Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. High-Resolution Computed Tomography
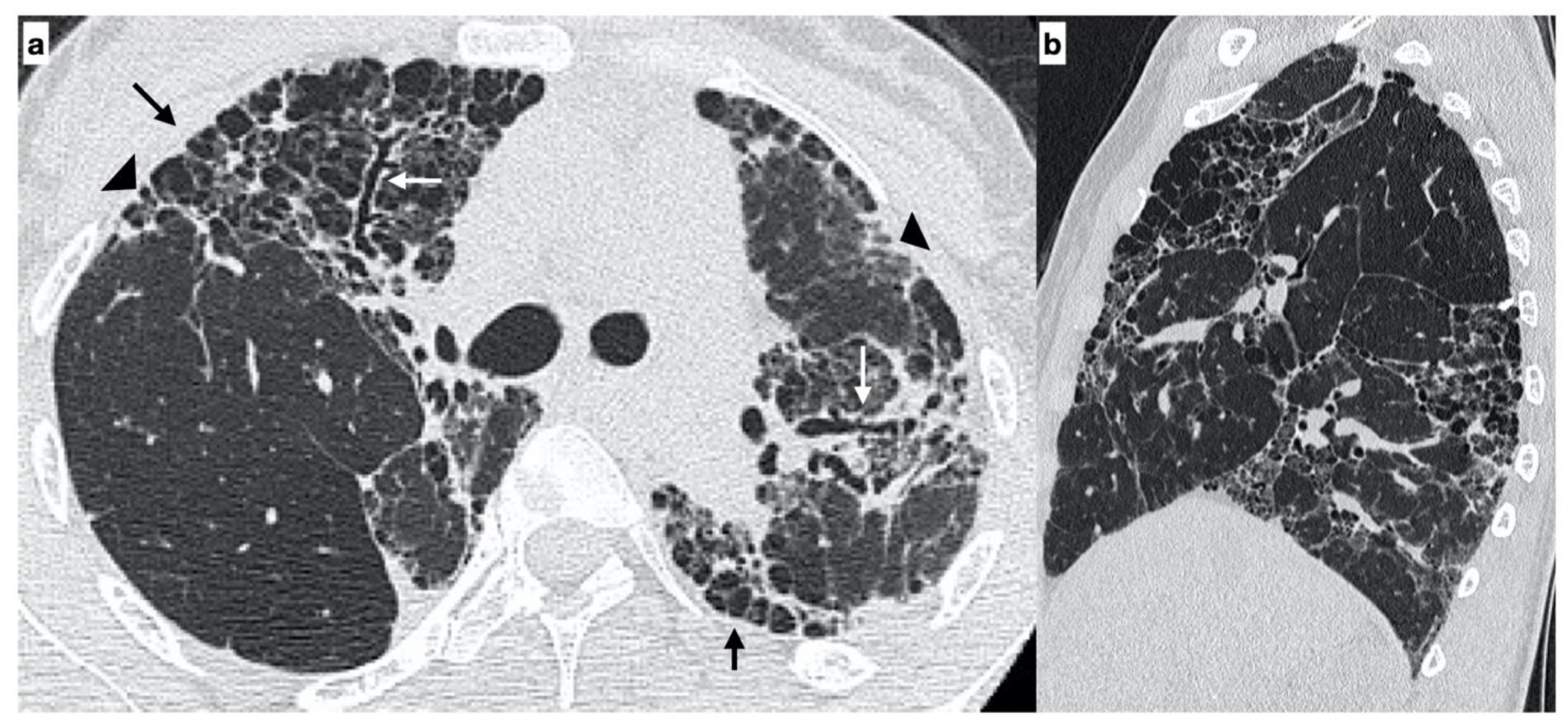
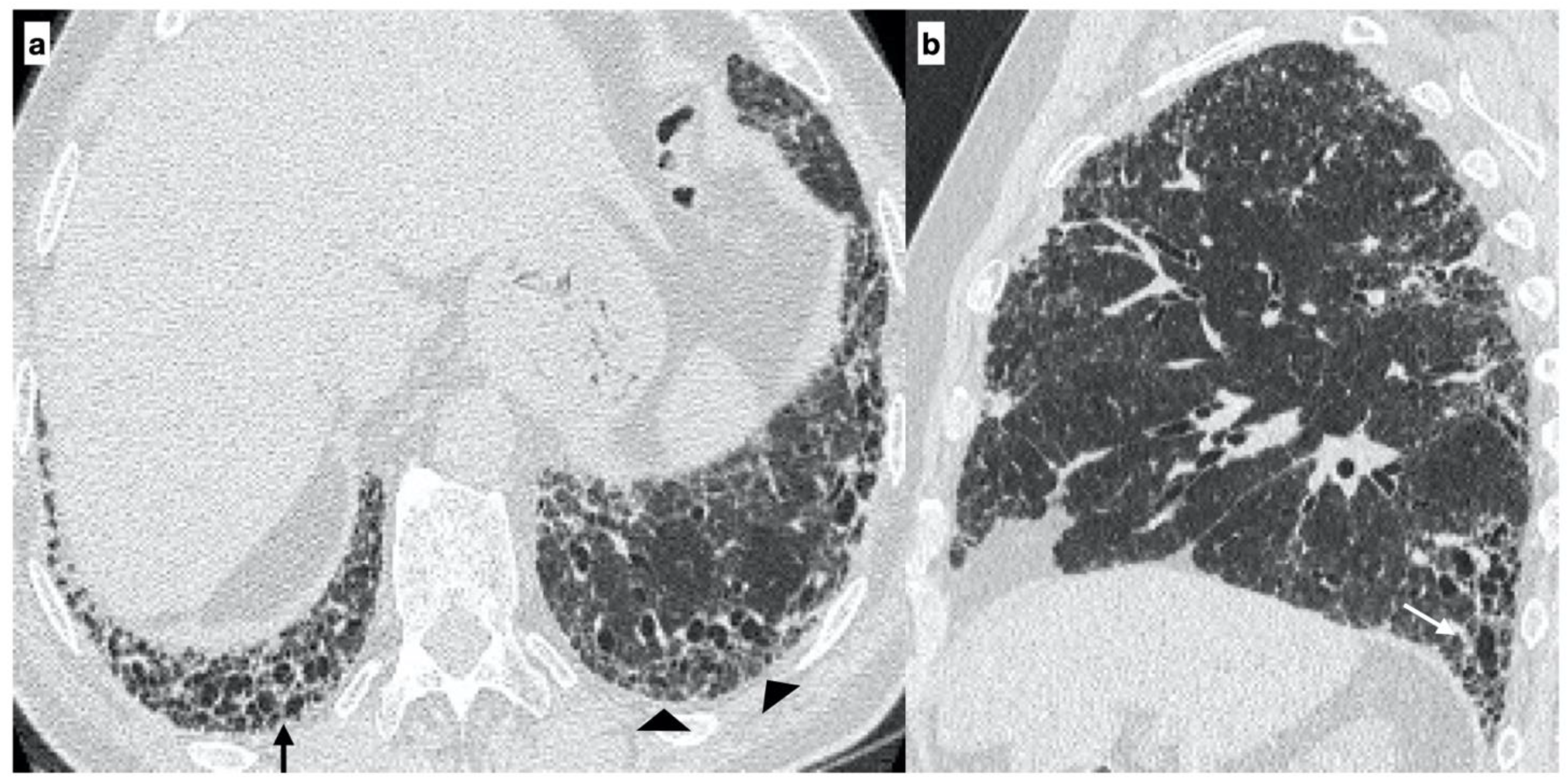
- Usual interstitial pneumonia (UIP): the presence of honeycombing in association with a reticular pattern with or without traction bronchiectasis/bronchiolectasis, with a subpleural and basal predominance;
- Probable UIP: the presence of a reticular pattern in association with traction bronchiectasis/bronchiolectasis without honeycombing and with a subpleural and basal predominance;
- Indeterminate UIP: the presence of subtle reticulation or ground-glass opacities, with a subpleural and basal predominance;
2.3. Genetic Analysis
2.4. Statistical Analysis
3. Results
- (a)
- the age at onset of the disease was lower in patients with the familial form than in those with the sporadic form;
- (b)
- there was no significant difference in gender for the familial form, whilst there was a prevalence of males in the sporadic form;
- (c)
- moreover, no current smokers were observed in the group of patients affected by the familial form, whilst there were numerous current smokers in the sporadic form group.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Hutchinson, J.P.; Fogarty, A.W.; Hubbard, R.B.; McKeever, T.M. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef]
- Diamantopoulos, A.; Wright, E.; Vlahopoulou, K.; Cornic, L.; Schoof, N.; Maher, T.M. The Burden of Illness of Idiopathic Pulmonary Fibrosis: A Comprehensive Evidence Review. Pharmacoeconomics 2018, 36, 779–807. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Ruaro, B.; Confalonieri, P.; Confalonieri, M. Epithelial–Mesenchymal Transition: A Major Pathogenic Driver in Idiopathic Pulmonary Fibrosis? Medecine 2020, 56, 608. [Google Scholar] [CrossRef]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Confalonieri, M.; Matucci-Cerinic, M.; Salton, F.; Confalonieri, P.; Santagiuliana, M.; Citton, G.; Baratella, E.; Bruni, C. The Treatment of Lung Involvement in Systemic Sclerosis. Pharmaceuticals 2021, 14, 154. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020, 66, 109482. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Walsh, S.L.F.; Calandriello, L.; Sverzellati, N.; Wells, A.U.; Hansell, D.M. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax 2015, 71, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Fiorese, I.; Marrocchio, C.; Salton, F.; Cova, M.A. Imaging Review of the Lung Parenchymal Complications in Patients with IPF. Medecine 2019, 55, 613. [Google Scholar] [CrossRef]
- Hobbs, S.; Chung, J.H.; Leb, J.; Kaproth-Joslin, K.; Lynch, D.A. Practical Imaging Interpretation in Patients Suspected of Having Idiopathic Pulmonary Fibrosis: Official Recommendations from the Radiology Working Group of the Pulmonary Fibrosis Foundation. Radiol. Cardiothorac. Imaging 2021, 3, e200279. [Google Scholar] [CrossRef]
- Moimas, S.; Salton, F.; Kosmider, B.; Ring, N.; Volpe, M.C.; Bahmed, K.; Braga, L.; Rehman, M.; Vodret, S.; Graziani, M.L.; et al. miR-200 family members reduce senescence and restore idiopathic pulmonary fibrosis type II alveolar epithelial cell transdifferentiation. ERJ Open Res. 2019, 5, 00138–02019. [Google Scholar] [CrossRef]
- Kropski, J.A.; Lawson, W.E.; Young, L.R.; Blackwell, T.S. Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis. Model. Mech. 2012, 6, 9–17. [Google Scholar] [CrossRef]
- Spagnolo, P.; Grunewald, J.; Du Bois, R.M. Genetic determinants of pulmonary fibrosis: Evolving concepts. Lancet Respir. Med. 2014, 2, 416–428. [Google Scholar] [CrossRef]
