Development of an Electronic Portal Imaging Device Dosimetry Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
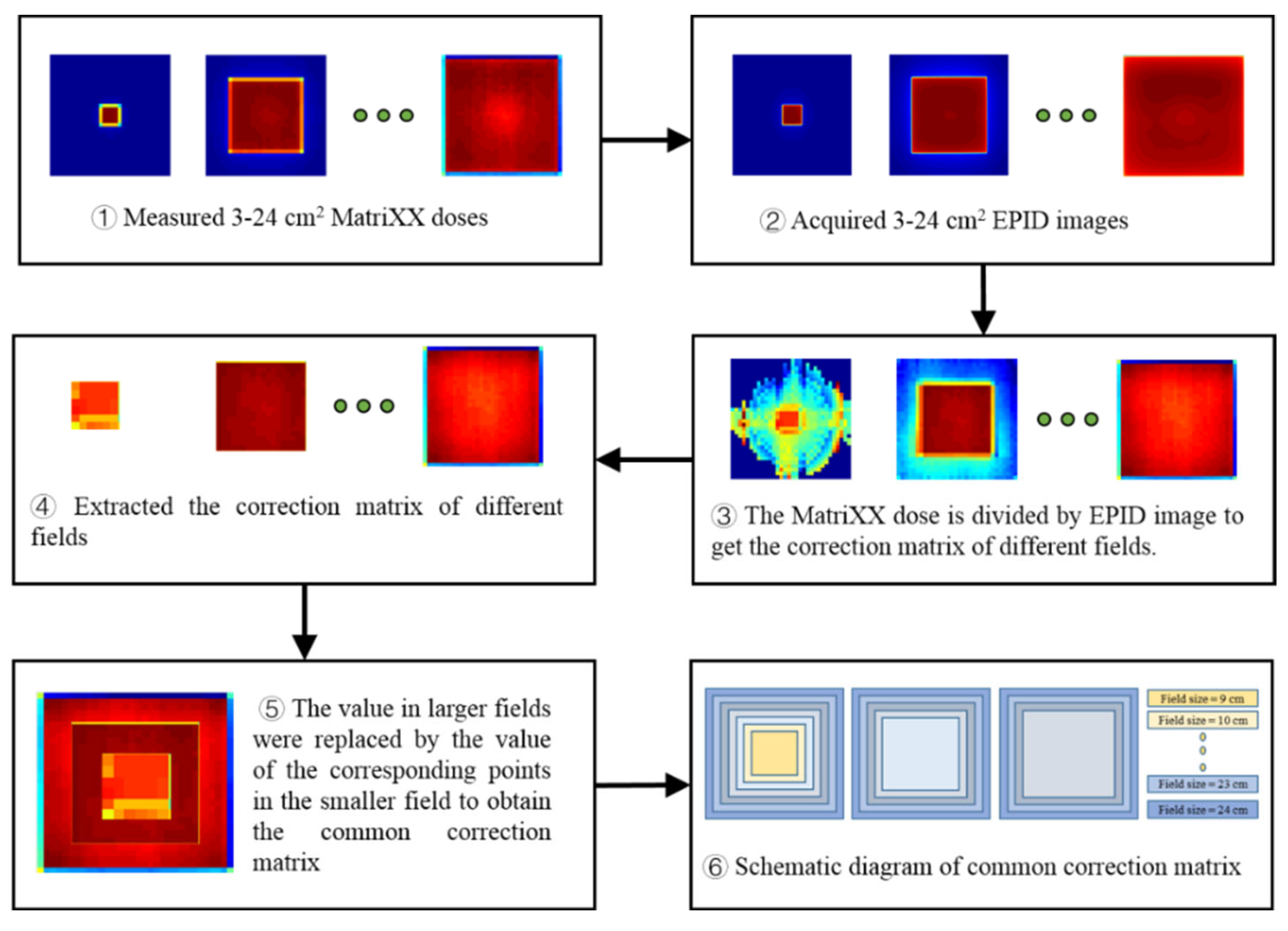
2.2.1. EPID Image Preprocessing
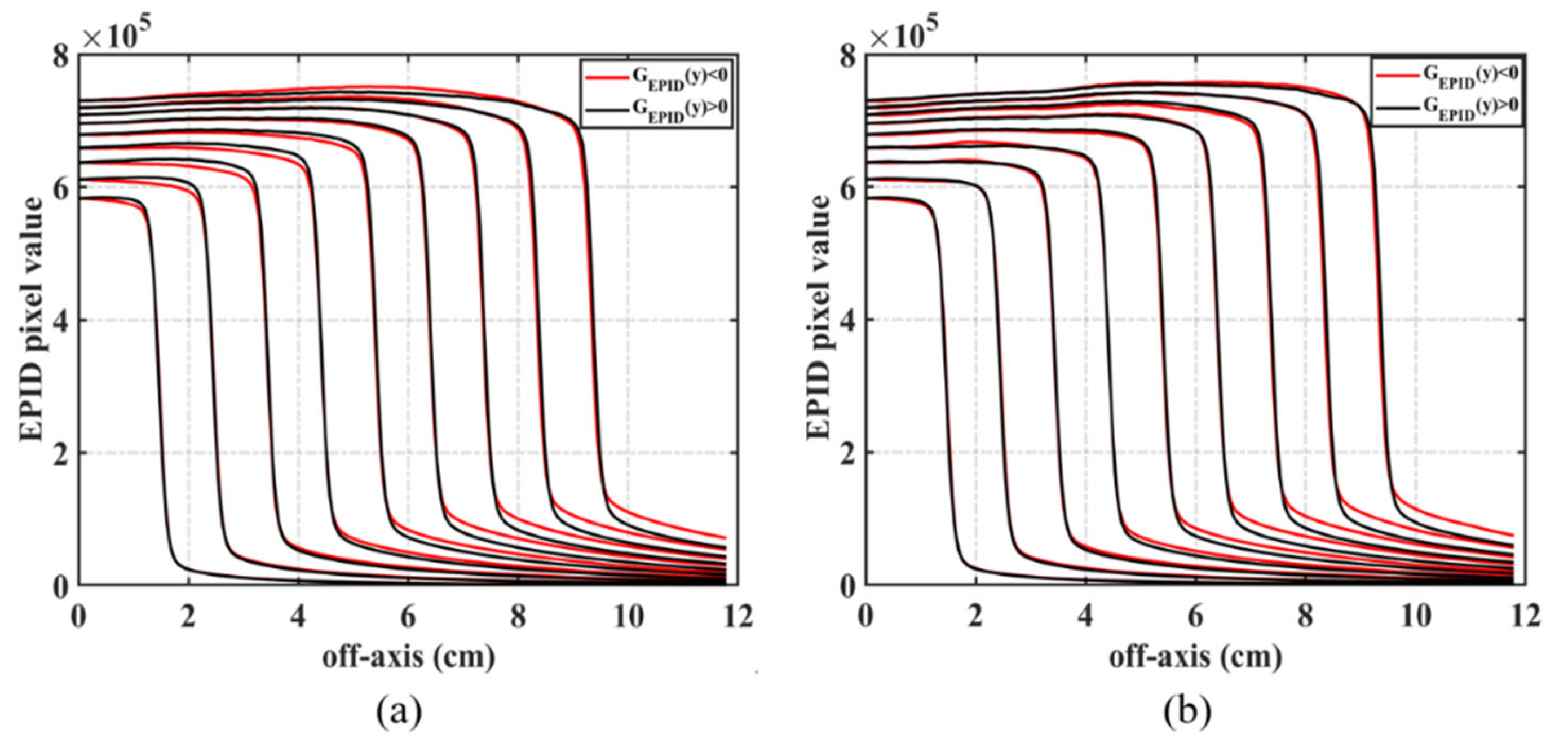
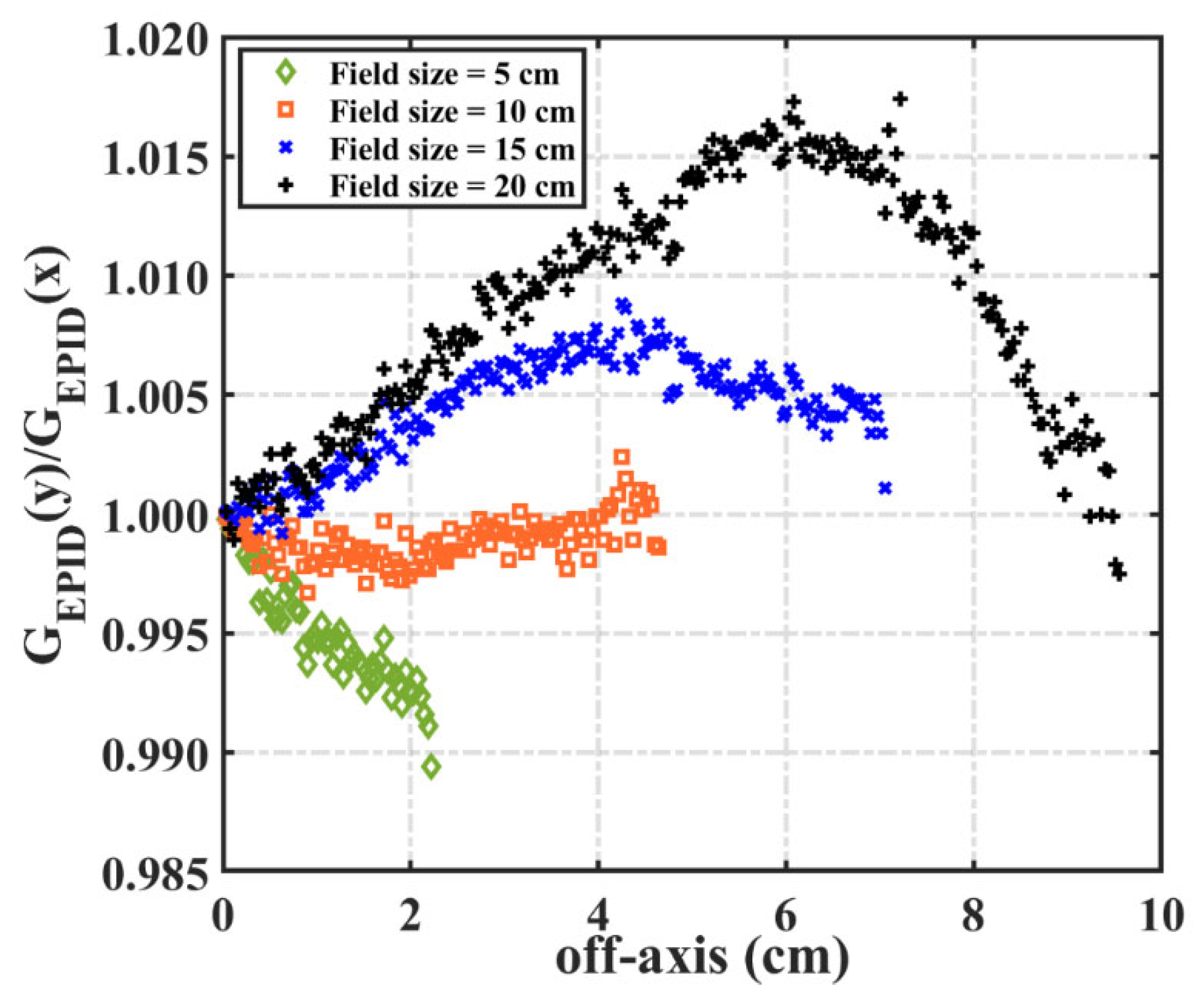
2.2.2. Backscatter and Off-Axis Response
2.2.3. Conversion of EPID Pixel Value and Dose Value
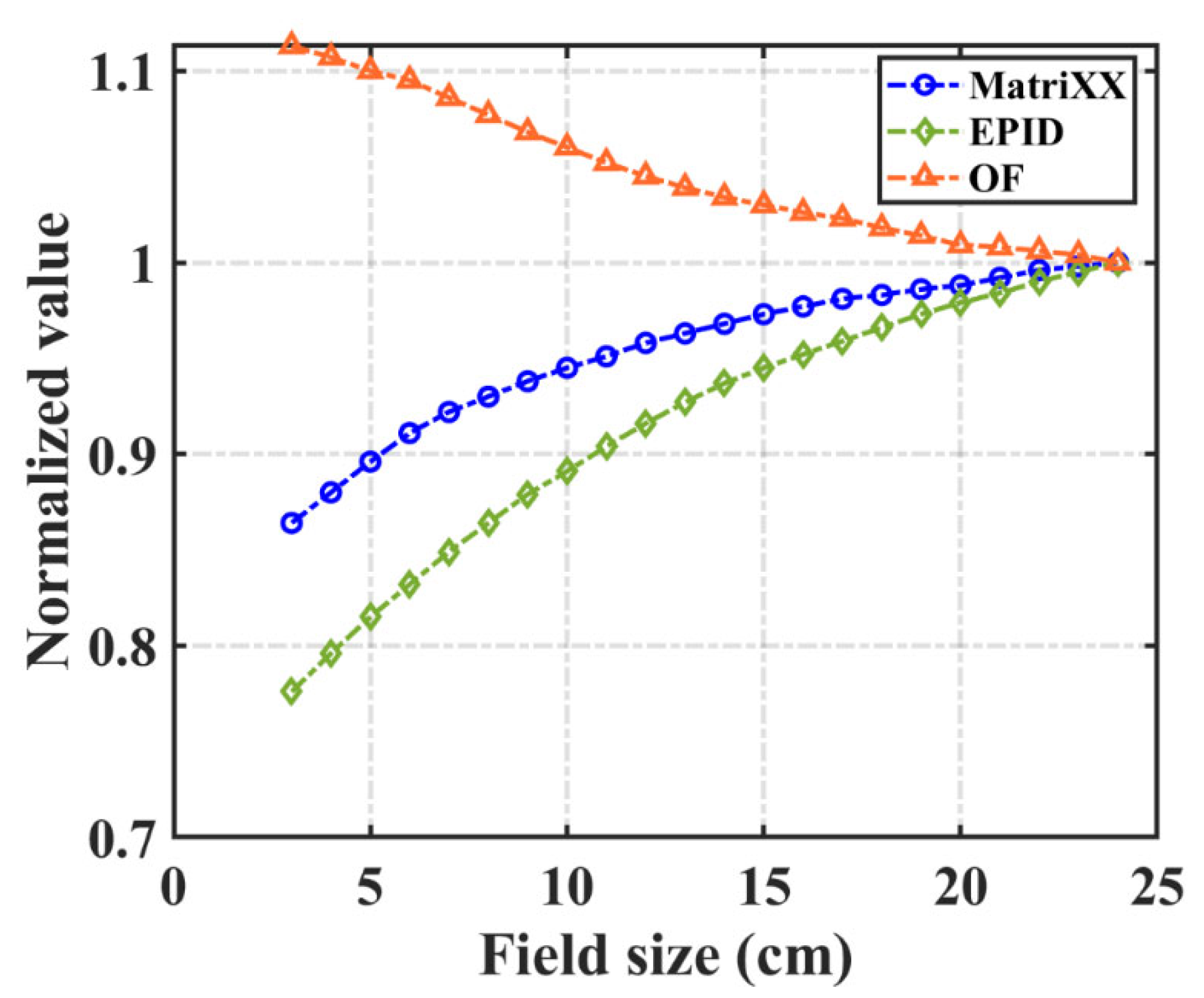
2.2.4. Field Output Factor
2.3. Validation
3. Results
3.1. Backscatter and Off-Axis Response Correction
3.2. Pixel Value and Dose Conversion Function
3.3. Field Output Factor
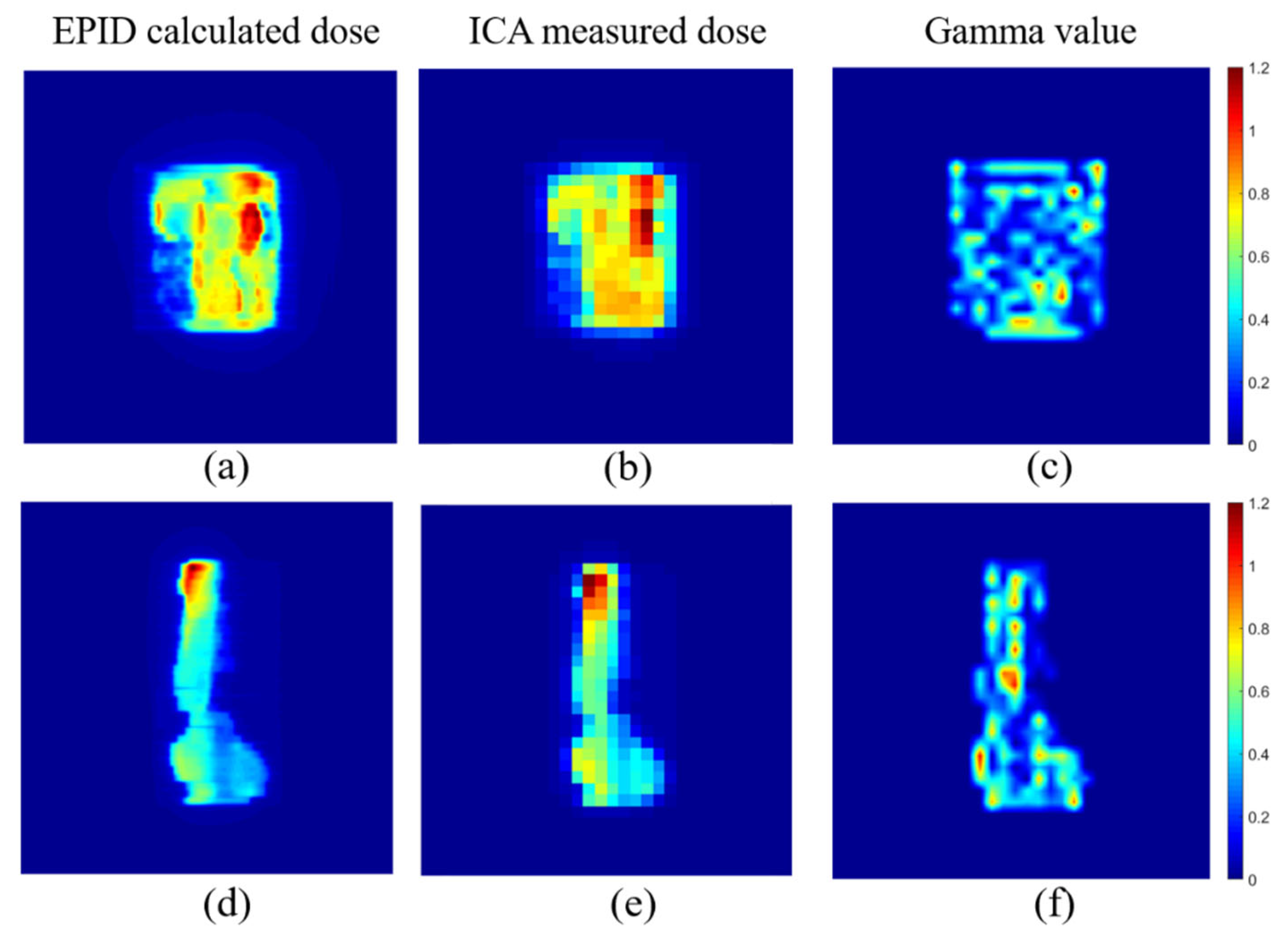
3.4. Dose Comparison for IMRT Fields
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, D.A.; Moran, J.M.; Dempsey, J.F.; Dong, L.; Oldham, M. Dosimetry tools and techniques for IMRT. Med. Phys. 2011, 38, 1313–1338. [Google Scholar] [CrossRef]
- Van Uytven, E.; Van Beek, T.; McCowan, P.M.; Chytyk-Praznik, K.; Greer, P.; McCurdy, B.M.C. Validation of a method for in vivo 3D dose reconstruction for IMRT and VMAT treatments using on-treatment EPID images and a model-based forward-calculation algorithm. Med. Phys. 2015, 42, 6945–6954. [Google Scholar] [CrossRef]
