sinha, S.; El-Azhary, R.A.; Nigwekar, S.U. Review: Update on the management of calciphylaxis. QJM 2019, 112, 29–34. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Brunelli, S.M.; Meade, D.; Wang, W.; Hymes, J.; Lacson, E., Jr. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin. J. Am. Soc. Nephrol. 2013, 8, 1162–1170. [Google Scholar] [CrossRef]
- Peng, T.; Zhuo, L.; Wang, Y.; Jun, M.; Li, G.; Wang, L.; Hong, D. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology 2018, 23, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Findlay, M.D.; Geddes, C.C.; Fox, J.G. The role of sodium thiosulphate in the treatment of calciphylaxis. Port. J. Nephrol. Hypert. 2012, 26, 245–254. [Google Scholar]
- Podymow, T.; Wherrett, C.; Burns, K.D. Hyperbaric oxygen in the treatment of calciphylaxis: A case series. Nephrol. Dial. Transplant. 2001, 16, 2176–2180. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Sinha, S.; Torregrosa, J.-V.; Garg, R.; Miller, S.; Canals, A.-Z.; Bahr, D.; Joubert, P.H.; Salcedo, C.; Carroll, K.J.; et al. Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: A phase 2 open-label study of patients with calciphylaxis. J. Nephrol. 2019, 32, 811–821. [Google Scholar] [CrossRef]
- Bellingeri, A.; Falciani, F.; Traspedini, P.; Moscatelli, A.; Russo, A.; Tino, G.; Chiari, P.; Peghetti, A. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: A single-blind RCT. J. Wound Care 2016, 25, 160, 162–166, 168. [Google Scholar] [CrossRef]
- Chan, L.N.; Lai, C.K. The effect of patient education with telephone follow-up on wound healing in adult patients with clean wounds: A randomized controlled trial. J. Wound Ostomy Cont. Nurs. 2014, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Taly, A.B.; Srivastava, A.; Kumar, S.; Thyloth, M. Efficacy of pulsed electromagnetic field therapy in healing of pressure ulcers: A randomized control trial. Neurol. India 2009, 57, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Izadi, M.; Sayyadi, N.; Rezaee, R.; Jafari, N.J.; Beiraghdar, F.; Zamani, A.; Sahebkar, A. Comparative trial of Aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. J. Wound Care 2015, 24, 455–459, 460–462. [Google Scholar] [CrossRef]
- Bates-Jensen, B.M.; Vredevoe, D.L.; Brecht, M.L. Validity and reliability of the Pressure Sore Status Tool. Decubitus 1992, 5, 20–28. [Google Scholar]
- Sussman, C.; Bates-Jensen, B.M. Chapter 6: Tools to measure wound healing. In Wound Care: A Collaborative Practice Manual for Health Professionals; Wolters Kluwer Health and Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 131–161. [Google Scholar]
- Harris, C.; Bates-Jensen, B.; Parslow, N.; Raizman, R.; Singh, M.; Ketchen, R. Bates-Jensen wound assessment tool: Pictorial guide validation project. J. Wound Ostomy Cont. Nurs. 2010, 37, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bates-Jensen, B.; Sussman, C. Chapter 5: Tools to Measure Wound Healing. In Wound Care: A Collaborative Practice Manual For Health Professionals; Sussman, C., Bates-Jensen, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 131–161. [Google Scholar]
- Tallis, A.; Motley, T.A.; Wunderlich, R.P.; Dickerson, J.E.; Waycaster, C., Jr.; Slade, H.B. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: Results of a randomized controlled study. Clin. Ther. 2013, 35, 1805–1820. [Google Scholar] [CrossRef] [PubMed]
- Bates-Jensen, B.M.; McNees, P. Toward an intelligent wound assessment system. Ostomy Wound Manag. 1995, 41, 80S–86S; Discussion 87S. [Google Scholar]


| BWAT Item | Scores | Included/Excluded in BWAT-CUA: Rationale |
|---|---|---|
| Necrotic tissue type | 1 = None visible; 2 = White/grey non-viable tissue and/or non-adherent yellow slough; 3 = Loosely adherent yellow slough; 4 = Adherent soft black eschar; 5 = Firmly adherent hard black eschar | Included Calciphylaxis is often diagnosed late, when necrosis is already present. Reduction of necrotic tissue may be a sensitive indicator of improvement. |
| Necrotic tissue amount | 1 = None visible; 2 = <25% of wound bed covered; 3 = 25% to 50% of wound covered; 4 = >50% but <75% of wound covered; 5 = 75% to 100% of wound covered | Included See necrotic tissue type. |
| Exudate type | 1 = None; 2 = Bloody; 3 = Serosanguineous: thin, watery, pale red/pink; 4 = Serous: thin, watery, clear; 5 = Purulent: thin or thick, opaque, tan/yellow, with or without odor | Included Particularly pertinent in calciphylaxis wounds, which have a high risk of infection; ~50% of patients with calciphylaxis have sepsis as an attributable cause of death. |
| Exudate amount | 1 = None, dry wound; 2 = Scant, wound moist but no observable exudate; 3 = Small; 4 = Moderate; 5 = Large | Included See exudate type. |
