Left Ventricular Deformation and Vortex Analysis in Heart Failure: From Ultrasound Technique to Current Clinical Application
Abstract
1. Introduction
2. Left Ventricular Speckle Tracking Echocardiography
2.1. Left Ventricular Mechanics
2.2. Left Ventricular Speckle Tracking Echocardiography: The Technique
2.3. Clinical Application of Left Ventricular Speckle Tracking Echocardiography to Heart Failure with Preserved Ejection Fraction
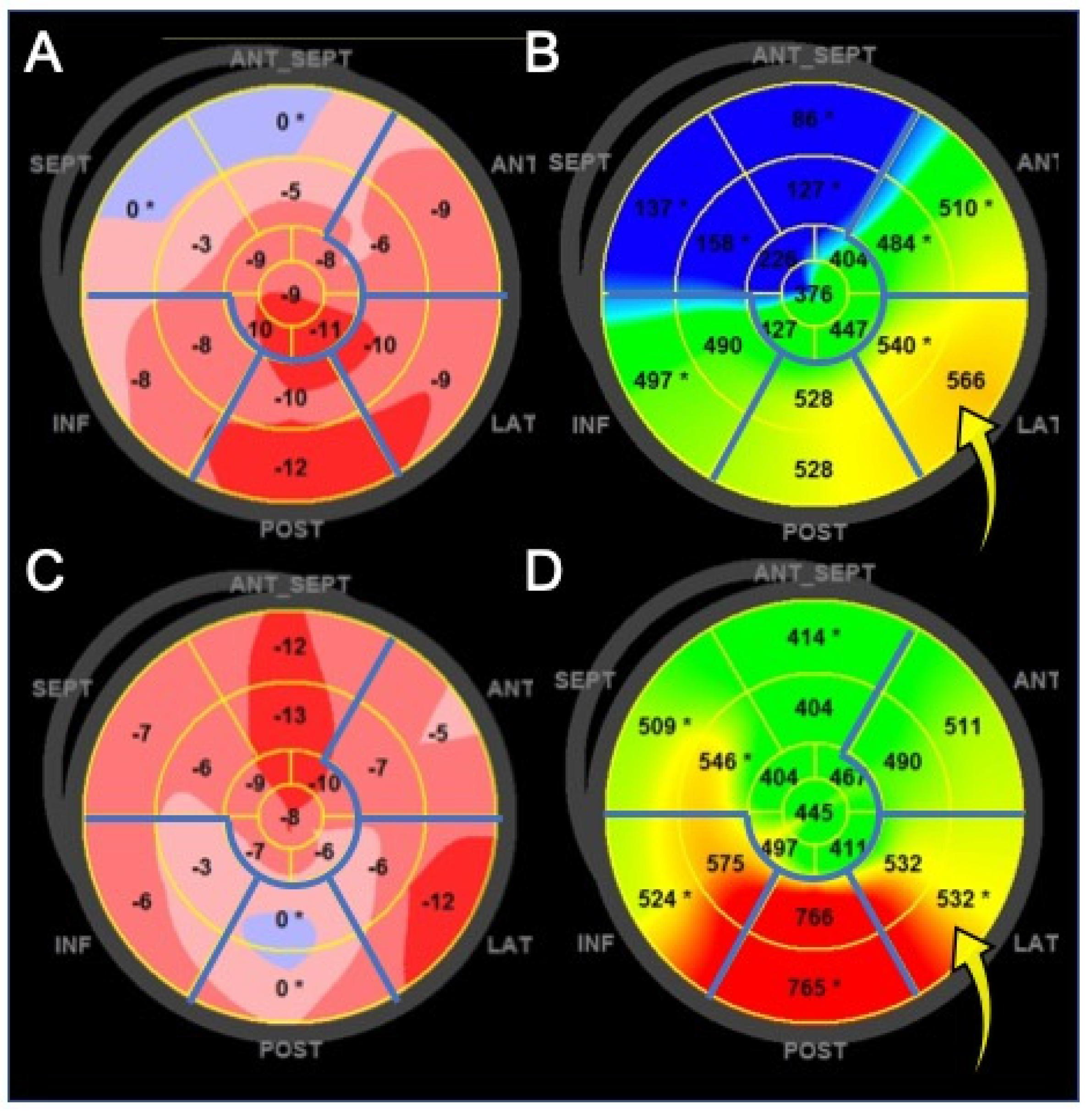
2.4. Clinical Application of Left Ventricular Speckle Tracking Echocardiography to Heart Failure with Reduced Ejection Fraction
3. Left Ventricular Color Doppler Flow Mapping
3.1. Left Ventricular Vortices
3.2. Color Doppler Flow Mapping: The Technique
3.3. Clinical Application of Color Doppler Flow Mapping to Heart Failure
4. New Ultrasound Technologies for Cardiac Resynchronization Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Omar, A.M.; Bansal, M.; Sengupta, P.P. Advances in Echocardiographic Imaging in Heart Failure with Reduced and Preserved Ejection Fraction. Circ. Res. 2016, 119, 357–374. [Google Scholar] [CrossRef]
- Bansal, M.; Kasliwal, R.R. How do I do it? Speckle-tracking echocardiography. Indian Heart J. 2013, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Sperlongano, S.; Pacileo, M.; Venturini, E.; Iannuzzo, G.; Gentile, M.; Sperlongano, R.; Vitale, G.; Maglione, M.; Cice, G.; et al. New Ultrasound Technologies for Ischemic Heart Disease Assessment and Monitoring in Cardiac Rehabilitation. J. Clin. Med. 2020, 9, 3131. [Google Scholar] [CrossRef] [PubMed]
- Mele, D.; Fiorencis, A.; Chiodi, E.; Gardini, C.; Benea, G.; Ferrari, R. Polar plot maps by parametric strain echocardiography allow accurate evaluation of non-viable transmural scar tissue in ischaemic heart disease. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, C.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Ma, X.-X.; Belyavskiy, E.; Kumar, R.A.; Kropf, M.; Kraft, R.; Frydas, A.; Osmanoglou, E.; Marquez, E.; Donal, E.; et al. Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: A meta-analysis. Open Heart 2017, 4, e000630. [Google Scholar] [CrossRef]
- Morris, D.A.; Boldt, L.H.; Eichstädt, H.; Ozcelik, C.; Haverkamp, W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ. Heart Fail. 2012, 5, 610–620. [Google Scholar] [CrossRef]
- Tschöpe, C.; Senni, M. Usefulness and clinical relevance of left ventricular global longitudinal systolic strain in patients with heart failure with preserved ejection fraction. Heart Fail. Rev. 2020, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; A De Boer, R.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- SShah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Liu, L.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015, 132, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Lund, L.H.; Oger, E.; Bosseau, C.; Reynaud, A.; Hage, C.; Drouet, E.; Daubert, J.-C.; Linde, C.; on behalf of the KaRen investigators. Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, F.; Yip, G.W.-K.; Sanderson, J.E.; Feng, W.; Xie, J.-M.; Luo, X.-X.; Lee, A.P.-W.; Lam, Y.-Y. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int. J. Cardiol. 2015, 178, 131–135. [Google Scholar] [CrossRef]