- McCowan, P.M.; Asuni, G.; Van Beek, T.; Van Uytven, E.; Kujanpaa, K.; McCurdy, B.M.C. A model-based 3D patient-specific pre-treatment QA method for VMAT using the EPID. Phys. Med. Biol. 2017, 62, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.; Gianoli, C.; Neppl, S.; Martins, J.; Veloza, S.; Podesta, M.; Verhaegen, F.; Reiner, M.; Belka, C.; Parodi, K. A novel approach to EPID-based 3D volumetric dosimetry for IMRT and VMAT QA. Phys. Med. Biol. 2018, 63, 115002. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, A.H.; Olch, A.J. Sensitivity study of an automated system for daily patient QA using EPID exit dose images. J. Appl. Clin. Med. Phys. 2018, 19, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Olaciregui-Ruiz, I.; Vivas-Maiques, B.; Kaas, J.; Perik, T.; Wittkamper, F.; Mijnheer, B.; Mans, A. Transit and non-transit 3D EPID dosimetry versus detector arrays for patient specific QA. J. Appl. Clin. Med. Phys. 2019, 20, 79–90. [Google Scholar] [CrossRef]
- Ślosarek, K.; Plaza, D.; Nas, A.; Reudelsdorf, M.; Wendykier, J.; Bekman, B.; Grządziel, A. Portal dosimetry in radiotherapy repeatability evaluation. J. Appl. Clin. Med. Phys. 2020, 22, 156–164. [Google Scholar] [CrossRef]
- Van Esch, A.; Huyskens, D.P.; Hirschi, L.; Scheib, S.; Baltes, C. Optimized Varian aSi portal dosimetry: Development of datasets for collective use. J. Appl. Clin. Med. Phys. 2013, 14, 4286. [Google Scholar] [CrossRef]