- Hodgson, U.; Laitinen, T.; Tukiainen, P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: Evidence of founder effect among multiplex families in Finland. Thorax 2002, 57, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.A.; Fox, G.; Bhatia, R.; Sala, E.; Noble, B.; Denic, N.; Fernandez, D.; Duguid, N.; Dohey, A.; Kamel, F.; et al. A Newfoundland cohort of familial and sporadic idiopathic pulmonary fibrosis patients: Clinical and genetic features. Respir. Res. 2012, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, M.A.; Shifren, A.; Huang, H.J.; Russell, T.D.; Mitra, R.D.; Zhang, Q.; Wegner, D.J.; Cole, F.S.; Hamvas, A. Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respir. Res. 2014, 1, e000057. [Google Scholar] [CrossRef]
- Nogee, L.M.; Dunbar, A.E.; Wert, S.E.; Askin, F.; Hamvas, A.; Whitsett, J.A. A Mutation in the Surfactant Protein C Gene Associated with Familial Interstitial Lung Disease. N. Engl. J. Med. 2001, 344, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.Q.; Lane, K.; Phillips, J.; Prince, M.; Markin, C.; Speer, M.; Schwartz, D.A.; Gaddipati, R.; Marney, A.; Johnson, J.; et al. Heterozygosity for a Surfactant Protein C Gene Mutation Associated with Usual Interstitial Pneumonitis and Cellular Nonspecific Interstitial Pneumonitis in One Kindred. Am. J. Respir. Crit. Care Med. 2002, 165, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Tanaka, T.; Ishida, M.; Kinoshita, A.; Fukuoka, J.; Takaki, M.; Sakamoto, N.; Ishimatsu, Y.; Kohno, S.; Hayashi, T.; et al. Surfactant protein C G100S mutation causes familial pulmonary fibrosis in Japanese kindred. Eur. Respir. J. 2011, 38, 861–869. [Google Scholar] [CrossRef]
