Microstructural MRI Correlates of Cognitive Impairment in Multiple Sclerosis: The Role of Deep Gray Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Neuropsychological Assessment
2.3. MRI Data Collection
2.4. MRI Data Processing and Features Extraction
2.5. Statistical Analysis
3. Results
3.1. Neuropsychological Performance between ACN and CI Patients
3.2. Qualitative Analysis of Microstructural Indices
3.3. Statistical Analysis of MRI-Derived Numerical Biomarkers
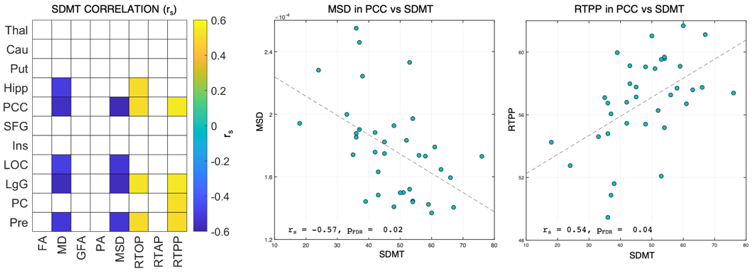
3.4. Associations between MRI-Derived Numerical Biomarkers and Neuropsychological Tests
4. Discussion
4.1. ACN and CI Groups Differed in Neuropsychological Performance
4.2. Between-Group Changes in Deep GM Are Depicted by dMRI Measures Only
4.3. Cortical Thickness and Microstructure Are Selectively Different between the Two Groups in Cortical Areas
4.4. dMRI Measures Correlate with the SDMT
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.G.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- McNicholas, N.; O’Connell, K.; Yap, S.M.; Killeen, R.P.; Hutchinson, M.; McGuigan, C. Cognitive dysfunction in early multiple sclerosis: A review. QJM 2018, 111, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Hakiki, B.; Giannini, M.; Pastò, L.; Razzolini, L. Cognitive impairment in early stages of multiple sclerosis. Neurol. Sci. 2010, 31, 211–214. [Google Scholar] [CrossRef]
- Kalb, R.; Beier, M.; Benedict, R.H.B.; Charvet, L.; Costello, K.; Feinstein, A.; Gingold, J.; Goverover, Y.; Halper, J.; Harris, C.; et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult. Scler. J. 2018, 1665–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillema, J.M.; Hulst, H.E.; Rocca, M.A.; Vrenken, H.; Steenwijk, M.D.; Damjanovic, D.; Enzinger, C.; Ropele, S.; Tedeschi, G.; Gallo, A.; et al. Regional cortical thinning in multiple sclerosis and its relation with cognitive impairment: A multicenter study. Mult. Scler. J. 2016, 22, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debernard, L.; Melzer, T.R.; Alla, S.; Eagle, J.; Van Stockum, S.; Graham, C.; Osborne, J.R.; Dalrymple-Alford, J.C.; Miller, D.H.; Mason, D.F. Deep grey matter MRI abnormalities and cognitive function in relapsing-remitting multiple sclerosis. Psychiatry Res. Neuroimaging 2015, 234, 352–361. [Google Scholar] [CrossRef]
- Calabrese, M.; Battaglini, M.; Giorgio, A.; Atzori, M.; Bernardi, V.; Mattisi, I.; Gallo, P.; De Stefano, N. Imaging distribution and frequency of cortical lesions in patients with multiple sclerosis. Neurology 2010, 75, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, O.; Pflugshaupt, T.; Bezzola, L.; Reuter, K.; Weller, D.; Schuknecht, B.; Brugger, P.; Linnebank, M. The relevance of cortical lesions in patients with multiple sclerosis. BMC Neurol. 2016, 16, 204. [Google Scholar] [CrossRef] [Green Version]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Rocca, M.A.; Amato, M.P.; De Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.-K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef]