- Martínez Ortega, J.; Pinto Monedero, M.; Gómez González, N.; Tolani, N.B.; Castro Tejero, P.; Castanedo Álvarez, M.; Núñez Martín, L.; Sánchez Montero, R. A collapsed-cone based transit EPID dosimetry method. Phys. Med. 2018, 46, 75–80. [Google Scholar] [CrossRef]
- Deshpande, S.; Blake, S.J.; Xing, A.; Metcalfe, P.; Holloway, L.C.; Vial, P. A simple model for transit dosimetry based on a water equivalent EPID. Med. Phys. 2018, 45, 1266–1275. [Google Scholar] [CrossRef]
- Nelms, B.E.; Rasmussen, K.H.; Tome, W.A. Evaluation of a fast method of EPID-based dosimetry for intensity-modulated radiation therapy. J. Appl. Clin. Med. Phys. 2010, 11, 3185. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, G.; Clivio, A.; Vanetti, E.; Krauss, H.; Fenoglietto, P.; Cozzi, L.; Fogliata, A. Evaluation of an aSi-EPID with flattening filter free beams: Applicability to the GLAaS algorithm for portal dosimetry and first experience for pretreatment QA of RapidArc. Med. Phys. 2013, 40, 111719. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.M.; González, N.G.; Tejero, P.C.; Monedero, M.P.; Tolani, N.B.; Martín, L.N.; Montero, R.S. A portal dosimetry dose prediction method based on collapsed cone algorithm using the clinical beam