Renal Complications Related to Checkpoint Inhibitors: Diagnostic and Therapeutic Strategies
Abstract
:1. Introduction
1.1. ICIs
1.2. Incidence of Renal irAEs
1.3. A Paradigm Shift from Renal “Toxicity”
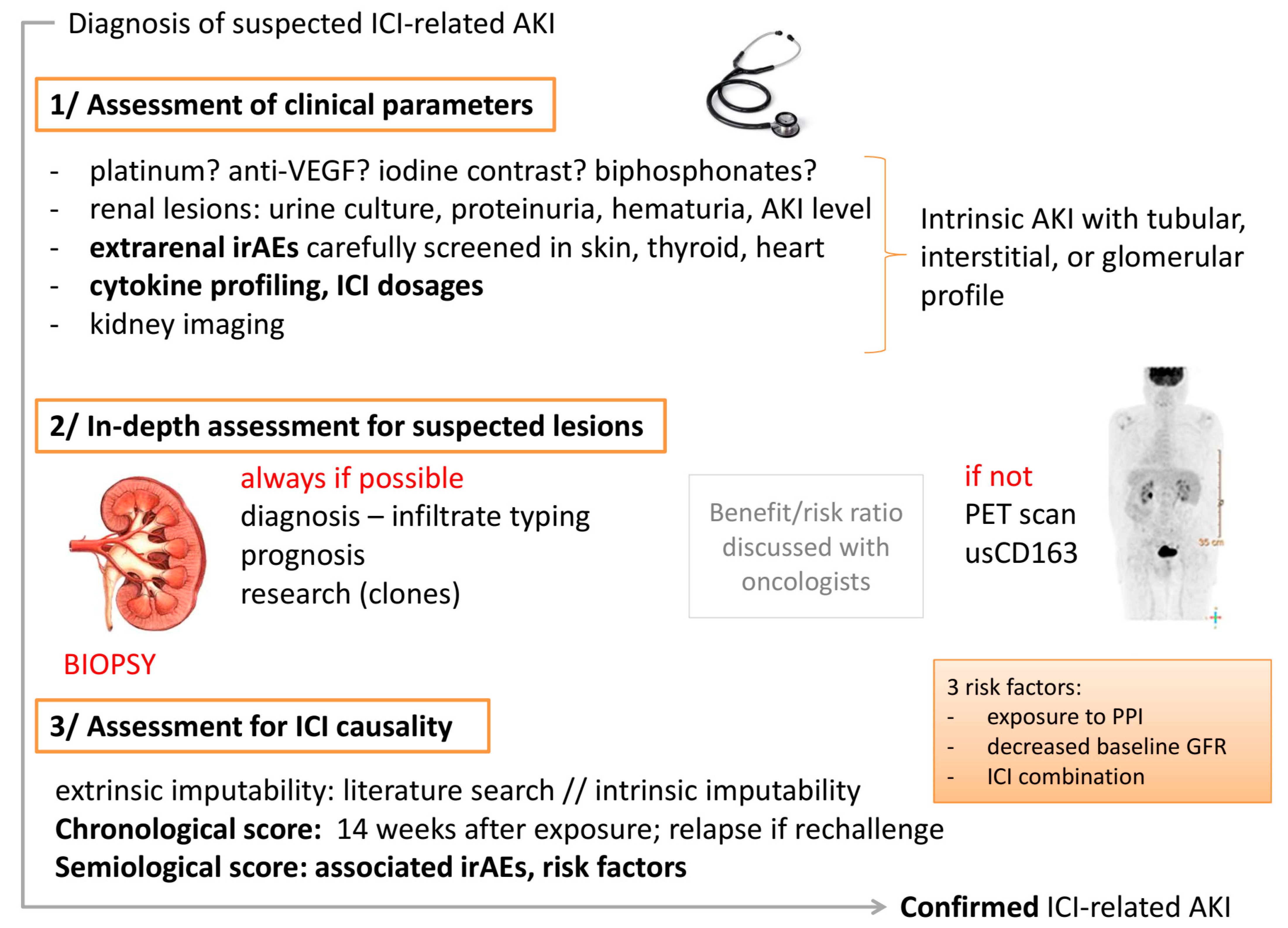
2. Diagnostic Strategies
2.1. First Step: Assessment for Clinical Renal Presentation
2.2. Second Step: In-Depth Assessment for Suspected Lesions
2.3. Third Step: Assessment for ICI Causality in Renal Lesions
- Extrinsic imputability: A literature search should be performed to identify similar cases.