- Rocca, M.A.; Comi, G.; Filippi, M. Surrogate Markers in Multiple Sclerosis: The Role of Magnetic Resonance Imaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128020074. [Google Scholar]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Vrenken, H.; Pouwels, P.J.W.; Geurts, J.J.G.; Knol, D.L.; Polman, C.H.; Barkhof, F.; Castelijns, J.A. Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: Cortical diffusion changes seem related to clinical deterioration. J. Magn. Reson. Imaging 2006, 23, 628–636. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Bruce, J.; Dwyer, M.G.; Weinstock-Guttman, B.; Tjoa, C.; Tavazzi, E.; Munschauer, F.E.; Zivadinov, R. Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult. Scler. J. 2007, 13, 722–730. [Google Scholar] [CrossRef]
- Tovar-Moll, F.; Evangelou, I.E.; Chiu, A.W.; Richert, N.D.; Ostuni, J.L.; Ohayon, J.M.; Auh, S.; Ehrmantraut, M.; Talagala, S.L.; McFarland, H.F.; et al. Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: A diffusion tensor imaging study at 3T. Am. J. Neuroradiol. 2009, 30, 1380–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, A.; Rocca, M.A.; Falini, A.; Tortorella, P.; Pagani, E.; Rodegher, M.; Comi, G.; Scotti, G.; Filippi, M. Normal-appearing white and grey matter damage in MS: A volumetric and diffusion tensor MRI study at 3.0 Tesla. J. Neurol. 2007, 254, 513–518. [Google Scholar] [CrossRef]
- Griffin, C.M.; Chard, D.T.; Ciccarelli, O.; Kapoor, R.; Barker, G.J.; Thompson, A.J.; Miller, D.H. Diffusion tensor imaging in early relapsing—Remitting multiple sclerosis. Mult. Scler. J. 2001, 7, 290–297. [Google Scholar]
- Brusini, L.; Boscolo Galazzo, I.; Akinci, M.; Cruciani, F.; Pitteri, M.; Ziccardi, S.; Bajrami, A.; Castellaro, M.; Salih, A.; Pizzini, F.B.; et al. Microstructural modulations in the hippocampus allow to characterizing relapsing-remitting versus primary progressive multiple sclerosis. In Proceedings of the MICCAI BrainLes 2020 Workshop, Lima, Peru, 4 October 2020. [Google Scholar] [CrossRef]
- Preziosa, P.; Rocca, M.A.; Pagani, E.; Stromillo, M.L.; Enzinger, C.; Gallo, A.; Hulst, H.E.; Atzori, M.; Pareto, D.; Riccitelli, G.C.; et al. Structural MRI correlates of cognitive impairment in patients with multiple sclerosis. Hum. Brain Mapp. 2016, 37, 1627–1644. [Google Scholar] [CrossRef]