- Armanios, M.Y.; Chen, J.J.-L.; Cogan, J.D.; Alder, J.K.; Ingersoll, R.G.; Markin, C.; Lawson, W.E.; Xie, M.; Vulto, I.; Phillips, J.A.; et al. Telomerase Mutations in Families with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2007, 356, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.K.; Stanley, S.E.; Wagner, C.L.; Hamilton, M.; Hanumanthu, V.S.; Armanios, M. Exome Sequencing Identifies Mutant TINF2 in a Family With Pulmonary Fibrosis. Chest 2015, 147, 1361–1368. [Google Scholar] [CrossRef]
- Fukuhara, A.; Tanino, Y.; Ishii, T.; Inokoshi, Y.; Saito, K.; Fukuhara, N.; Sato, S.; Saito, J.; Ishida, T.; Yamaguchi, H.; et al. Pulmonary fibrosis in dyskeratosis congenita withTINF2gene mutation. Eur. Respir. J. 2013, 42, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Cogan, J.D.; Kropski, J.A.; Zhao, M.; Mitchell, D.B.; Rives, L.; Markin, C.; Garnett, E.T.; Montgomery, K.H.; Mason, W.R.; Mckean, D.F.; et al. Rare Variants inRTEL1Are Associated with Familial Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2015, 191, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Fingerlin, T.E.; Murphy, E.; Zhang, W.; Peljto, A.L.; Brown, K.K.; Steele, M.P.; Loyd, J.E.; Cosgrove, G.P.; Lynch, D.A.; Groshong, S.D.; et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet. 2013, 45, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Mushiroda, T.; Wattanapokayakit, S.; Takahashi, A.; Nukiwa, T.; Kudoh, S.; Ogura, T.; Taniguchi, H.; Kubo, M.; Kamatani, N.; Nakamura, Y.; et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J. Med. Genet. 2008, 45, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Noth, I.; Zhang, Y.; Ma, S.-F.; Flores, C.; Barber, M.; Huang, Y.; Broderick, S.M.; Wade, M.S.; Hysi, P.; Scuirba, J.; et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: A genome-wide association study. Lancet Respir. Med. 2013, 1, 309–317. [Google Scholar] [CrossRef]