- Intrinsic imputability with the following two criteria: (i) Chronological score: ICI-related renal complications have a long latency period. In a series by Cortazar et al., the median (interquartile range) time from immune checkpoint inhibitor initiation to AKI was 14 (6–37) weeks [22] (as opposed to 4 weeks for skin diseases and 6 weeks for colitis). Practitioners should bear in mind that renal complications are possible even after the reintroduction of an ICI [24]. If a rechallenge is performed and AKI occurs again, the score is higher. (ii) Semiological score: Firstly, the patient exhibits known risk factors that have been previously established. A lower baseline eGFR, proton pump inhibitor use, and combination immune checkpoint inhibitor therapy were each independently associated with an increased risk of immune checkpoint inhibitor-associated AKI in the largest series [22]. The following other risk factors should be assessed: pembrolizumab and liver disease [47], as well as age > 65 years. Secondly, the patient experiences or has recently experienced extrarenal irAEs in 40–87% of the cases (hypereosinophilia [48]; immune thrombocytopenic purpura [49]).
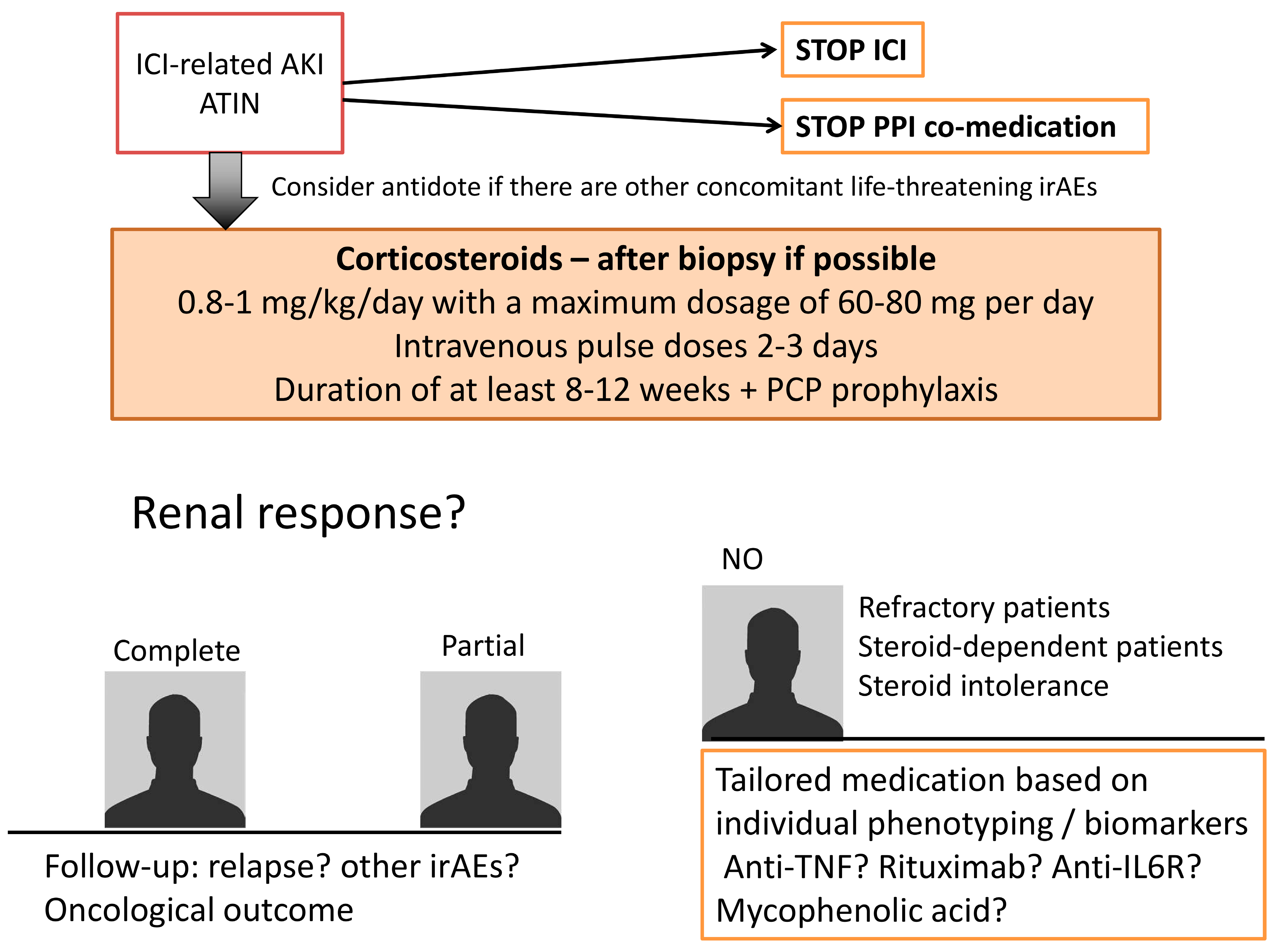
3. Treatment Strategies
3.1. Stop Exposure to ICI
3.2. Stop Immune Response Triggered by ICI
3.3. Tailor Immunosuppression to the Patient
3.3.1. Refractory Patients
3.3.2. Steroid-Dependent Patients or Patients with an Intolerance to Prolonged Steroid Schemes
3.4. Follow the Patient
3.5. Prevent Relapse If ICI Must Be Re-Started: The “Rechallenge”
3.6. Prevent the Disease in Future Patients
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brennan, D.C.; Aguado, J.M.; Potena, L.; Jardine, A.G.; Legendre, C.; Saemann, M.D.; Mueller, N.J.; Merville, P.; Emery, V.; Nashan, B. Effect of maintenance immunosuppressive drugs on virus pathobiology: Evidence and potential mechanisms. Rev. Med. Virol. 2013, 23, 97–125. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; Van Den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.-C.; Li, P.-C.; Fan, J.-Q.; Lin, G.-H.; Liu, Q. Durvalumab and tremelimumab combination therapy versus durvalumab or tremelimumab monotherapy for patients with solid tumors. Medicine 2020, 99, e21273. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.; Kudo, M.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.; Qin, S.; Tai, D.; Lim, H.; Yau, T.; et al. O-6 The novel regimen of tremelimumab in combination with durvalumab provides a favorable safety profile and clinical activity for patients with advanced hepatocellular carcinoma. Ann. Oncol. 2020, 31, 233–234. [Google Scholar] [CrossRef]
- Perets, R.; Bar, J.; Rasco, D.W.; Ahn, M.J.; Yoh, K.; Kim, D.W.; Nagrial, A.; Satouchi, M.; Lee, D.H.; Spigel, D.R.; et al. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann. Oncol. 2021, 32, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Yoh, K.; Bar, J.; Nagrial, A.; Spigel, D.R.; Gutierrez, M.; Kim, D.-W.; Kotasek, D.; Rasco, D.; Niu, J.; et al. Results from a phase I study of MK-1308 (ANTI–CTLA-4) plus pembrolizumab in previously treated advanced small cell lung cancer. Ann Oncol. 2019, 30, xi36–xi37. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574. [Google Scholar] [CrossRef]