- Pagani, E.; Rocca, M.A.; De Meo, E.; Horsfield, M.A.; Colombo, B.; Rodegher, M.; Comi, G.; Filippi, M. Structural connectivity in multiple sclerosis and modeling of disconnection. Mult. Scler. J. 2020, 26, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Daams, M.; Steenwijk, M.D.; Schoonheim, M.M.; Wattjes, M.P.; Balk, L.J.; Tewarie, P.K.; Killestein, J.; Uitdehaag, B.M.J.; Geurts, J.J.G.; Barkhof, F. Multi-parametric structural magnetic resonance imaging in relation to cognitive dysfunction in long-standing multiple sclerosis. Mult. Scler. J. 2016, 22, 608–619. [Google Scholar] [CrossRef]
- Klinke, M.E.; Hafsteinsdóttir, T.B.; Hjaltason, H.; Jónsdóttir, H. Ward-based interventions for patients with hemispatial neglect in stroke rehabilitation: A systematic literature review. Int. J. Nurs. Stud. 2015, 52, 1375–1403. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, M.; Ziccardi, S.; Dapor, C.; Guandalini, M.; Calabrese, M. Lost in Classification: Lower Cognitive Functioning in Apparently Cognitive Normal Newly Diagnosed RRMS Patients. Brain Sci. 2019, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis. Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitteri, M.; Romualdi, C.; Magliozzi, R.; Monaco, S.; Calabrese, M. Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult. Scler. J. 2017, 23, 848–854. [Google Scholar] [CrossRef]
- Magliozzi, R.; Pitteri, M.; Ziccardi, S.; Pisani, A.I.; Montibeller, L.; Marastoni, D.; Rossi, S.; Mazziotti, V.; Guandalini, M.; Dapor, C.; et al. CSF parvalbumin levels reflect interneuron loss linked with cortical pathology in multiple sclerosis. Ann. Clin. Transl. Neurol. 2021, 8, 534–547. [Google Scholar] [CrossRef]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Ricchiuti, L.; De Caro, M.F.; Patti, F.; Vecchio, R.; Sorbi, S.; Trojano, M. The Rao’ s Brief Repeatable Battery and Stroop test: Normative values with age, education and gender corrections in an Italian population. Mult. Scler. J. 2006, 12, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. A short version of the Stroop test: Normative data in an Italian population sample. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Eshaghi, A.; Prados, F.; Brownlee, W.J.; Altmann, D.R.; Tur, C.; Cardoso, M.J.; De Angelis, F.; van de Pavert, S.H.; Cawley, N.; De Stefano, N.; et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann. Neurol. 2018, 83, 210–222. [Google Scholar] [CrossRef] [Green Version]
- Batista, S.; Zivadinov, R.; Hoogs, M.; Bergsland, N.; Heininen-Brown, M.; Dwyer, M.G.; Weinstock-Guttman, B.; Benedict, R.H.B. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J. Neurol. 2012, 259, 139–146. [Google Scholar] [CrossRef]
- Özarslan, E.; Koay, C.G.; Shepherd, T.M.; Komlosh, M.E.; Irfanoǧlu, M.O.; Pierpaoli, C.; Basser, P.J. Mean apparent propagator (MAP) MRI: A novel diffusion imaging method for mapping tissue microstructure. Neuroimage 2013, 78, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Zucchelli, M.; Brusini, L.; Andrés Méndez, C.; Daducci, A.; Granziera, C.; Menegaz, G. What lies beneath? Diffusion EAP-based study of brain tissue microstructure. Med. Image Anal. 2016, 32, 145–156. [Google Scholar] [CrossRef]
