Ultrasound for the Diagnosis of Biliary Atresia: From Conventional Ultrasound to Artificial Intelligence
Abstract
:1. Introduction
2. Conventional Ultrasound
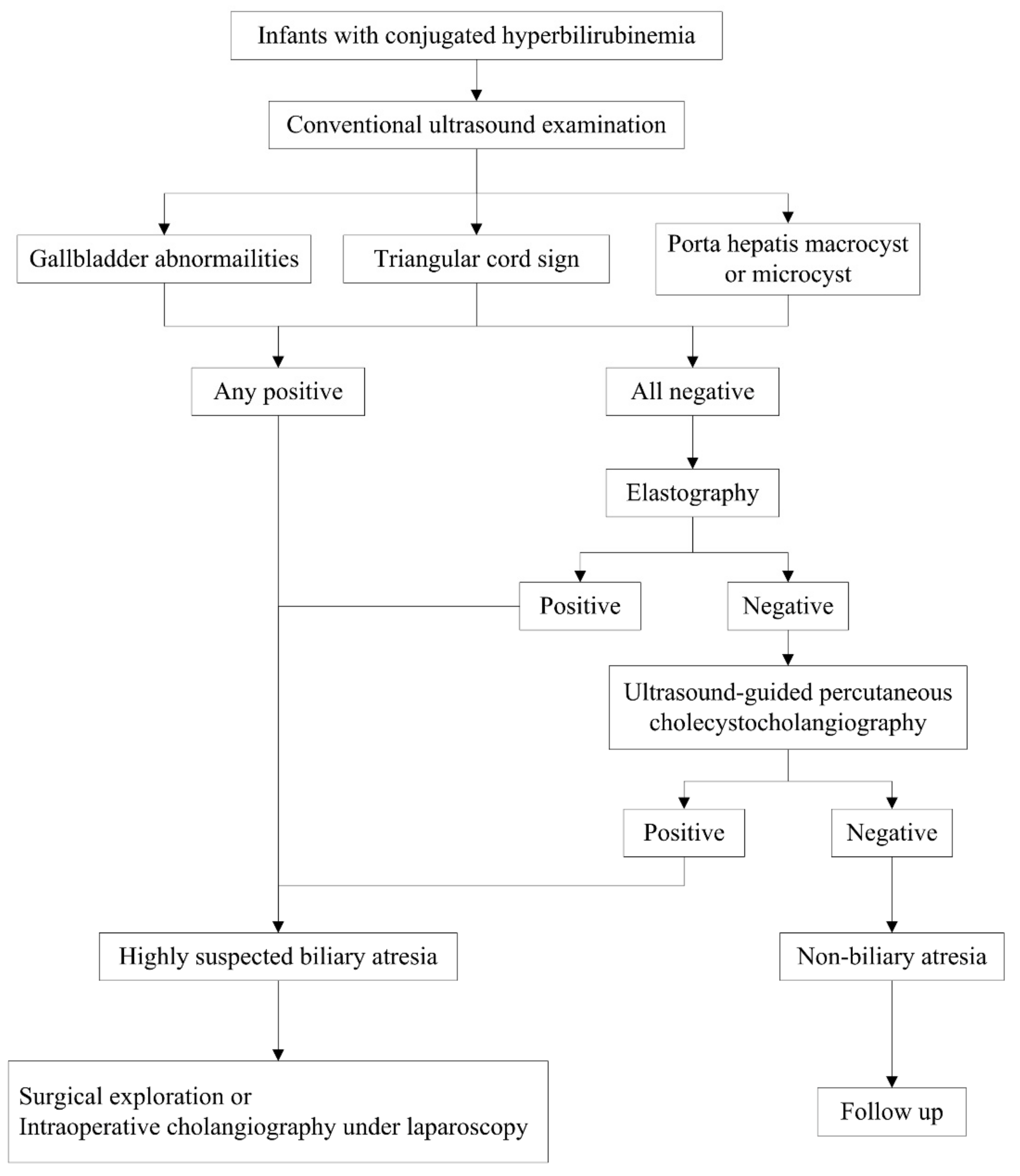
2.1. Gallbladder Abnormalities
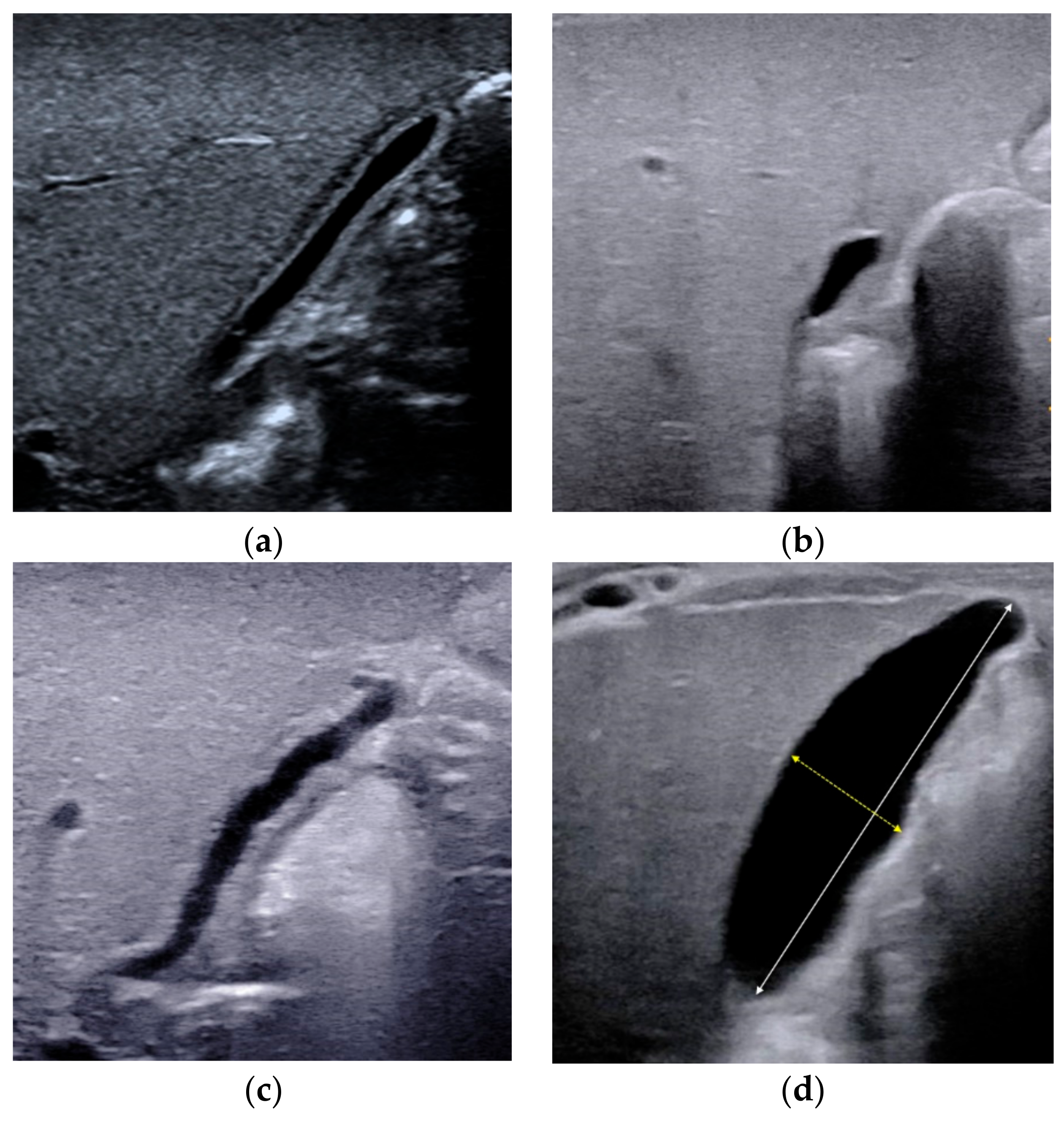
2.2. Triangular Cord Sign
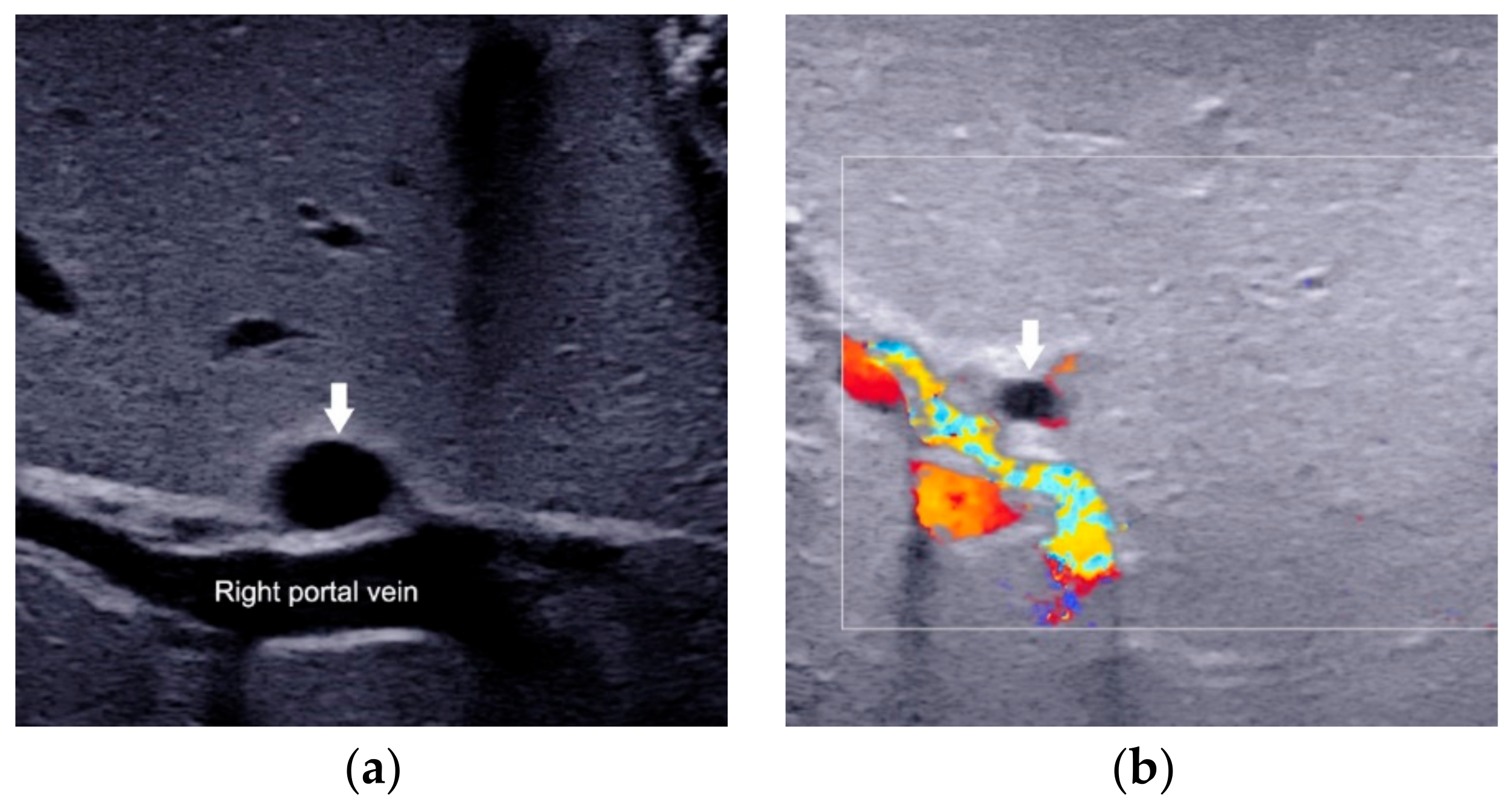