- Sengeløv, M.; Jørgensen, P.G.; Jensen, J.S.; Bruun, N.E.; Olsen, F.J.; Fritz-Hansen, T.; Nochioka, K.; Biering-Sørensen, T. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc. Imaging 2015, 8, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.H.; Naeemi, R.; Moaref, A.; Attar, A. Global longitudinal strain for prediction of ventricular arrhythmia in patients with heart failure. ESC Heart Fail. 2020, 7, 2956–2961. [Google Scholar] [CrossRef]
- Park, J.J.; Park, J.-B.; Park, J.-H.; Cho, G.-Y. Global Longitudinal Strain to Predict Mortality in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar] [CrossRef]
- Mazzetti, S.; Scifo, C.; Abete, R.; Margonato, D.; Chioffi, M.; Rossi, J.; Pisani, M.; Passafaro, G.; Grillo, M.; Poggio, D.; et al. Short-term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with sacubitril/valsartan. ESC Heart Fail. 2020, 7, 964–972. [Google Scholar] [CrossRef]
- Hung, C.-L.; Verma, A.; Uno, H.; Shin, S.-H.; Bourgoun, M.; Hassanein, A.H.; McMurray, J.J.; Velazquez, E.J.; Kober, L.; Pfeffer, M.A.; et al. Longitudinal and Circumferential Strain Rate, Left Ventricular Remodeling, and Prognosis After Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 56, 1812–1822. [Google Scholar] [CrossRef]
- Mele, D.; Smarrazzo, V.; Pedrizzetti, G.; Capasso, F.; Pepe, M.; Severino, S.; Luisi, G.A.; Maglione, M.; Ferrari, R. Intracardiac Flow Analysis: Techniques and Potential Clinical Applications. J. Am. Soc. Echocardiogr. 2019, 32, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-R.; Pedrizzetti, G.; Tonti, G.; Li, P.; Wei, Z.; Kim, J.K.; Baweja, A.; Liu, S.; Chung, N.; Houle, H.; et al. Characterization and Quantification of Vortex Flow in the Human Left Ventricle by Contrast Echocardiography Using Vector Particle Image Velocimetry. JACC Cardiovasc. Imaging 2008, 1, 705–717. [Google Scholar] [CrossRef]
- Kheradvar, A.; Houle, H.; Pedrizzetti, G.; Tonti, G.; Belcik, T.; Ashraf, M.; Lindner, J.R.; Gharib, M.; Sahn, D. Echocardiographic Particle Image Velocimetry: A Novel Technique for Quantification of Left Ventricular Blood Vorticity Pattern. J. Am. Soc. Echocardiogr. 2010, 23, 86–94. [Google Scholar] [CrossRef]
- Bermejo, J.; Benito, Y.; Alhama, M.; Yotti, R.; Martínez-Legazpi, P.; Del Villar, C.P.; Pérez-David, E.; González-Mansilla, A.; Santa-Marta, C.; Barrio, A.; et al. Intraventricular vortex properties in nonischemic dilated cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2014, 306, H718–H729. [Google Scholar] [CrossRef]
