Retinal and Optic Disc Vascular Changes in Patients Using Long-Term Tadalafil: A Prospective Non-Randomized Matched-Pair Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- (a)
- Patients suffering from iatrogenic ED < 70 years old;
- (b)
- Continuous administration of tadalafil 20 mg OAD for >6 months;
- (c)
- No evidence of retinopathy.
2.2. Study Techniques
2.2.1. Spectral Domain-Optical Coherence Tomography
2.2.2. Subfoveal Choroidal Thickness Measurement
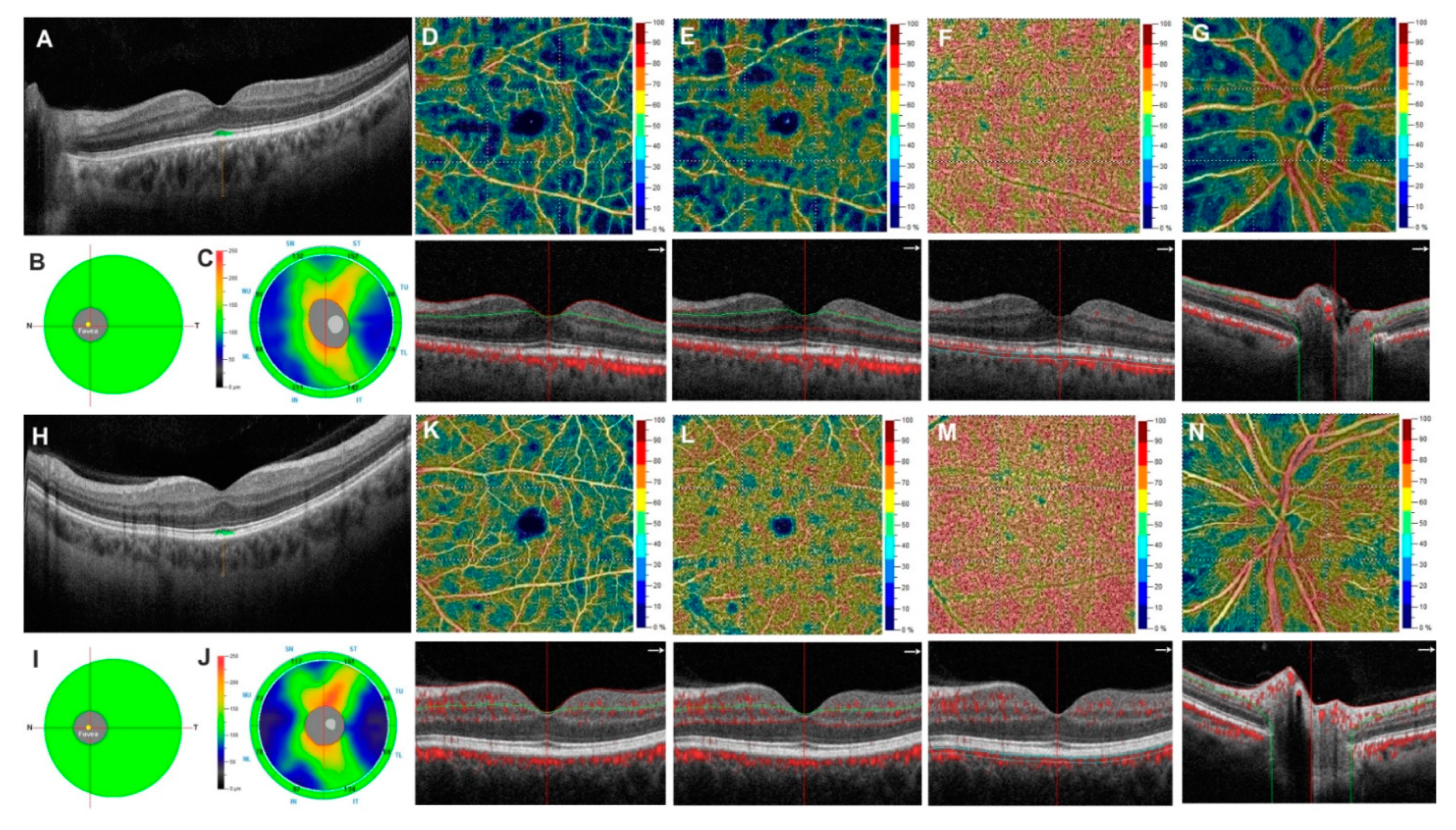
2.2.3. Optical Coherence Tomography Angiography
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappelleri, J.C.; Tseng, L.-J.; Stecher, V.; Goldstein, I. Enriching the Interpretation of the Erectile Dysfunction Inventory of Treatment Satisfaction: Characterizing Success in Treatment Satisfaction. J. Sex. Med. 2018, 15, 732–740. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and Their Regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Nitoda, E. Pathophysiology of visual disorders induced by phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drug Des. Dev. Ther. 2016, 10, 3407–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederen, R.M.H.; La Heij, E.C.; Markerink-van Ittersum, M.; Kijlstra, A.; Hendrikse, F.; de Vente, J. Selective blockade of phosphodiesterase types 2, 5 and 9 results in cyclic 3’5’ guanosine monophosphate accumulation in retinal pigment epithelium cells. Br. J. Ophthalmol. 2006, 91, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Marmor, M.F.; Kessler, R. Sildenafil (Viagra) and ophthalmology. Surv. Ophthalmol. 1999, 44, 153–162. [Google Scholar] [CrossRef]
- Campbell, U.B.; Walker, A.M.; Gaffney, M.; Petronis, K.R.; Creanga, D.; Quinn, S.; Klein, B.E.; Laties, A.M.; Lewis, M.; Sharlip, I.D.; et al. Acute Nonarteritic Anterior Ischemic Optic Neuropathy and Exposure to Phosphodiesterase Type 5 Inhibitors. J. Sex. Med. 2015, 12, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhu, L.; Zhong, J.; Zeng, G.; Deng, T. The Association between Phosphodiesterase Type 5 Inhibitor Use and Risk of Non-Arteritic Anterior Ischemic Optic Neuropathy: A Systematic Review and Meta-Analysis. Sex. Med. 2018, 6, 185–192. [Google Scholar] [CrossRef]
- Nathoo, N.A.; Etminan, M.; Mikelberg, F.S. Association between Phosphodiesterase-5 Inhibitors and Nonarteritic Ante-rior Ischemic Optic Neuropathy. J. Neuro-Ophthalmol. 2015, 35, 12–15. [Google Scholar] [CrossRef]