- Wei, R.; Li, C.; Zhang, M.; Jones-Hall, Y.L.; Myers, J.L.; Noth, I.; Liu, W.; Jones, Y.L. Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Transl. Res. 2014, 163, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.M.; Hatabu, H.; Okajima, Y.; Gao, W.; Dupuis, J.; Latourelle, J.C.; Nishino, M.; Araki, T.; Zazueta, O.E.; Kurugol, S.; et al. MUC5B Promoter Polymorphism and Interstitial Lung Abnormalities. N. Engl. J. Med. 2013, 368, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.P.; Speer, M.C.; Loyd, J.E.; Brown, K.K.; Herron, A.; Slifer, S.H.; Burch, L.H.; Wahidi, M.M.; Phillips, J.A.; Sporn, T.A.; et al. Clinical and Pathologic Features of Familial Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 172, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Scholand, M.B.; Coon, H.; Wolff, R.; Cannon-Albright, L. Use of a Genealogical Database Demonstrates Heritability of Pulmonary Fibrosis. Lung 2013, 191, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Dressen, A.; Abbas, A.R.; Cabanski, C.; Reeder, J.; Ramalingam, T.R.; Neighbors, M.; Bhangale, T.R.; Brauer, M.J.; Hunkapiller, J.; Reeder, J.; et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: A candidate gene sequencing study. Lancet Respir. Med. 2018, 6, 603–614. [Google Scholar] [CrossRef]
- Cottin, V.; Reix, P.; Khouatra, C.; Thivolet-Béjui, F.; Feldmann, D.; Cordier, J.-F. Combined pulmonary fibrosis and emphysema syndrome associated with familial SFTPC mutation. Thorax 2011, 66, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Gottardi, M.; Corbo, V.; Fassan, M.; Mafficini, A.; Malpeli, G.; Lawlor, R.T.; Scarpa, A. DNA Qualification Workflow for Next Generation Sequencing of Histopathological Samples. PLoS ONE 2013, 8, e62692. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Patel, V.M.; Coon, M.; Nguyen, T.; Land, S.J.; Ruden, D.M.; Lu, X. Using Drosophila melanogaster as a Model for Genotoxic Chemical Mutational Studies with a New Program, SnpSift. Front. Genet. 2012, 3, 35. [Google Scholar] [CrossRef]