- Merlet, S.L.; Deriche, R. Continuous diffusion signal, EAP and ODF estimation via Compressive Sensing in diffusion MRI. Med. Image Anal. 2013, 17, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Alexander, A.L. Hybrid diffusion imaging. Neuroimage 2007, 36, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavi, S.; Petracca, M.; Sun, P.; Fleysher, L.; Cocozza, S.; El Mendili, M.M.; Signori, A.; Babb, J.S.; Podranski, K.; Song, S.-K.; et al. Non-invasive quantification of inflammation, axonal and myelin injury in multiple sclerosis. Brain 2021, 144, 213–223. [Google Scholar] [CrossRef]
- Artemiadis, A.; Anagnostouli, M.; Zalonis, I.; Chairopoulos, K.; Triantafyllou, N. Structural MRI correlates of cognitive function in multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Fischer, M.; Kunkel, A.; Bublak, P.; Faiss, J.H.; Hoffmann, F.; Sailer, M.; Schwab, M.; Zettl, U.K.; Köhler, W. How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis? J. Neurol. Sci. 2014, 343, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y. Imaging laminar structures in the gray matter with diffusion MRI. Neuroimage 2019, 197, 677–688. [Google Scholar] [CrossRef]
- Roebroeck, A.; Miller, K.L.; Aggarwal, M. Ex vivo diffusion MRI of the human brain: Technical challenges and recent advances. NMR Biomed. 2019, 32, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontillo, G.; Lanzillo, R.; Russo, C.; Stasi, M.D.; Paolella, C.; Vola, E.A.; Criscuolo, C.; Borrelli, P.; Palma, G.; Tedeschi, E.; et al. Determinants of deep gray matter atrophy in multiple sclerosis: A multimodal MRI study. Am. J. Neuroradiol. 2019, 40, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorefice, L.; Carta, E.; Frau, J.; Contu, F.; Casaglia, E.; Coghe, G.; Barracciu, M.A.; Cocco, E.; Fenu, G. The impact of deep grey matter volume on cognition in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102351. [Google Scholar] [CrossRef]
- Costa, S.L.; Genova, H.M.; DeLuca, J.; Chiaravalloti, N.D. Information processing speed in multiple sclerosis: Past, present, and future. Mult. Scler. J. 2016, 23, 772–789. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Carolus, K.; Benedict, R.H.B.; Bergsland, N.; Ramasamy, D.; Jakimovski, D.; Weinstock-Guttman, B.; Kuceyeski, A.; Zivadinov, R.; Dwyer, M.G.; et al. Impact of Focal White Matter Damage on Localized Subcortical Gray Matter Atrophy in Multiple Sclerosis: A 5-Year Study. Am. J. Neuroradiol. 2018, 39, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Kuceyeski, A.F.; Vargas, W.; Dayan, M.; Monohan, E.; Blackwell, C.; Raj, A.; Fujimoto, K.; Gauthier, S.A. Modeling the relationship among gray matter atrophy, abnormalities in connecting white matter, and cognitive performance in early multiple sclerosis. Am. J. Neuroradiol. 