- Agati, L.; Cimino, S.; Tonti, G.; Cicogna, F.; Petronilli, V.; De Luca, L.; Iacoboni, C.; Pedrizzetti, G. Quantitative analysis of intraventricular blood flow dynamics by echocardiographic particle image velocimetry in patients with acute myocardial infarction at different stages of left ventricular dysfunction. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Mangual, J.O.; Kraigher-Krainer, E.; De Luca, A.; Toncelli, L.; Shah, A.; Solomon, S.; Galanti, G.; Domenichini, F.; Pedrizzetti, G. Comparative numerical study on left ventricular fluid dynamics after dilated cardiomyopathy. J. Biomech. 2013, 46, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Delgado, V.; Bertini, M.; Marsan, N.A.; Van De Veire, N.R.; Ng, A.C.; Siebelink, H.-M.J.; Schalij, M.J.; Holman, E.R.; Sengupta, P.P.; et al. Left Ventricular Muscle and Fluid Mechanics in Acute Myocardial Infarction. Am. J. Cardiol. 2010, 106, 1404–1409. [Google Scholar] [CrossRef]
- Abe, H.; Caracciolo, G.; Kheradvar, A.; Pedrizzetti, G.; Khandheria, B.K.; Narula, J.; Sengupta, P.P. Contrast echocardiography for assessing left ventricular vortex strength in heart failure: A prospective cohort study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1049–1060. [Google Scholar] [CrossRef]
- Poh, K.K.; Lee, L.C.; Shen, L.; Chong, E.; Tan, Y.L.; Chai, P.; Yeo, T.C.; Wood, M.J. Left ventricular fluid dynamics in heart failure: Echocardiographic measurement and utilities of vortex formation time. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 385–393. [Google Scholar] [CrossRef]
- Gharib, M.; Rambod, E.; Kheradvar, A.; Sahn, D.J.; Dabiri, J.O. Optimal vortex formation as an index of cardiac health. Proc. Natl. Acad. Sci. USA 2006, 103, 6305–6308. [Google Scholar] [CrossRef]
- Kim, I.-C.; Hong, G.-R.; Pedrizzetti, G.; Shim, C.Y.; Kang, S.-M.; Chung, N. Usefulness of Left Ventricular Vortex Flow Analysis for Predicting Clinical Outcomes in Patients with Chronic Heart Failure: A Quantitative Vorticity Imaging Study Using Contrast Echocardiography. Ultrasound Med. Biol. 2018, 44, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.G.F.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; Dicarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Sipahi, I.; Carrigan, T.P.; Rowland, D.Y.; Stambler, B.S.; Fang, J.C. Impact of QRS Duration on Clinical Event Reduction With Cardiac Resynchronization Therapy. Arch. Intern. Med. 2011, 171, 1454–1462. [Google Scholar] [CrossRef]
- Sipahi, I.; Chou, J.C.; Hyden, M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: Meta-analysis of randomized controlled trials. Am. Heart J. 2012, 163, 260–267. [Google Scholar] [CrossRef]
- Marek, J.; Gandalovičová, J.; Kejřová, E.; Pšenička, M.; Linhart, A.; Paleček, T. Echocardiography and cardiac resynchronization therapy. Cor et Vasa 2016, 58, e340–e351. [Google Scholar] [CrossRef]