- Seethapathy, H.; Zhao, S.; Chute, D.F.; Zubiri, L.; Oppong, Y.; Strohbehn, I.; Cortazar, F.B.; Leaf, D.E.; Mooradian, M.J.; Villani, A.C.; et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 2019, 14, 1692–1700. [Google Scholar] [CrossRef]
- Perazella, M.A.; Shirali, A.C. Immune checkpoint inhibitor nephrotoxicity: What do we know and what should we do? Kidney Int. 2020, 97, 62–74. [Google Scholar] [CrossRef]
- Wanchoo, R.; Karam, S.; Uppal, N.N.; Barta, V.S.; Deray, G.; Devoe, C.; Launay-Vacher, V.; Jhaveri, K.D. Cancer and Kidney International Network Workgroup on immune checkpoint inhibitors: Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am. J. Nephrol. 2017, 45, 160–169. [Google Scholar] [CrossRef]
- Rosner, M.H.; Jhaveri, K.D.; McMahon, B.A.; Perazella, M.A. Onconephrology: The intersections between the kidney and cancer. Cancer J. Clin. 2021, 71, 47–77. [Google Scholar] [CrossRef]
- Centanni, M.; Moes, D.J.A.R.; Trocóniz, I.F.; Ciccolini, J.; van Hasselt, J.G.C. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin. Pharmacokinet. 2019, 58, 835–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E.; Ghezzi, P.; Saxena, A.; et al. The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: Where do we stand? Front. Immunol. 2020, 11, 574271. [Google Scholar] [CrossRef] [PubMed]
- Fadel, F.; El Karoui, K.; Knebelmann, B. Anti-CTLA4 antibody–induced lupus nephritis. N. Engl. J. Med. 2009, 361, 211–212. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, X.; Gao, W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin. Immunol. 2005, 115, 184–191. [Google Scholar] [CrossRef]
- Hakroush, S.; Kopp, S.B.; Tampe, D.; Gersmann, A.K.; Korsten, P.; Zeisberg, M.; Tampe, B. Variable expression of programmed cell death protein 1-Ligand 1 in kidneys independent of immune checkpoint inhibition. Front. Immunol. 2021, 11, 2021. [Google Scholar] [CrossRef]
- Patel, V.; Elias, R.; Formella, J.; Schwartzman, W.; Christie, A.; Cai, Q.; Malladi, V.; Kapur, P.; Vazquez, M.; McKay, R.; et al. Acute interstitial nephritis, a potential predictor of response to immune checkpoint inhibitors in renal cell carcinoma. J. Immunother. Cancer 2020, 8, e001198. [Google Scholar] [CrossRef]
- Koda, R.; Watanabe, H.; Tsuchida, M.; Iino, N.; Suzuki, K.; Hasegawa, G.; Imai, N.; Narita, I. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: A case report. BMC Nephrol. 2018, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; Jhaveri, K.D.; Wadhwani, S.; Deshpande, P.; Harel, Z.; Kishibe, T.; Henriksen, K.; Wanchoo, R. A systematic review of immune checkpoint inhibitor–associated glomerular disease. Kidney Int. Rep. 2021, 6, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Mulroy, M.; Ghafouri, S.; Sisk, A.; Ribas, A.; Goshtaseb, R.; Cherry, G.; Shen, J. Acute interstitial nephritis and PR3-ANCA following reintroduction of pembrolizumab: A case report. Immunotherapy 2021, 13, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.; Connolly, E.; Hui, M.; Chadban, S. Minimal change disease in a patient receiving checkpoint inhibition: Another possible manifestation of kidney autoimmunity? Cancer Rep. 2020, 3, e1250. [Google Scholar] [CrossRef] [PubMed]