2015, 36, 702–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulst, H.E.; Schoonheim, M.M.; Van Geest, Q.; Uitdehaag, B.M.J.; Barkhof, F.; Geurts, J.J.G. Memory impairment in multiple sclerosis: Relevance of hippocampal activation and hippocampal connectivity. Mult. Scler. J. 2015, 21, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, M.M.; Hulst, H.E.; Brandt, R.B.; Strik, M.; Wink, A.M.; Uitdehaag, B.M.J.; Barkhof, F.; Geurts, J.J.G. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 2015, 84, 776–783. [Google Scholar] [CrossRef]
- Rimkus, C.M.; Schoonheim, M.M.; Steenwijk, M.D.; Vrenken, H.; Eijlers, A.J.C.; Killestein, J.; Wattjes, M.P.; Leite, C.C.; Barkhof, F.; Tijms, B.M. Gray matter networks and cognitive impairment in multiple sclerosis. Mult. Scler. J. 2019, 25, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Benedict, R.H.B. Frontal Cortex Atrophy Predicts Cognitive Impairment in Multiple Sclerosis. J. Neuropsychiatr. 2002, 14, 44–51. [Google Scholar] [CrossRef]
- Pitteri, M.; Dapor, C.; Pisani, A.I.; Castellaro, M.; DeLuca, J.; Chiaravalloti, N.; Guandalini, M.; Ziccardi, S.; Calabrese, M. Executive functioning affects verbal learning process in multiple sclerosis patients: Behavioural and imaging results. J. Neuropsychol. 2019, 14, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.J.; Yarkoni, T.; Khaw, M.W.; Sanfey, A.G. Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cereb. Cortex 2013, 23, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Liu, X.; Van Dam, N.T.; Hof, P.R.; Fan, J. Cognition-emotion integration in the anterior insular cortex. Cereb. Cortex 2013, 23, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, M.; Agosta, F.; Rinaldi, F.; Mattisi, I.; Grossi, P.; Favaretto, A.; Atzori, M.; Bernardi, V.; Barachino, L.; Rinaldi, L.; et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 2009, 66, 1144–1150. [Google Scholar] [CrossRef] [Green Version]
- Van Den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Eijlers, A.J.C.; Meijer, K.A.; Van Geest, Q.; Geurts, J.J.G.; Schoonheim, M.M. Determinants of cognitive impairment in patients with multiple sclerosis with and without atrophy. Radiology 2018, 288, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliozzi, R.; Reynolds, R.; Calabrese, M. MRI of cortical lesions and its use in studying their role in MS pathogenesis and disease course. Brain Pathol. 2018, 28, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Brusini, L.; Cruciani, F.; Boscolo Galazzo, I.; Pitteri, M.; Storti, S.F.; Calabrese, M.; Lorenzi, M.; Menegaz, G. Multivariate data analysis suggests the link between brain microstructure and cognitive impairment in multiple sclerosis. In Proceedings of the ISBI 2021, Nice, France, 13–16 April 2021. [Google Scholar] [CrossRef]