model. Med. Phys. 2017, 44, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Greer, P.B. Correction of pixel sensitivity variation and off-axis response for amorphous silicon EPID dosimetry. Med. Phys. 2005, 32, 3558–3568. [Google Scholar] [CrossRef] [PubMed]
- McCowan, P.M.; McCurdy, B.M. Frame average optimization of cine-mode EPID images used for routine clinical in vivo patient dose verification of VMAT deliveries. Med. Phys. 2016, 43, 254. [Google Scholar] [CrossRef] [PubMed]
- Olaciregui-Ruiz, I.; Rozendaal, R.; van Oers, R.F.; Mijnheer, B.; Mans, A. Virtual patient 3D dose reconstruction using in air EPID measurements and a back-projection algorithm for IMRT and VMAT treatments. Phys. Med. 2017, 37, 49–57. [Google Scholar] [CrossRef]
- Han, B.; Ding, A.; Lu, M.; Xing, L. Pixel response-based EPID dosimetry for patient specific QA. J. Appl. Clin. Med. Phys. 2017, 18, 9–17. [Google Scholar] [CrossRef]
- Nailon, W.H.; Welsh, D.; McDonald, K.; Burns, D.; Forsyth, J.; Cooke, G.; Cutanda, F.; Carruthers, L.J.; McLaren, D.B.; Puxeu Vaqué, J.; et al. EPID-based in vivo dosimetry using Dosimetry Check™: Overview and clinical experience in a 5-yr study including breast, lung, prostate, and head and neck cancer patients. J. Appl. Clin. Med. Phys. 2019, 20, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mijnheer, B.; Jomehzadeh, A.; González, P.; Olaciregui-Ruiz, I.; Rozendaal, R.; Shokrani, P.; Spreeuw, H.; Tielenburg, R.; Mans, A. Error detection during VMAT delivery using EPID-based 3D transit dosimetry. Phys. Med. 2018, 54, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Olaciregui-Ruiz, I.; Rozendaal, R.; Mijnheer, B.; Mans, A. Site-specific alert criteria to detect patient-related errors with 3D EPID transit dosimetry. Med. Phys. 2019, 46, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.L.; Polvorosa, C.S.; Wuu, C.S. A field size specific backscatter correction algorithm for accurate EPID dosimetry. Med. Phys. 2010, 37, 2425–2434. [Google Scholar] [CrossRef]
- Rowshanfarzad, P.; McCurdy, B.M.C.; Sabet, M.; Lee, C.; O’Connor, D.J.; Greer, P.B. Measurement and modeling of the effect of support arm backscatter on dosimetry with a Varian EPID. Med. Phys. 2010, 37, 2269–2278. [Google Scholar] [CrossRef]
- King, B.W.; Morf, D.; Greer, P.B. Development and testing of an improved dosimetry system using a backscatter shielded electronic portal imaging device. Med. Phys. 2012, 39, 2839–2847. [Google Scholar] [CrossRef]
- Rowshanfarzad, P.; Sabet, M.; O’Connor, D.J.; Greer, P.B. Improvement of Varian a-Si EPID dosimetry measurements using a lead-shielded support-arm. Med. Dosim. 2012, 37, 145–151. [Google Scholar] [CrossRef]