- McLaren, W.; Pritchard, B.; Rios, D.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef]
- Geraghty, P.; Wallace, A.; D’Armiento, J.M. Induction of the unfolded protein response by cigarette smoke is primarily an activating transcription factor 4-C/EBP homologous protein mediated process. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 309–319. [Google Scholar] [CrossRef]
- Hengstermann, A.; Müller, T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic. Biol. Med. 2008, 44, 1097–1107. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, J.; Shan, L.; Jorgensen, E.D. Measuring the Impact of Cigarette Smoke on the UPR. Methods Enzymol. 2011, 489, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Margaritopoulos, G.A.; Harari, S.; Caminati, A.; Antoniou, K.M. Smoking-related idiopathic interstitial pneumonia: A review. Respirology 2015, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kropski, J.A.; Blackwell, T.S.; Loyd, J.E. The genetic basis of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Idiopathic Pulmonary Fibrosis Is A Complex Genetic Disorder. Trans. Am. Clin. Clim. Assoc. 2016, 127, 34–45. [Google Scholar]
- Kropski, J.A.; Young, L.R.; Cogan, J.D.; Mitchell, D.B.; Lancaster, L.H.; Worrell, J.A.; Markin, C.; Na Liu, M.; Mason, W.R.; Fingerlin, T.E.; et al. Genetic Evaluation and Testing of Patients and Families with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 195, 1423–1428. [Google Scholar] [CrossRef] [PubMed]

| Variables | Familial (n = 19) | Sporadic (n = 46) | p-Value |
|---|---|---|---|
| Age of Diagnosis (mean ± SD) | 62 ± 15 | 70 ± 9 | 0.012 |
| Gender (N, %) | |||
| Female | 9 (47.4%) | 13 (28.3%) | 0.159 |
| Male | 10 (52.6%) | 33 (71.7%) | |
| Smoking Status (N, %) | |||