- Benedict, R.H.; DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. J. 2017, 23, 721–733. [Google Scholar] [CrossRef]
- Benedict, R.H.; Cookfair, D.; Gavett, R.; Gunther, M.; Munschauer, F.; Garg, N.; Weinstock-Guttman, B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J. Int. Neuropsychol. Soc. 2006, 12, 549–558. [Google Scholar] [CrossRef]
- Genova, H.M.; Hillary, F.G.; Wylie, G.; Rypma, B.; Deluca, J. Examination of processing speed deficits in multiple sclerosis using functional magnetic resonance imaging. J. Int. Neuropsychol. Soc. 2009, 15, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 413–427. [Google Scholar] [CrossRef] [Green Version]
- Meijer, K.A.; Eijlers, A.J.C.; Douw, L.; Uitdehaag, B.M.J.; Barkhof, F.; Geurts, J.J.G.; Schoonheim, M.M. Increased connectivity of hub networks and cognitive impairment in multiple sclerosis. Neurology 2017, 88, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Eijlers, A.J.C.; Meijer, K.A.; Wassenaar, T.M.; Steenwijk, M.D.; Uitdehaag, B.M.J.; Barkhof, F.; Wink, A.M.; Geurts, J.J.G.; Schoonheim, M.M. Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 2017, 88, 952–960. [Google Scholar] [CrossRef]
- Louapre, C.; Perlbarg, V.; García-Lorenzo, D.; Urbanski, M.; Benali, H.; Assouad, R.; Galanaud, D.; Freeman, L.; Bodini, B.; Papeix, C.; et al. Brain networks disconnection in early multiple sclerosis cognitive deficits: An anatomofunctional study. Hum. Brain Mapp. 2014, 35, 4706–4717. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, S.; Gallo, A.; Sacco, R.; Della Corte, M.; Bisecco, A.; Docimo, R.; Lavorgna, L.; Corbo, D.; Di Costanzo, A.; Tortora, F.; et al. Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult. Scler. J. 2011, 17, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Pardini, M.; Castellazzi, G.; Lascialfari, A.; Chard, D.; D’Angelo, E.; Gandini Wheeler-Kingshott, C.A.M. Default Mode Network Structural Integrity and Cerebellar Connectivity Predict Information Processing Speed Deficit in Multiple Sclerosis. Front. Cell. Neurosci. 2019, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Van Geest, Q.; Douw, L.; van ‘t Klooster, S.; Leurs, C.E.; Genova, H.M.; Wylie, G.R.; Steenwijk, M.D.; Killestein, J.; Geurts, J.J.G.; Hulst, H.E. Information processing speed in multiple sclerosis: Relevance of default mode network dynamics. NeuroImage Clin. 2018, 19, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.A.; van Geest, Q.; Eijlers, A.J.C.; Geurts, J.J.G.; Schoonheim, M.M.; Hulst, H.E. Is impaired information processing speed a matter of structural or functional damage in MS? NeuroImage Clin. 2018, 20, 844–850. [Google Scholar] [CrossRef] [PubMed]