- Mele, D.; Trevisan, F.; Fiorencis, A.; Smarrazzo, V.; Bertini, M.; Ferrari, R. Current Role of Echocardiography in Cardiac Resynchronization Therapy: From Cardiac Mechanics to Flow Dynamics Analysis. Curr. Heart Fail. Rep. 2020, 17, 384–396. [Google Scholar] [CrossRef]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.-P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef]
- Suffoletto, M.S.; Dohi, K.; Cannesson, M.; Saba, S.; Gorcsan, J. Novel Speckle-Tracking Radial Strain From Routine Black-and-White Echocardiographic Images to Quantify Dyssynchrony and Predict Response to Cardiac Resynchronization Therapy. Circulation 2006, 113, 960–968. [Google Scholar] [CrossRef]
- Gorcsan, J., 3rd; Oyenuga, O.; Habib, P.J.; Tanaka, H.; Adelstein, E.C.; Hara, H.; McNamara, D.M.; Saba, S. Relationship of Echocardiographic Dyssynchrony to Long-Term Survival After Cardiac Resynchronization Therapy. Circulation 2010, 122, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nesser, H.-J.; Buck, T.; Oyenuga, O.; Jánosi, R.A.; Winter, S.; Saba, S.; Gorcsan, J. Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: Results of the Speckle Tracking and Resynchronization (STAR) study. Eur. Heart J. 2010, 31, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; van Bommel, R.J.; Bertini, M.; Borleffs, C.J.W.; Marsan, N.A.; Ng, A.C.; Nucifora, G.; van de Veire, N.R.; Ypenburg, C.; Boersma, E.; et al. Relative Merits of Left Ventricular Dyssynchrony, Left Ventricular Lead Position, and Myocardial Scar to Predict Long-Term Survival of Ischemic Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. Circulation 2011, 123, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- Mele, D.; Toselli, T.; Capasso, F.; Stabile, G.; Piacenti, M.; Piepoli, M.; Giatti, S.; Klersy, C.; Sallusti, L.; Ferrari, R. Comparison of myocardial deformation and velocity dyssynchrony for identification of responders to cardiac resynchronization therapy. Eur. J. Heart Fail. 2009, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kirn, B.; Jansen, A.; Bracke, F.; Van Gelder, B.; Arts, T.; Prinzen, F.W. Mechanical discoordination rather than dyssynchrony predicts reverse remodeling upon cardiac resynchronization. Am. J. Physiol. Circ. Physiol. 2008, 295, H640–H646. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-L.; Wu, C.-T.; Yeh, Y.-H.; Wu, L.-S.; Chang, C.-J.; Ho, W.-J.; Hsu, L.-A.; Luqman, N.; Kuo, C.-T. Recoordination Rather than Resynchronization Predicts Reverse Remodeling after Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2010, 23, 611–620. [Google Scholar] [CrossRef]
- Mele, D.; Luisi, G.A.; Malagù, M.; Laterza, A.; Ferrari, R.; Bertini, M. Echocardiographic evaluation of cardiac dyssynchrony: Does it still matter? Echocardiography 2018, 35, 707–715. [Google Scholar] [CrossRef]
- Risum, N.; Jons, C.; Olsen, N.T.; Fritz-Hansen, T.; Bruun, N.E.; Hojgaard, M.V.; Valeur, N.; Kronborg, M.B.; Kisslo, J.; Sogaard, P. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: Rationale, initial results, and advantages. Am. Heart J. 2012, 163, 697–704. [Google Scholar] [CrossRef]