- Camilleri, J.; Mazurier, J.; Franck, D.; Dudouet, P.; Latorzeff, I.; Franceries, X. 2D EPID dose calibration for pretreatment quality control of conformal and IMRT fields: A simple and fast convolution approach. Phys. Med. 2016, 32, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Greer, P.B.; Cadman, P.; Lee, C.; Bzdusek, K. An energy fluence-convolution model for amorphous silicon EPID dose prediction. Med. Phys. 2009, 36, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.A.; Siebers, J.V. Verification of the optimal backscatter for an aSi electronic portal imaging device. Phys. Med. Biol. 2005, 50, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Renner, W.D.; Norton, K.; Holmes, T. A method for deconvolution of integrated electronic portal images to obtain incident fluence for dose reconstruction. J. Appl. Clin. Med. Phys. 2005, 6, 22–39. [Google Scholar] [CrossRef]
- Cai, B.; Goddu, S.M.; Yaddanapudi, S.; Caruthers, D.; Wen, J.; Noel, C.; Mutic, S.; Sun, B. Normalize the response of EPID in pursuit of linear accelerator dosimetry standardization. J. Appl. Clin. Med. Phys. 2018, 19, 73–85. [Google Scholar] [CrossRef]
- Ko, L.; Kim, J.O.; Siebers, J.V. Investigation of the optimal backscatter for an aSi electronic portal imaging device. Phys. Med. Biol. 2004, 49, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, L.; Chen, A.; Luo, G.; Deng, X.; Liu, X. Fast 3D dosimetric verifications based on an electronic portal imaging device using a GPU calculation engine. Radiat. Oncol. 2015, 10, 85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mans, A.; Wendling, M.; McDermott, L.N.; Sonke, J.-J.; Tielenburg, R.; Vijlbrief, R.; Mijnheer, B.; van Herk, M.; Stroom, J.C. Catching errors within vivoEPID dosimetry. Med. Phys. 2010, 37, 2638–2644. [Google Scholar] [CrossRef] [PubMed]




| Off-Axis (cm) | EPID-UNCORRECTED | EPID-CORRECTED | ||
|---|---|---|---|---|
| a | b | a | b | |
| 0 | 0.001398 | 3.975 | 0.001398 | 3.975 |
| 1.142 | 0.001401 | 3.953 | 0.001399 | 3.953 |
| 1.904 | 0.001404 | 4.148 | 0.001400 | 4.148 |
| 2.666 | 0.001406 | 4.311 | 0.001401 | 4.311 |
| 3.428 | 0.001412 | 4.337 | 0.001401 | 4.337 |
| 4.190 | 0.001419 | 4.424 | 0.001403 | 4.424 |
| 4.952 | 0.001423 | 4.465 | 0.001402 | 4.466 |
| 5.714 | 0.001424 | 4.504 | 0.001402 | 4.307 |
| 6.476 | 0.001429 | 4.633 | 0.001401 | 4.432 |
| 7.237 | 0.001435 | 4.583 | 0.001402 | 4.483 |
| 7.999 | 0.001443 | 4.641 | 0.001402 | 4.340 |
| 8.761 | 0.00145 | 4.701 | 0.001402 | 4.300 |