2.3. Porta Hepatis Macro- or Microcyst
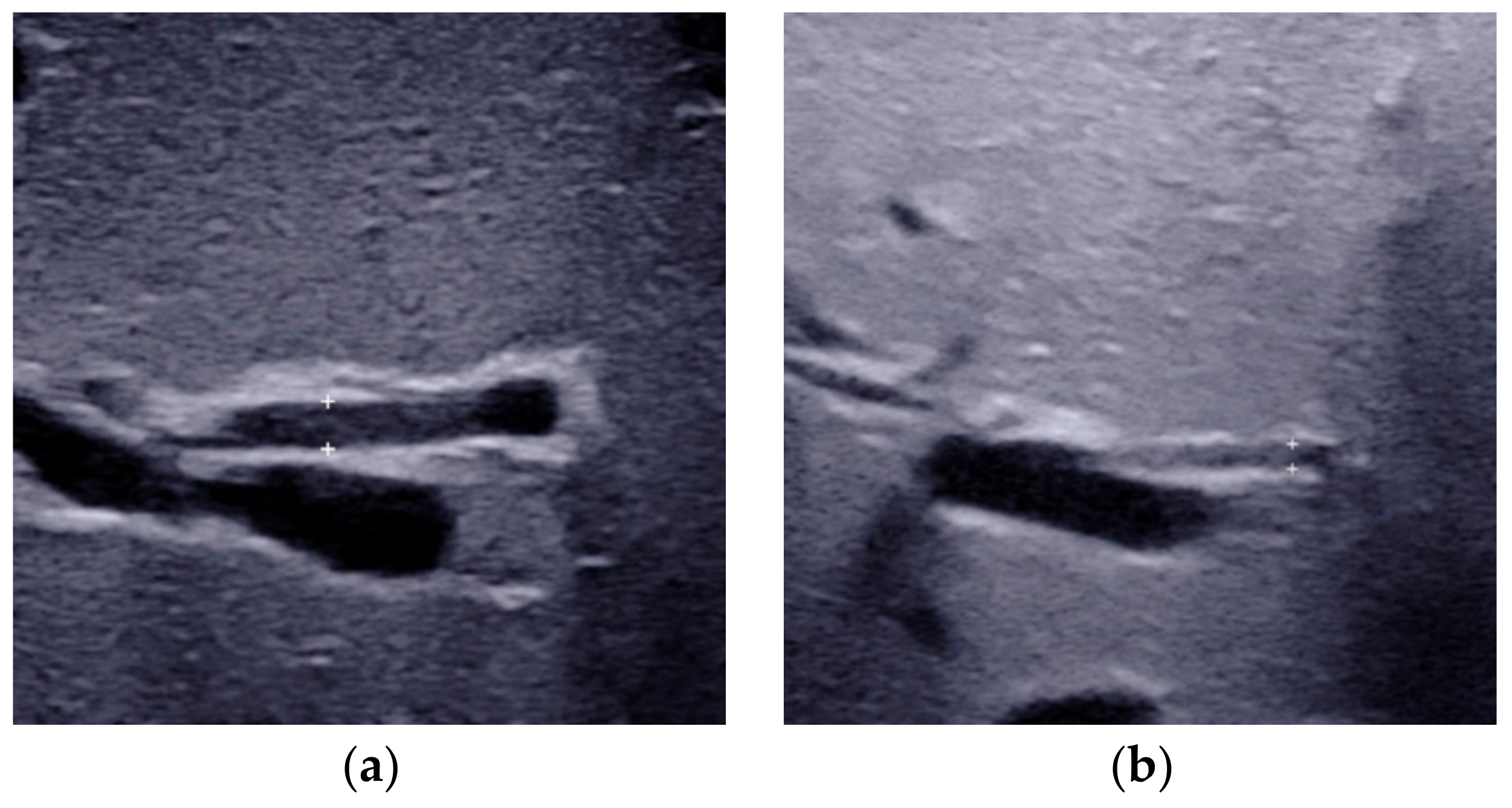
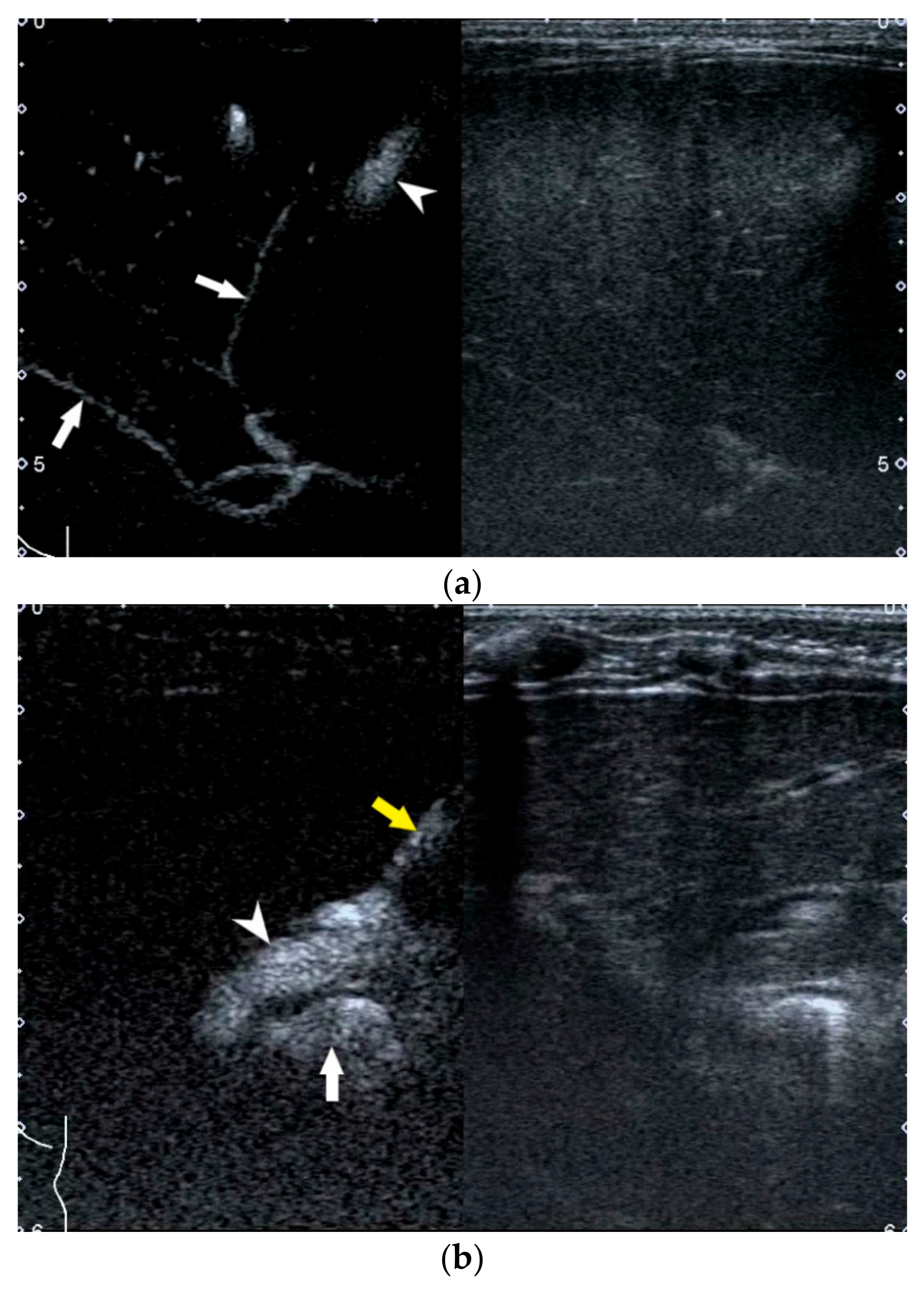
2.4. Enlarged Hepatic Hilar Lymph Node (LN)
2.5. Other Helpful US Features
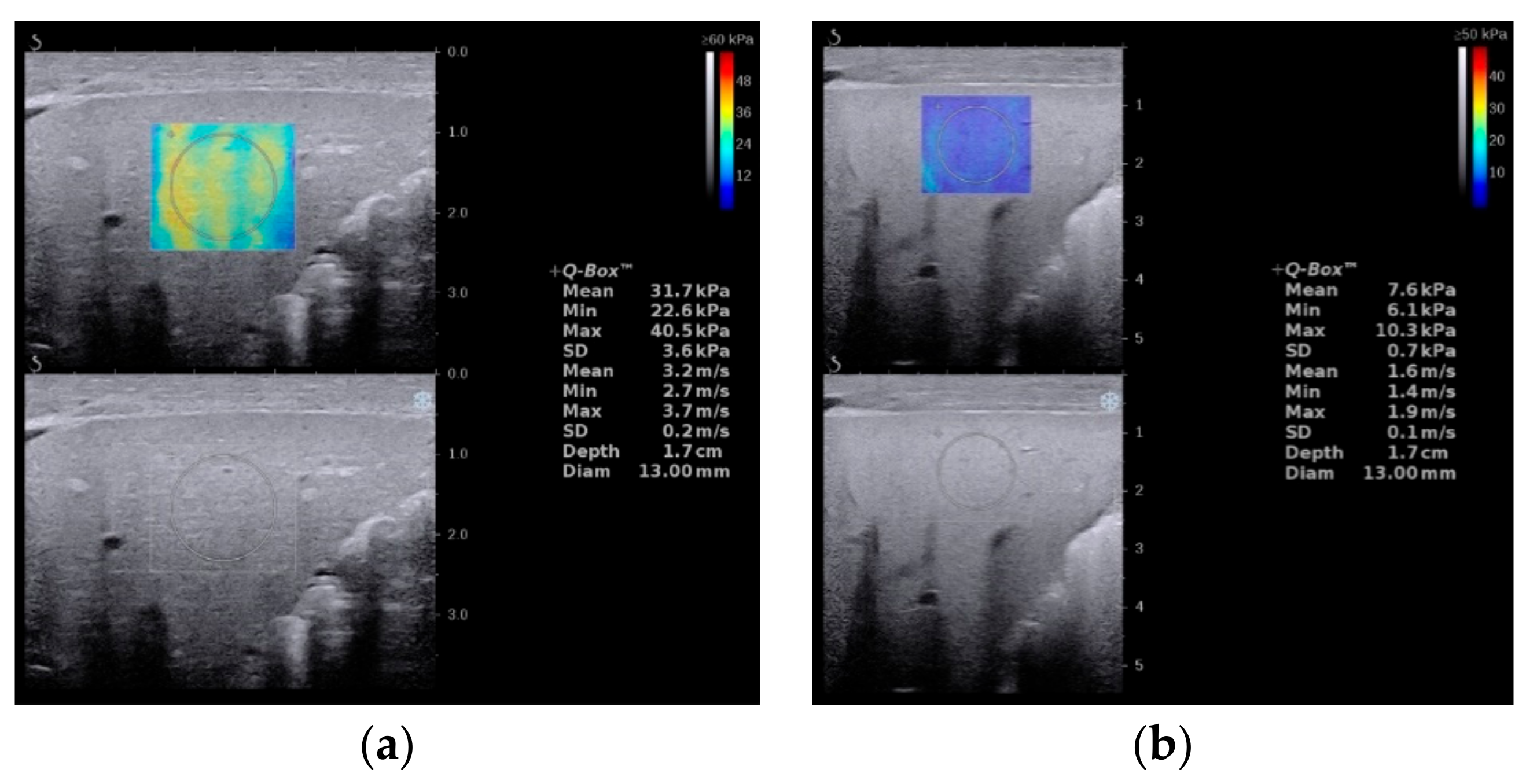
3. Elastography
4. US-Guided Percutaneous Cholecystocholangiography with Microbubbles
5. Artificial Intelligence Based on US Gallbladder Images