- Capece, M.; Verze, P.; Creta, M.; La Rocca, R.; Persico, F.; Spirito, L.; Cardi, A.; Mirone, V. Efficacy and safety of low-intensity shockwave therapy plus tadalafil 5 mg once daily in men with type 2 diabetes mellitus and erectile dysfunc-tion: A matched-pair comparison study. Asian J. Androl. 2020, 22, 379. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Chopra, I.; Patel, D.; Hassan, T.A.; Tang, W.Y. Phosphodiesterase Type-5 Inhibitor Prescription Patterns in the United States Among Men With Erectile Dysfunction: An Update. J. Sex. Med. 2020, 17, 941–948. [Google Scholar] [CrossRef]
- Fusco, F.; D’Anzeo, G.; Sessa, A.; Pace, G.; Rossi, A.; Capece, M.; d’Emmanuele di Villa Bianca, R. BPH/LUTS and ED: Com-mon Pharmacological Pathways for a Common Treatment. J. Sex. Med. 2013, 10, 2382–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coscas, F.; Coscas, G.; Zucchiatti, I.; Bandello, F.; Soubrane, G.; Souïed, E. Optical coherence tomography in tadalafil-associated retinal toxicity. Eur. J. Ophthalmol. 2012, 22, 853–856. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Zhang, Y.; Qian, Y.W.; Zhang, J.F.; Wang, Z.L. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express 2019, 10, 1532–1544. [Google Scholar] [CrossRef]
- Bille, J.F. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Cennamo, G.; Montorio, D.; Velotti, N.; Sparnelli, F.; Reibaldi, M.; Cennamo, G. Optical coherence tomography angiography in pre-perimetric open-angle glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1787–1793. [Google Scholar] [CrossRef]
- Branchini, L.; Regatieri, C.V.; Flores-Moreno, I.; Baumann, B.; Fujimoto, J.G.; Duker, J.S. Reproducibility of Cho-roidal Thickness Measurements across Three Spectral Domain Optical Coherence Tomography Systems. Ophthalmology 2012, 119, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cennamo, G.; Montorio, D.; D’Alessandro, A.; Napolitano, P.; D’Andrea, L.; Tranfa, F. Prospective Study of Vessel Density by Optical Coherence Tomography Angiography after Intravitreal Bevacizumab in ExudativeAge-Related Macular Degeneration. Ophthalmol. Ther. 2019, 9, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Jia, Y.; Gao, S.S.; Lumbroso, B.; Rispoli, M. Optical Coherence Tomography Angiography Using the Optovue Device. Dev. Ophthalmol. 