- Glutsch, V.; Grän, F.; Weber, J.; Gesierich, A.; Goebeler, M.; Schilling, B. Response to combined ipilimumab and nivolumab after development of a nephrotic syndrome related to PD-1 monotherapy. J. Immunother. Cancer 2019, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Mamlouk, O.; Selamet, U.; Machado, S.; Abdelrahim, M.; Glass, W.F.; Tchakarov, A.; Gaber, L.; Lahoti, A.; Workeneh, B.; Chen, S.; et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J. Immunother. Cancer 2019, 7, 2. [Google Scholar] [CrossRef]
- Tanabe, K.; Kanzaki, H.; Wada, T.; Nakashima, Y.; Sugiyama, H.; Okada, H.; Wada, J. Nivolumab-induced IgA nephropathy in a patient with advanced gastric cancer: A case report. Medicine 2020, 99, e20464. [Google Scholar] [CrossRef]
- Takahashi, N.; Tsuji, K.; Tamiya, H.; Shinohara, T.; Kuroda, N.; Takeuchi, E. Goodpasture’s disease in a patient with advanced lung cancer treated with nivolumab: An autopsy case report. Lung Cancer 2018, 122, 22–24. [Google Scholar] [CrossRef]
- Cruz-Whitley, J.; Giehl, N.; Jen, K.-Y.; Young, B. Membranoproliferative glomerulonephritis associated with nivolumab therapy. Case Rep. Nephrol. 2020, 2020, 2638283. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishna, P.; Villegas, A. Hypokalemic paralysis secondary to immune checkpoint inhibitor therapy. Case Rep. Oncol. Med. 2017, 2017, 5063405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bitar, S.; Weerasinghe, C.; El-Charabaty, E.; Odaimi, M. Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep. Oncol. Med. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Bruin, M.A.C.; Korse, C.M.; van Wijnen, B.; de Jong, V.M.T.; Linn, S.C.; van Triest, B.; Rosing, H.; Beijnen, J.H.; van den Broek, D.; Huitema, A.D.R. A real or apparent decrease in glomerular filtration rate in patients using olaparib? Eur. J. Clin. Pharmacol. 2021, 77, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Contreras-Sandoval, A.M.; Martisova, E.; Debets, R.; Smerdou, C.; Garrido, M.J. Quantification of pharmacokinetic profiles of pd-1/pd-l1 antibodies by validated elisas. Pharmaceutics 2020, 12, 595. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [Green Version]
- Izzedine, H.; Mathian, A.; Champiat, S.; Picard, C.; Mateus, C.; Routier, E.; Varga, A.; Malka, D.; Leary, A.; Michels, J.; et al. Renal toxicities associated with pembrolizumab. Clin. Kidney J. 2019, 12, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.G.; Lim, K.; Lee, Y.J.; Yang, J.; Oh, S.W.; Cho, W.Y.; Jo, S.K. M2 macrophages predict worse long-term outcomes in human acute tubular necrosis. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Okawa, S.; Fujiwara, K.; Shimonishi, A.; Matsuura, H.; Ozeki, T.; Nishimura, J.; Kayatani, H.; Minami, D.; Shinno, Y.; Sato, K.; et al. Rapidly progressive acute kidney injury associated with nivolumab treatment. Case Rep. Oncol. 2020, 13, 85–90. [Google Scholar] [CrossRef]