- Beela, A.S.; Ünlü, S.; Duchenne, J.; Ciarka, A.; Daraban, A.M.; Kotrc, M.; Aarones, M.; Szulik, M.; Winter, S.; Penicka, M.; et al. Assessment of mechanical dyssynchrony can improve the prognostic value of guideline-based patient selection for cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging 2018, 20, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mada, R.O.; Lysyansky, P.; Duchenne, J.; Beyer, R.; Mada, C.; Muresan, L.; Rosianu, H.; Serban, A.; Winter, S.; Fehske, W.; et al. New Automatic Tools to Identify Responders to Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2016, 29, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Leyva, F.; Foley, P.W.; Chalil, S.; Ratib, K.; Smith, R.E.; Prinzen, F.; Auricchio, A. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2011, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Zwicker, C.; Kaminski, M.; Napp, A.; Altiok, E.; Ocklenburg, C.; Friedman, Z.; Adam, D.; Schauerte, P.; Marx, N.; et al. Dependency of Cardiac Resynchronization Therapy on Myocardial Viability at the LV Lead Position. JACC Cardiovasc. Imaging 2011, 4, 366–374. [Google Scholar] [CrossRef]
- Khan, F.Z.; Virdee, M.S.; Palmer, C.R.; Pugh, P.J.; O’Halloran, D.; Elsik, M.; Read, P.A.; Begley, D.; Fynn, S.P.; Dutka, D.P. Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2012, 59, 1509–1518. [Google Scholar] [CrossRef]
- Saba, S.; Marek, J.; Schwartzman, D.; Jain, S.; Adelstein, E.; White, P.; Oyenuga, O.A.; Onishi, T.; Soman, P.; Gorcsan, J. Echocardiography-Guided Left Ventricular Lead Placement for Cardiac Resynchronization Therapy. Circ. Heart Fail. 2013, 6, 427–434. [Google Scholar] [CrossRef]
- Mele, D.; Nardozza, M.; Malagù, M.; Leonetti, E.; Fragale, C.; Rondinella, A.; Ferrari, R.; Bertini, M. Left Ventricular Lead Position Guided by Parametric Strain Echocardiography Improves Response to Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2017, 30, 1001–1011. [Google Scholar] [CrossRef]
- Mele, D.; Smarrazzo, V.; Pedrizzetti, G.; Bertini, M.; Ferrari, R. Intracardiac flow analysis in cardiac resynchronization therapy: A new challenge? Echocardiography 2019, 36, 1919–1929. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Martiniello, A.R.; Bianchi, V.; D’Onofrio, A.; Caso, P.; Tonti, G. Cardiac fluid dynamics anticipates heart adaptation. J. Biomech. 2015, 48, 388–391. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Martiniello, A.R.; Bianchi, V.; D’Onofrio, A.; Caso, P.; Tonti, G. Changes in electrical activation modify the orientation of left ventricular flow momentum: Novel observations using echocardiographic particle image velocimetry. Eur. Heart J. Cardiovasc. Imaging 2015, 17, 203–209. [Google Scholar] [CrossRef]