| 9.523 | 0.001462 | 4.624 | 0.001403 | 4.403 |
| 10.285 | 0.001471 | 4.496 | 0.001403 | 4.415 |
| 11.047 | 0.001472 | 4.496 | 0.001403 | 4.423 |
| Off-Axis (cm) | MatriXX (mGy) | EPID-UNCORRECTED (mGy) | Error | EPID-CORRECTED (mGy) | Error |
|---|---|---|---|---|---|
| 0 | 1060.77 | 1056.2 | 0.4% | 1056.2 | 0.4% |
| 5 | 1102.98 | 1078.7 | 2.2% | 1097.2 | 0.5% |
| 11 | 1094.20 | 1035.6 | 5.4% | 1088.9 | 0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, Z.; Zhang, X.; Yang, R.; Wen, J. Development of an Electronic Portal Imaging Device Dosimetry Method. Diagnostics 2021, 11, 1654. https://doi.org/10.3390/diagnostics11091654
Zhang J, Fan Z, Zhang X, Yang R, Wen J. Development of an Electronic Portal Imaging Device Dosimetry Method. Diagnostics. 2021; 11(9):1654. https://doi.org/10.3390/diagnostics11091654
Chicago/Turabian StyleZhang, Jun, Ziting Fan, Xile Zhang, Ruijie Yang, and Junhai Wen. 2021. "Development of an Electronic Portal Imaging Device Dosimetry Method" Diagnostics 11, no. 9: 1654. https://doi.org/10.3390/diagnostics11091654
APA StyleZhang, J., Fan, Z., Zhang, X., Yang, R., & Wen, J. (2021). Development of an Electronic Portal Imaging Device Dosimetry Method. Diagnostics, 11(9), 1654. https://doi.org/10.3390/diagnostics11091654