- Tabei, A.; Watanabe, M.; Ikeuchi, H.; Nakasatomi, M.; Sakairi, T.; Kaneko, Y.; Maeshima, A.; Kaira, K.; Hirato, J.; Nojima, Y.; et al. The analysis of renal infiltrating cells in acute tubulointerstitial nephritis induced by anti-PD-1 antibodies: A case report and review of the literature. Intern. Med. 2018, 57, 3135–3139. [Google Scholar] [CrossRef] [Green Version]
- Endo, N.; Tsuboi, N.; Furuhashi, K.; Shi, Y.; Du, Q.; Abe, T.; Hori, M.; Imaizumi, T.; Kim, H.; Katsuno, T.; et al. Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol. Dial. Transplant. 2016, 31, 2023–2033. [Google Scholar] [CrossRef] [Green Version]
- Villacorta, J.; Lucientes, L.; Goicoechea, E.; Acevedo, M.; Cavero, T.; Sanchez-Camara, L.; Díaz-Crespo, F.; Gimenez-Moyano, S.; García-Bermejo, L.; Fernandez-Juarez, G. Urinary soluble CD163 as a biomarker of disease activity and relapse in antineutrophil cytoplasm antibody-associated glomerulonephritis. Clin. Kidney J. 2021, 14, 212–219. [Google Scholar] [CrossRef]
- Qualls, D.; Seethapathy, H.; Bates, H.; Tajmir, S.; Heidari, P.; Endres, P.; Reynolds, K.; Lawrence, D.; Sise, M. Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J. Immunother. Cancer 2019, 7, 356. [Google Scholar] [CrossRef]
- Singh, P.; Brito, A.; Abdulnour, R.-E.E.; Grover, S.; Yenulevich, E.; Stuver, S.O.; Kehl, K.L.; LeBoeuf, N.R.; Jacobson, J.O.; Rahma, O.E. Defining real-world criteria for immune-related adverse events (irAEs). J. Clin. Oncol. 2019, 37, e14172. [Google Scholar] [CrossRef]
- Gawai, P.P. Overview of important methods used for causality assessment of adverse drug events in pharmacovigilance. J. Pharmacovigil. Drug Res. 2020, 1, 6–11. [Google Scholar]
- Shimamura, Y.; Watanabe, S.; Maeda, T.; Abe, K.; Ogawa, Y.; Takizawa, H. Incidence and risk factors of acute kidney injury, and its effect on mortality among Japanese patients receiving immune check point inhibitors: A single-center observational study. Clin. Exp. Nephrol. 2021, 25, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Scanvion, Q.; Béné, J.; Gautier, S.; Grandvuillemin, A.; Le Beller, C.; Chenaf, C.; Etienne, N.; Brousseau, S.; Cortot, A.B.; Mortier, L.; et al. Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 Cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. Oncoimmunology 2020, 9, 1722022. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, A.; Mirili, C.; Bilici, M.; Tekin, S.B. Possible atezolizumab-associated acute kidney injury and immune thrombocytopenia. J. Oncol. Pharm. Pract. 2020, 26, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, D.W.; Visser, S.; Cornelissen, R.; van Gelder, T.; Vansteenkiste, J.; von der Thusen, J. Aerts JGJV: Renal toxicity from pemetrexed and pembrolizumab in the era of combination therapy in patients with metastatic nonsquamous cell NSCLC. J. Thorac. Oncol. 2020, 15, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Selamet, U.; Bui, P.; Sun, S.F.; Shenouda, O.; Nobakht, N.; Barsoum, M.; Arman, F.; Rastogi, A. Acute Kidney Injury after Pembrolizumab-Induced Adrenalitis and Adrenal Insufficiency. Case Rep. Nephrol. Dial. 2018, 8, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Zakaroff, A.G.; Meyer, N.; Delas, A.; Faguer, S.; Kamar, N.; Belliere, J. Cytokine storm induced by a PD1 inhibitor in a renal transplant patient. Am. J. Transplant. 