2016, 56, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Pradhan, Z.S.; Weinreb, R.N.; Reddy, H.B.; Riyazuddin, M.; Dasari, S.; Palakurthy, M.; Puttaiah, N.K.; Rao, D.A.; Webers, C.A. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2016, 171, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.; Pomeranz, H.D. Food and Drug Administration Adverse Event Reports of Retinal Vascular Occlusions Associated With Phosphodiesterase Type 5 Inhibitor Use. J. Neuro-Ophthalmol. 2016, 36, 480–481. [Google Scholar] [CrossRef]
- Lee, W.J.; Seong, M. Sildenafil citrate and choroidal thickness. Retina 2011, 31, 1742. [Google Scholar] [CrossRef]
- Harris, A.; Kagemann, L.; Ehrlich, R.; Ehrlich, Y.; Lopez, C.R.; Purvin, V. The effect of sildenafil on ocular blood flow. Br. J. Ophthalmol. 2008, 92, 469–473. [Google Scholar] [CrossRef]
- Yiu, G.; Vuong, V.S.; Tran, S.; Migacz, J.; Cunefare, D.; Farsiu, S.; Khandelwal, N.; Agrawal, R.; Cheung, C.M.G. Vascular Response to Sildenafil Citrate in Aging and Age-Related Macular Degeneration. Sci. Rep. 2019, 9, 5049. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Silverman, R.H.; Chan, R.V.P.; Khanifar, A.A.; Rondeau, M.; Lloyd, H.; Schlegel, P.; Coleman, D.J. Measurement of choroidal perfusion and thickness following systemic sildenafil (Viagra®). Acta Ophthalmol. 2012, 91, 183–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, S.K.; Imamura, Y.; Freund, K.B. The Effects of Sildenafil Citrate on Choroidal Thickness as Determined by Enhanced Depth Imaging Optical Coherence Tomography. Retina 2011, 31, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Aslan, F.; Topcuoğlu, M.; Öktem, Ç.Ğ.; Akkoç, A.; Uçar, M. Can subfoveal choroidal thickness replace subjective tests in pa-tients using tadalafil to treat erectile dysfunction? Andrologia 2020, 52, 332–335. [Google Scholar] [CrossRef]
- Sarhan, N.R.; Omar, N.M. An immunohistochemical and ultrastructural analysis of the retina in tadalafil (Cialis) treated rats. Acta Histochem. 2018, 120, 312–322. [Google Scholar] [CrossRef] [PubMed]
- European Association of Urology Guidelines on Sexual and Reproductive Health. 2020. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Sexual-and-Reproductive-Health-2020.pdf (accessed on 25 March 2020).