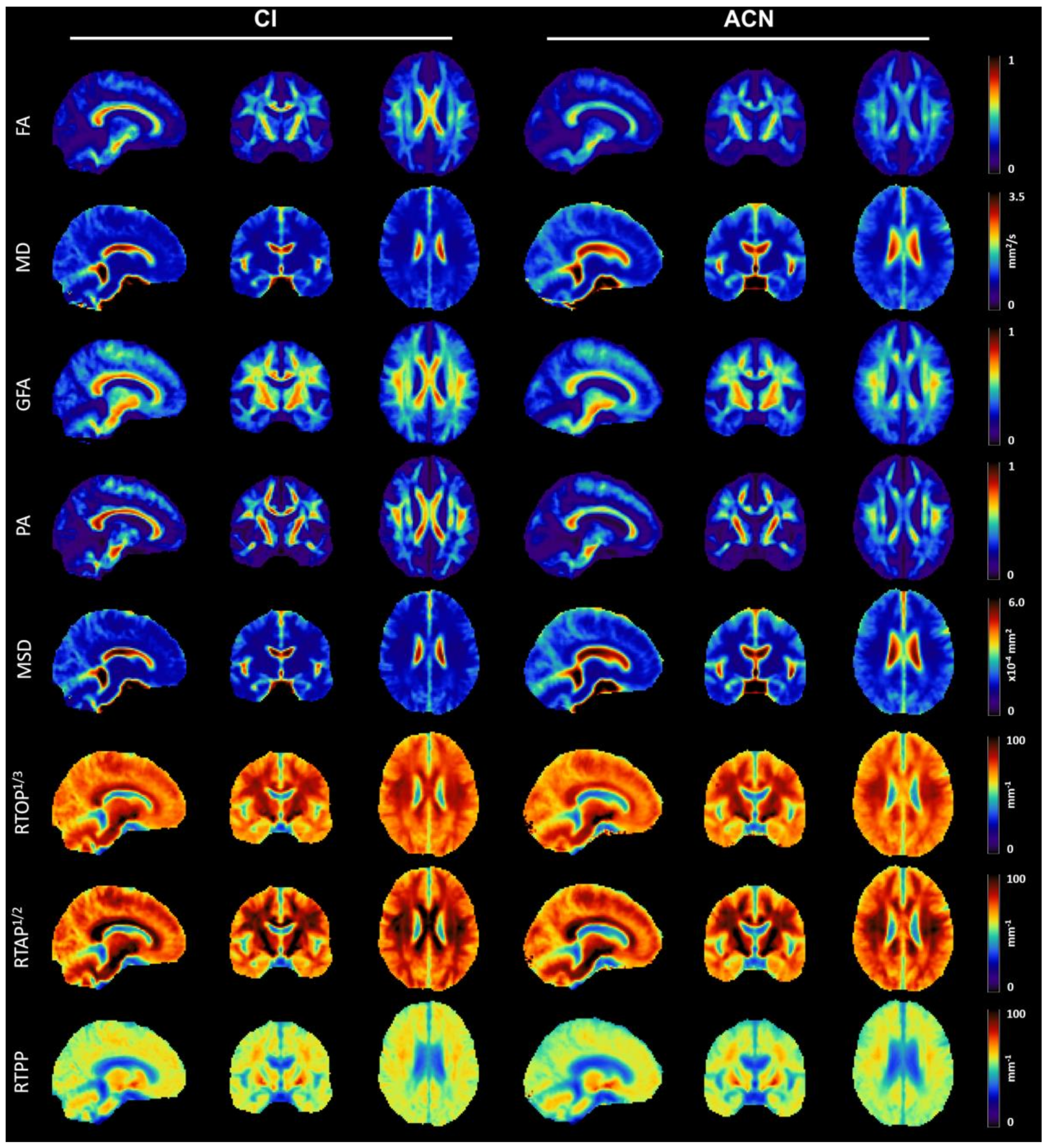
| MS Cohort (=36) | ACN-MS (=11) | CI-MS (=25) | p-Value | |
|---|---|---|---|---|
| Age (years) | 46.1 ± 8.1 | 41.9 ± 8.7 | 48.0 ± 7.2 | p = 0.04 * |
| Education (years) | 13.0 ± 3.5 | 13.9 ± 3.8 | 12.6 ± 3.3 | p = 0.29 |
| Gender (M/F) | 12/24 | 2/9 | 10/15 | p = 0.20 |
| Disease duration (years) | 9.1 ± 7.7 | 8.6 ± 8.3 | 9.2 ± 7.6 | p = 0.83 |
| EDSS | 2.5 (0–7) | 1.5 (0–3.5) | 2.5 (0–7) | p = 0.05 * |
| Index | ROI | Mean Difference (CI—ACN) | Std. Error | Sig. | Effect Size (Hedge’s g) |
|---|---|---|---|---|---|
| FA | Cau | 0.019 | 0.006 | 0.004 | 1.030 |
| Put | 0.017 | 0.007 | 0.008 | 0.947 | |
| GFA | Cau | 0.027 | 0.014 | 0.050 | 0.723 |
| Put | 0.031 | 0.014 | 0.026 | 0.807 | |
| PC | −0.027 | 0.014 | 0.045 | −0.724 | |
| Pre | −0.030 | 0.014 | 0.030 | −0.804 | |
| PA | Thal | 0.022 | 0.006 | 0.001 | 1.047 |
| Cau | 0.020 | 0.006 | 0.002 | 1.039 | |
| Put | 379.60 | 173.941 | 0.030 | 0.775 | |
| PCC | −423.12 | 173.941 | 0.015 | −0.865 | |
| RTAP [mm−2] | Ins | −369.61 | 173.941 | 0.034 | −0.754 |
| LgG | −390.49 | 173.941 | 0.025 | −0.797 | |
| PC | −378.01 | 169.358 | 0.026 | −0.773 | |
| Pre | −445.32 | 169.358 | 0.009 | −0.910 | |
| Put | 40,367.72 | 17,133.29 | 0.019 | 0.835 | |
| PCC | −39,119.08 | 17,133.29 | 0.023 | −0.809 | |
| RTOP [mm−3] | LgG | −36,666.03 | 17,133.29 | 0.033 | −0.748 |
| PC | −32,923.28 | 16,681.81 | 0.049 | −0.681 | |
| Pre | −41,099.81 | 16,681.81 | 0.014 | −0.851 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitteri, M.; Galazzo, I.B.; Brusini, L.; Cruciani, F.; Dapor, C.; Marastoni, D.; Menegaz, G.; Calabrese, M. Microstructural MRI Correlates of Cognitive Impairment in Multiple Sclerosis: The Role of Deep Gray Matter. Diagnostics 2021, 11, 1103. https://doi.org/10.3390/diagnostics11061103
Pitteri M, Galazzo IB, Brusini L, Cruciani F, Dapor C, Marastoni D, Menegaz G, Calabrese M. Microstructural MRI Correlates of Cognitive Impairment in Multiple Sclerosis: The Role of Deep Gray Matter. Diagnostics. 2021; 11(6):1103. https://doi.org/10.3390/diagnostics11061103
Chicago/Turabian StylePitteri, Marco, Ilaria Boscolo Galazzo, Lorenza Brusini, Federica Cruciani, Caterina Dapor, Damiano Marastoni, Gloria Menegaz, and Massimiliano Calabrese. 2021. "Microstructural MRI Correlates of Cognitive Impairment in Multiple Sclerosis: The Role of Deep Gray Matter" Diagnostics 11, no. 6: 1103. https://doi.org/10.3390/diagnostics11061103
APA StylePitteri, M., Galazzo, I. B., Brusini, L., Cruciani, F., Dapor, C., Marastoni, D., Menegaz, G., & Calabrese, M. (2021). Microstructural MRI Correlates of Cognitive Impairment in Multiple Sclerosis: The Role of Deep Gray Matter. Diagnostics, 11(6), 1103. https://doi.org/10.3390/diagnostics11061103