2021. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Ghamrawi, R.; Chengappa, M.; Goksu, B.N.B.; Kottschade, L.; Finnes, H.; Dronca, R.; Leventakos, K.; Herrmann, J.; Herrmann, S.M. Acute interstitial nephritis and checkpoint inhibitor therapy. Kidney360 2020, 1, 16–24. [Google Scholar] [CrossRef]
- Fisher, J.; Zeitouni, N.; Fan, W.; Samie, F.H. Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review. J. Am. Acad. Dermatol. 2020, 82, 1490–1500. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Ortuno, S.; Lebrun-Vignes, B.; Johnson, D.B.; Moslehi, J.J.; Hertig, A.; Salem, J.E. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur. J. Cancer 2021, 148, 36–47. [Google Scholar] [CrossRef]
- Salem, J.E.; Allenbach, Y.; Kerneis, M. Abatacept for severe immune checkpoint inhibitor–associated myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.M.; Perazella, M.A. Immune checkpoint inhibitors and immune-related adverse renal events. Kidney Int. Rep. 2020, 5, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; Michielin, O.; Peters, S.; Coukos, G.; Spertini, F.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef] [Green Version]
- Murakami, N.; Borges, T.J.; Yamashita, M.; Riella, L.V. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin. Kidney J. 2016, 9, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daanen, R.A.; Maas, R.J.H.; Koornstra, R.H.T.; Steenbergen, E.J.; Van Herpen, C.M.L.; Willemsen, A.E.C.A.B. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: A case report. J. Immunother. 2017, 40, 345–348. [Google Scholar] [CrossRef]
- Mamlouk, O.; Lin, J.S.; Abdelrahim, M.; Tchakarov, A.S.; Glass, W.F.; Selamet, U.; Buni, M.; Abdel-Wahab, N.; Abudayyeh, A. Checkpoint inhibitor-related renal vasculitis and use of rituximab. J. Immunother. Cancer 2020, 8, e000750. [Google Scholar] [CrossRef]
- Bottlaender, L.; Breton, A.L.; Laforcade, L.; Dijoud, F.; Thomas, L.; Dalle, S. Acute interstitial nephritis after sequential ipilumumab—Nivolumab therapy of metastatic melanoma. J. Immunother. Cancer 2017, 5, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsung, I.; Worden, F.P.; Fontana, R.J. A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma. Oncologist 2021, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mejia, C.D.; Frank, A.M.; Singh, P.; Yadav, A. Immune checkpoint inhibitor therapy-associated graft intolerance syndrome in a failed kidney transplant recipient. Am. J. Transplant. 2021, 21, 1322–1325. [Google Scholar] [CrossRef] [PubMed]

| ICI Class | Molecule | Date of Approval | Type of Indications |
|---|---|---|---|
| anti-CTLA4 | Ipilimumab Tremelimumab Quavonlimab | 2011 2015 current folder | melanoma, renal cell carcinoma, CRC mesothelioma, in combination with durvalumab in advanced non-small cell lung cancer, head and neck squamous cell carcinoma, advanced hepatocellular carcinoma |