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nashar, H.Y.; Hemeda, S. Assessment of peripapillary vessel density in acute non-arteritic anterior ischemic optic neu-ropathy. Int. Ophthalmol. 2020, 40, 1269–1276. [Google Scholar] [CrossRef]
- Baker, J.C.; Fintelmann, R.; Sharifi, R.; Lee, M. Precautions and Monitoring of Patients Taking Phosphodiesterase Type 5 In-hibitors Who are at Risk of Increased Intraocular Pressure. Drugs Aging 2019, 36, 991–997. [Google Scholar] [CrossRef]
- Pomeranz, H.D. Erectile Dysfunction Agents and Nonarteritic Anterior Ischemic Optic Neuropathy. Neurol. Clin. 2017, 35, 17–27. [Google Scholar] [CrossRef]
| Group B | Group A | p Value | |
|---|---|---|---|
| Eye (n.) | 54 | 54 | - |
| Age (years) | 64.1 ± 9 | 63.7 ± 6.9 | 0.933 |
| BCVA (logMAR) | 0.06 ± 0.08 | 0.04 ± 0.08 | 0.301 |
| Mean spherical equivalent (diopters) | −0.50 ± 1.75 | 0.75 ± 1.30 | 0.532 |
| Dyslipidemia (n. subjects) | 7 | 8 | 0.761 |
| Smoking habits (n. subjects) | 4 | 6 | 0.483 |
| Group B | Group A | p Value | |
|---|---|---|---|
| GCC (µm) | |||
| Average | 98.18 ± 6.27 | 96.68 ± 8.16 | 0.449 |
| Superior | 96.90 ± 6.15 | 96.22 ± 8.23 | 0.897 |
| Inferior | 98.05 ± 6.06 | 97.11 ± 8.25 | 0.680 |
| RNFL (µm) | |||
| Average | 99.94 ± 8.21 | 98.33 ± 7.89 | 0.625 |
| Superior | 102.74 ± 8.63 | 101.16 ± 8.87 | 0.518 |
| Inferior | 96.40 ± 6.80 | 95.33 ± 8.41 | 0.493 |
| Subfoveal choroidal thickness (µm) | 255.38 ± 64.24 | 389.05 ± 76.67 | <0.001 |
| Group B | Group A | p Value | |
|---|---|---|---|
| SPC (%) | |||
| Whole image | 51.63 ± 4.75 | 48.54 ± 4.22 | 0.002 |
| Parafovea | 53.63 ± 2.18 | 50.77 ± 6.97 | 0.013 |
| Fovea | 26.19 ± 4.58 | 22.53 ± 8.17 | 0.012 |
| DPC (%) | |||
| Whole image | 54.20 ± 2.82 | 51.20 ± 7.25 | 0.028 |
| Parafovea | 57.14 ± 2.52 | 54.31 ± 6.74 | 0.019 |
| Fovea | 44.28 ± 4.06 | 40.29 ± 9.61 | 0.021 |
| CC (%) | |||
| Whole image | 73.12 ± 3.19 | 73.16 ± 3.14 | 0.975 |
| Parafovea | 71.62 ± 5.16 | 71.47 ± 3.57 | 0.393 |
| Fovea | 70.03 ± 4.29 | 69.87 ± 5.60 | 0.601 |
| RPC (%) | |||
| Whole image | 50.87 ± 6.62 | 47.95 ± 2.53 | 0.044 |
| Inside Disc | 53.52 ± 3.33 | 51.32 ± 4.97 | 0.022 |
| Peripapillary | 52.82 ± 4.21 | 50.41 ± 2.64 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capece, M.; Montorio, D.; Comune, C.; Aveta, A.; Melchionna, A.; Celentano, G.; Imbimbo, C.; Crocetto, F.; Califano, G.; Cennamo, G. Retinal and Optic Disc Vascular Changes in Patients Using Long-Term Tadalafil: A Prospective Non-Randomized Matched-Pair Study. Diagnostics 2021, 11, 802. https://doi.org/10.3390/diagnostics11050802
Capece M, Montorio D, Comune C, Aveta A, Melchionna A, Celentano G, Imbimbo C, Crocetto F, Califano G, Cennamo G. Retinal and Optic Disc Vascular Changes in Patients Using Long-Term Tadalafil: A Prospective Non-Randomized Matched-Pair Study. Diagnostics. 2021; 11(5):802. https://doi.org/10.3390/diagnostics11050802
Chicago/Turabian StyleCapece, Marco, Daniela Montorio, Chiara Comune, Achille Aveta, Alberto Melchionna, Giuseppe Celentano, Ciro Imbimbo, Felice Crocetto, Gianluigi Califano, and Gilda Cennamo. 2021. "Retinal and Optic Disc Vascular Changes in Patients Using Long-Term Tadalafil: A Prospective Non-Randomized Matched-Pair Study" Diagnostics 11, no. 5: 802. https://doi.org/10.3390/diagnostics11050802