| Non-smoker | 7 (41.2%) | 11 (23.9%) | |
| Former smoker | 9 (52.9%) | 16 (34.8%) | 0.027 |
| Smoker | 1 (5.9%) | 19 (41.3%) | |
| Not available | 2 | 0 | |
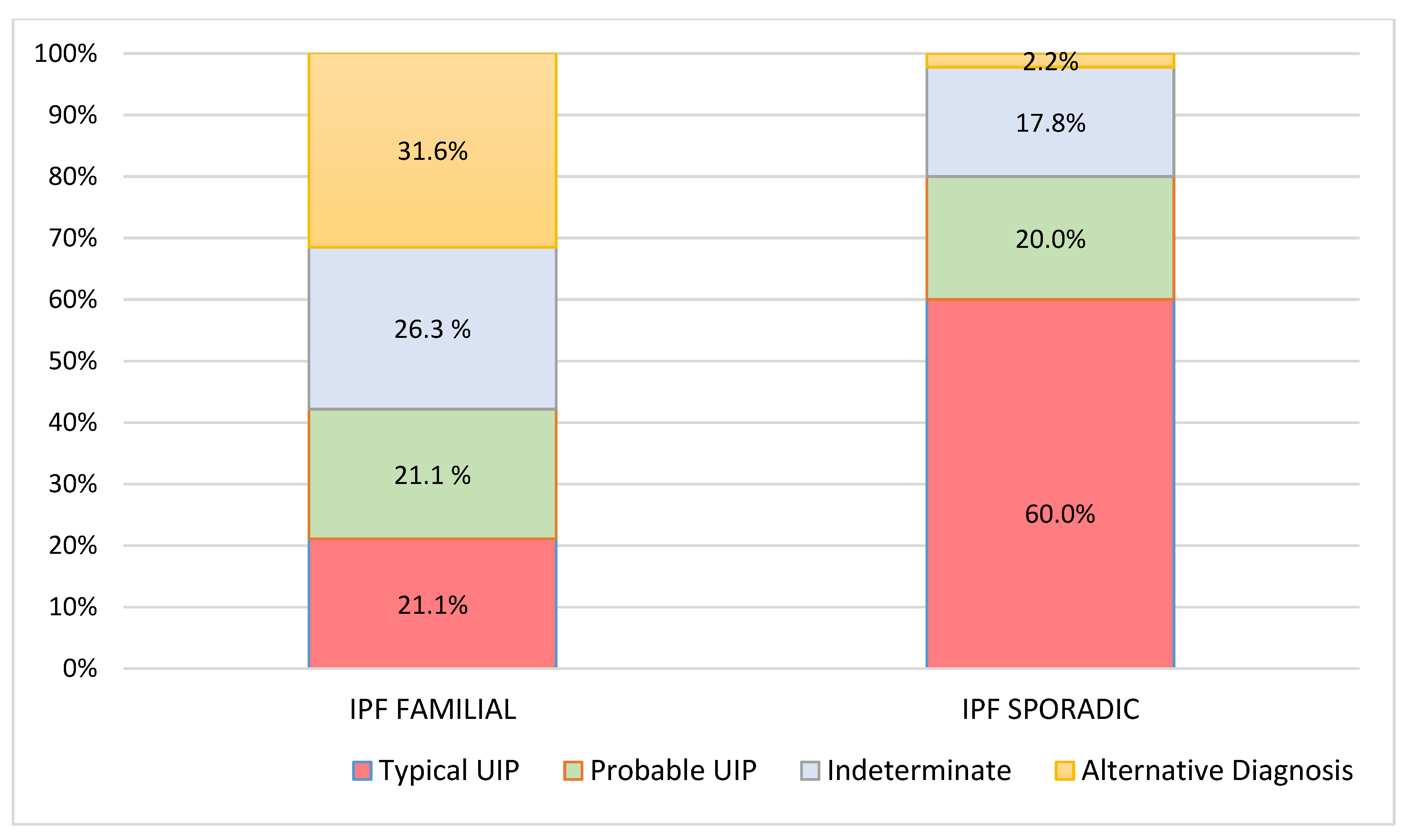
| Pattern at Diagnosis (N, %) | |||
| typical UIP | 4 (21.1%) | 27 (60.0%) | |
| probable UIP | 4 (21.1%) | 9 (20.0%) | 0.001 |
| Indeterminate | 5 (26.3%) | 8 (17.8%) | |
| Alternative Diagnosis | 6 (31.6%) | 1 (2.2%) | |
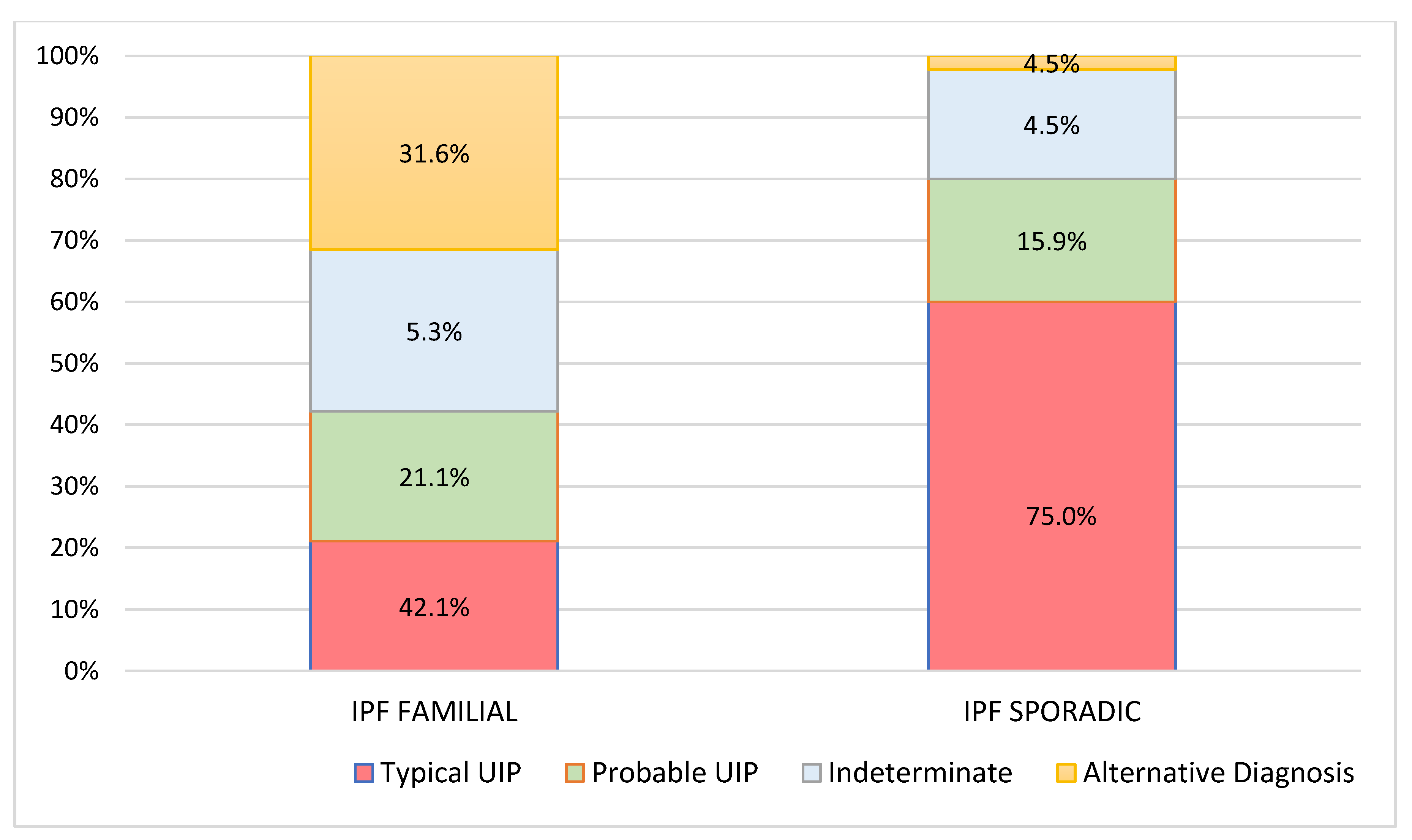
| Pattern at 2 years (N, %) | |||
| Typical UIP | 8 (42.1%) | 33 (75.0%) | 0.014 |
| Probable UIP | 4 (21.1%) | 7 (15.9%) | |
| Indeterminate | 1 (5.3%) | 2 (4.5%) | |
| Alternative Diagnosis | 6 (31.6%) | 2 (4.5%) | |
| Worsening of the pattern after two years (N, %) | |||
| Yes | 12 (63.2%) | 30 (69.8%) | 0.769 |
| Invariate | 7 (36.8%) | 13 (30.2%) |
| Gene | N of Mutation | % |
|---|---|---|
| PROMOTER-TERT | 15 | 83.3% |
| SFTPA2 | 12 | 66.7% |
| SFTPC | 8 | 44.4% |
| PROMOTER-MUC5B | 6 | 33.3% |
| TERT | 3 | 16.7% |
| TERC | 3 | 16.7% |
| ABCA3 | 1 | 5.6% |
| DKC1 | 0 | 0.0% |
| RTEL1 | 0 | 0.0% |
| Mutation | Polymorphism | N | % |
|---|---|---|---|
| SFTPA2 | rs1965708 (Q223K) | 4 | 33.3% |
| rs17886395 (A91P) | 3 | 25.0% | |
| rs1965708 (Q223K); rs17886395 (A91P) | 5 | 41.7% | |
| ABCA3 | Gly964Asp | 1 | 100% |
| Promoter-MUC5B | rs35705950 | 1 | 100% |
| Promoter-TERT | rs143938607 | 1 | 6.7% |
| Promoter-TERT | rs2735940 | 4 | 26.7% |
| rs2735940; rs2736109 | 6 | 40.0% | |
| rs2735940; rs3215401; rs2736109 | 2 | 13.3% | |
| rs2736109 | 1 | 6.7% | |
| rs33977403 | 1 | 6.7% | |
| SFTPC | rs4715 (T138N); rs1124 (S186N) | 2 | 25.0% |
| rs4715 (T138N) | 6 | 75.0% | |
| TERT | rs35719940 (A1062T) | 1 | 16.7% |
| R756C | 2 | 33.3% | |
| TERC | rs2293607 | 3 | 50.0% |
| Variables | Typical UIP | Probable UIP | Indeterminate | Alternative Diagnosis | p-Value |