- Cimino, S.; Palombizio, D.; Cicogna, F.; Cantisani, D.; Reali, M.; Filomena, D.; Petronilli, V.; Iacoboni, C.; Agati, L. Significant increase of flow kinetic energy in “nonresponders” patients to cardiac resynchronization therapy. Echocardiography 2017, 34, 709–715. [Google Scholar] [CrossRef] [PubMed]



| HF Phenotype | LV Damage | Deformation Imaging | Diagnostic Value of GLS | Prognostic Value of GLS |
|---|---|---|---|---|
| HFpEF | Subendocardial | Longitudinal dysfunction | <16% (minor criteria) [12] | Predictor of poor prognosis when impaired (HF hospitalization, CV death, cardiac arrest) [8,13,14,15] |
| HFrEF | Transmural | Longitudinal and circumferential dysfunction | - | Predictor of poor prognosis when impaired (all-cause mortality, ventricular arrhythmias) [16,17,18] Early marker of response to therapy for HF [19] |
| Index | Cut-Off Value | Imaging Method | Echocardiographic View | Parameter to Evaluate |
|---|---|---|---|---|
| ASPWD | 130 ms | 2D STE | PSAX (papillary muscles) | LV radial dyssynchrony |
| SDI | 25% | 2D STE | Apical 4-, 2-, and 3-C | LV longitudinal dyssynchrony |
| Tε-SD (MDI) | 60 ms | 2D STE | Apical 4-, 2-, and 3-C | LV longitudinal dyssynchrony |
| Septal flash | 130 ms | 2D STE | Apical 4-C | LV discoordination/ anomalous wall movements |
| Apical rocking | 9.8% | 2D STE | Apical 4-C or 3-C | LV discoordination/ anomalous wall movements |
| RDI | >40% | 2D STE | PSAX (papillary muscles) | LV radial discoordination |
| SRS | 4.7% | 2D STE | Apical 4-C | LV discoordination/ anomalous wall movements |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperlongano, S.; D’Andrea, A.; Mele, D.; Russo, V.; Pergola, V.; Carbone, A.; Ilardi, F.; Di Maio, M.; Bottino, R.; Giallauria, F.; et al. Left Ventricular Deformation and Vortex Analysis in Heart Failure: From Ultrasound Technique to Current Clinical Application. Diagnostics 2021, 11, 892. https://doi.org/10.3390/diagnostics11050892
Sperlongano S, D’Andrea A, Mele D, Russo V, Pergola V, Carbone A, Ilardi F, Di Maio M, Bottino R, Giallauria F, et al. Left Ventricular Deformation and Vortex Analysis in Heart Failure: From Ultrasound Technique to Current Clinical Application. Diagnostics. 2021; 11(5):892. https://doi.org/10.3390/diagnostics11050892
Chicago/Turabian StyleSperlongano, Simona, Antonello D’Andrea, Donato Mele, Vincenzo Russo, Valeria Pergola, Andreina Carbone, Federica Ilardi, Marco Di Maio, Roberta Bottino, Francesco Giallauria, and et al. 2021. "Left Ventricular Deformation and Vortex Analysis in Heart Failure: From Ultrasound Technique to Current Clinical Application" Diagnostics 11, no. 5: 892. https://doi.org/10.3390/diagnostics11050892
APA StyleSperlongano, S., D’Andrea, A., Mele, D., Russo, V., Pergola, V., Carbone, A., Ilardi, F., Di Maio, M., Bottino, R., Giallauria, F., Bossone, E., & Golino, P. (2021). Left Ventricular Deformation and Vortex Analysis in Heart Failure: From Ultrasound Technique to Current Clinical Application. Diagnostics, 11(5), 892. https://doi.org/10.3390/diagnostics11050892