| Skin color surrounding wound | 1 = Pink or normal for ethnic group; 2 = Bright red and/or blanches to touch; 3 = White or grey pallor or hypopigmented; 4 = Dark red or purple and/or non-blanchable; 5 = Black or hyperpigmented | Included Erythema is often seen in calciphylaxis wounds; it can assist with the diagnostic process as well as with monitoring wound progression and infection. |
| Peripheral tissue edema | 1 = No swelling or edema; 2 = Non-pitting edema extends <4 cm around wound; 3 = Non-pitting edema extends >4 cm around wound; 4 = Pitting edema extends <4 cm around wound; 5 = Crepitus and/or pitting edema extends >4 cm around wound | Included Edema is often seen in calciphylaxis wounds; it can assist with the diagnostic process as well as with monitoring wound progression and infection. |
| Peripheral tissue induration | 1 = None present; 2 = Induration <2 cm in any area around wound; 3 = Induration 2–4 cm extending <50% around wound; 4 = Induration 2–4 cm extending >50% around wound; 5 = Induration >4 cm in any area around wound | Included Induration is often seen in calciphylaxis wounds; it can assist with the diagnostic process as well as with monitoring wound progression and infection. |
| Granulation tissue | 1 = Skin intact or partial thickness wound; 2 = Bright, beefy red; 75% to 100% of wound filled and/or tissue overgrowth; 3 = Bright, beefy red; >25% to <75% of wound filled; 4 = Pink, and/or dull, dusky red and/or fills <25% of wound; 5 = No granulation tissue present | Included As calciphylaxis wounds improve it is expected that there will be increased granulation tissue. Granulation tissue indicates commencement of healing, particularly for slow-healing calciphylaxis wounds. |
| Excluded Items | ||
| Undermining | 0 = Healed, resolved wound; 1 = None; 2 = Undermining <2 cm in any area; 3 = Undermining 2–4 cm involving <50% wound margins; 4 = Undermining 2–4 cm involving >50% wound margins; 5 = Undermining >4 cm or tunneling in any area | Excluded Undermining is not a characteristic feature of calciphylaxis wounds. |
| Size | 0 = Healed, resolved wound; 1 = Length × width <4 cm2; 2 = Length × width 4 to <16 cm2; 3 = Length × width 16.1 to <36 cm2; 4 = Length × width 36.1 to <80 cm2; 5 = Length × width >80 cm2 | Excluded Ranges are broad and wound size is not a sensitive measure for slow-healing wounds like calciphylaxis. |
| Depth | 0 = Healed, resolved wound; 1 = Non-blanchable erythema on intact skin; 2 = Partial thickness skin loss involving epidermis and/or dermis; 3 = Full thickness skin loss involving damage or necrosis of subcutaneous tissue; may extend down to but not through underlying fascia; and/or mixed partial & full thickness and/or tissue layers obscured by granulation tissue; 4 = Obscured by necrosis; 5 = Full thickness skin loss with extensive destruction, tissue necrosis or damage to muscle, bone or supporting structures | Excluded The depth descriptions for BWAT correspond to pressure ulcer stages. Calciphylaxis lesions usually present as either necrotic or full-thickness lesions; thus, this item was most likely to be binary (4 or 5) and redundant with the BWAT item for necrotic tissue type. |
| Edges | 0 = Healed, resolved wound; 1 = Indistinct, diffuse, none clearly visible; 2 = Distinct, outline clearly visible, attached, even with wound base; 3 = Well-defined, not attached to wound base; 4 = Well-defined, not attached to base, rolled under, thickened; 5 = Well-defined, fibrotic, scarred or hyperkeratotic | Excluded Edges are less relevant in slow-healing calciphylaxis wounds, which tend to heal from the base up. |
| Epithelialization | 1 = 100% wound covered, surface intact; 2 = 75% to <100% wound covered and/or epithelial tissue extends >0.5 cm into wound bed; 3 = 50% to <75% wound covered and/or epithelial tissue extends to <0.5 cm into wound bed; 4 = 25% to <50% wound covered; 5 = <25% wound covered | Excluded Late feature of wound healing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, L.J.; Serena, T.E.; Sinha, S. Development of the BWAT-CUA Scale to Assess Wounds in Patients with Calciphylaxis. Diagnostics 2021, 11, 730. https://doi.org/10.3390/diagnostics11040730
Gould LJ, Serena TE, Sinha S. Development of the BWAT-CUA Scale to Assess Wounds in Patients with Calciphylaxis. Diagnostics. 2021; 11(4):730. https://doi.org/10.3390/diagnostics11040730
Chicago/Turabian StyleGould, Lisa J., Thomas E. Serena, and Smeeta Sinha. 2021. "Development of the BWAT-CUA Scale to Assess Wounds in Patients with Calciphylaxis" Diagnostics 11, no. 4: 730. https://doi.org/10.3390/diagnostics11040730
APA StyleGould, L. J., Serena, T. E., & Sinha, S. (2021). Development of the BWAT-CUA Scale to Assess Wounds in Patients with Calciphylaxis. Diagnostics, 11(4), 730. https://doi.org/10.3390/diagnostics11040730