|---|---|---|---|---|---|
| Promoter-TERT | |||||
| Yes | 2 | 3 | 5 | 5 | p = 0.651 |
| No | 1 | 1 | 0 | 1 | |
| SFTPA2 | |||||
| Yes | 2 | 3 | 4 | 3 | p = 0.830 |
| No | 1 | 1 | 1 | 3 | |
| SFTPC | p = 0.168 | ||||
| Yes | 0 | 1 | 4 | 3 | |
| No | 3 | 3 | 1 | 3 | |
| Promoter-MUC5B | |||||
| Yes | 1 | 0 | 3 | 2 | p = 0.364 |
| No | 2 | 4 | 2 | 4 | |
| TERT | |||||
| Yes | 2 | 1 | 0 | 0 | p = 0.033 |
| No | 1 | 3 | 5 | 6 | |
| TERC | |||||
| Yes | 2 | 0 | 1 | 1 | p = 0.853 |
| No | 1 | 4 | 4 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratella, E.; Ruaro, B.; Giudici, F.; Wade, B.; Santagiuliana, M.; Salton, F.; Confalonieri, P.; Simbolo, M.; Scarpa, A.; Tollot, S.; et al. Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis. Diagnostics 2021, 11, 762. https://doi.org/10.3390/diagnostics11050762
Baratella E, Ruaro B, Giudici F, Wade B, Santagiuliana M, Salton F, Confalonieri P, Simbolo M, Scarpa A, Tollot S, et al. Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis. Diagnostics. 2021; 11(5):762. https://doi.org/10.3390/diagnostics11050762
Chicago/Turabian StyleBaratella, Elisa, Barbara Ruaro, Fabiola Giudici, Barbara Wade, Mario Santagiuliana, Francesco Salton, Paola Confalonieri, Michele Simbolo, Aldo Scarpa, Saverio Tollot, and et al. 2021. "Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis" Diagnostics 11, no. 5: 762. https://doi.org/10.3390/diagnostics11050762
APA StyleBaratella, E., Ruaro, B., Giudici, F., Wade, B., Santagiuliana, M., Salton, F., Confalonieri, P., Simbolo, M., Scarpa, A., Tollot, S., Marrocchio, C., Cova, M. A., & Confalonieri, M. (2021). Evaluation of Correlations between Genetic Variants and High-Resolution Computed Tomography Patterns in Idiopathic Pulmonary Fibrosis. Diagnostics, 11(5), 762. https://doi.org/10.3390/diagnostics11050762