| anti-PD1 | Pembrolizumab Nivolumab cemiplimab | 2014 2014 2018 | melanoma, hepatocellular carcinoma, cervical cancer, advanced NSCLC, gastric cancers, Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, urothelial cancer, cutaneous squamous cell carcinoma melanoma, head and neck, hepatocellular carcinoma, renal cell carcinoma, CRC, small lung cancer, advanced NSCLC cutaneous squamous cell carcinoma |
| anti-PDL1 | Atezolizumab Avelumab Durvalumab | 2016 2017 2017 | advanced small cell lung cancer, advanced NSCLC, triple negative breast cancer, urothelial cancer Merkel cell carcinoma, urothelial cancer urothelial cancer, locally advanced NSCLC, advanced SCLC |
| anti-LAG3 | Eftilagimod alpha Relatlimab | FDA approval March 2020 current folder | metastatic RCC, metastatic breast cancer, melanoma, advanced NSCLC and head and neck squamous cell carcinoma clinical trials recruiting |
| anti-TIM3 | TSR-022 MBG453 Sym023 INCAGN2390 LY3321367 BMS-9862 SHR-170258 RO7121661 | current folder | clinical trials recruiting |
| anti-TIGIT | Tiragolumab MK-7684 Etigilimab BMS-986207 AB-154 ASP-8374 | FDA approval January 2021 current folder | PD-L1-high non-small cell lung cancer clinical trials recruiting |
| anti-VISTA | JNJ-61610588 CA-170 | current folder | clinical trials recruiting |
| anti-B7-H3 | Enoblituzumab | FDA approval December 2020 | Patients with Pretreated Metastatic HER2-Positive Breast Cancer |
| ICI Class | Drug Name | Number of ICSR, n = | Number of ICSR with Renal or Urinary Adverse Effects n = (%) |
|---|---|---|---|
| anti-CTLA4 | Ipilimumab (alone or in combination) | 22,641 | 1021 (4.5%) |
| Ipilimumab (combined with nivolumab) | 11,536 | 686 (5.9%) | |
| tremelimumab | 408 | 22 (5.4%) | |
| anti-PD1 | pembrolizumab | 29,633 | 1397 (4.7%) |
| nivolumab | 51,705 | 2350 (4.5%) | |
| cemiplimab | 655 | 50 (7.6%) | |
| anti-PDL1 | atezolizumab | 8193 | 431 (5.3%) |
| avelumab | 1300 | 82 (6.3%) | |
| durvalumab | 4372 | 116 (2.7%) | |
| Anti-LAG3 * | relatlimab | 65 | 5 (7.7%) |
| anti-TIGIT | Tiragolumab | 8 | 0 (0%) |
| anti-B7-H3 | Enoblituzumab | 2 | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belliere, J.; Mazieres, J.; Meyer, N.; Chebane, L.; Despas, F. Renal Complications Related to Checkpoint Inhibitors: Diagnostic and Therapeutic Strategies. Diagnostics 2021, 11, 1187. https://doi.org/10.3390/diagnostics11071187
Belliere J, Mazieres J, Meyer N, Chebane L, Despas F. Renal Complications Related to Checkpoint Inhibitors: Diagnostic and Therapeutic Strategies. Diagnostics. 2021; 11(7):1187. https://doi.org/10.3390/diagnostics11071187
Chicago/Turabian StyleBelliere, Julie, Julien Mazieres, Nicolas Meyer, Leila Chebane, and Fabien Despas. 2021. "Renal Complications Related to Checkpoint Inhibitors: Diagnostic and Therapeutic Strategies" Diagnostics 11, no. 7: 1187. https://doi.org/10.3390/diagnostics11071187
APA StyleBelliere, J., Mazieres, J., Meyer, N., Chebane, L., & Despas, F. (2021). Renal Complications Related to Checkpoint Inhibitors: Diagnostic and Therapeutic Strategies. Diagnostics, 11(7), 1187. https://doi.org/10.3390/diagnostics11071187