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chardot, C.; Carton, M.; Spire-Bendelac, N.; Le Pommelet, C.; Golmard, J.-L.; Auvert, B. Epidemiology of biliary atresia in France: A national study 1986–96. J. Hepatol. 1999, 31, 1006–1013. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Chang, M.H.; Chen, H.-L.; Lee, H.-C.; Wu, T.-C.; Lin, C.-C.; Yang, Y.-J.; Chen, A.-C.; Tiao, M.-M.; Lau, B.-H.; et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology 2007, 47, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, P.J.; Baker, A.J.; A Kelly, D. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet 2000, 355, 25–29. [Google Scholar] [CrossRef]
- Hartley, J.L.; Davenport, M.; A Kelly, D. Biliary atresia. Lancet 2009, 374, 1704–1713. [Google Scholar] [CrossRef]
- Shneider, B.L.; Brown, M.B.; Haber, B.; Whitington, P.F.; Schwarz, K.; Squires, R.; Bezerra, J.; Shepherd, R.; Rosenthal, P.; Hoofnagle, J.H.; et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J. Pediatr. 2006, 148, 467–474.e1. [Google Scholar] [CrossRef]
- Brumbaugh, D.; Mack, C. Conjugated Hyperbilirubinemia in Children. Pediatr. Rev. 2012, 33, 291–302. [Google Scholar] [CrossRef]
- Harpavat, S.; Garcia-Prats, J.A.; Anaya, C.; Brandt, M.L.; Lupo, P.J.; Finegold, M.J.; Obuobi, A.; Elhennawy, A.A.; Jarriel, W.S.; Shneider, B.L. Diagnostic Yield of Newborn Screening for Biliary Atresia Using Direct or Conjugated Bilirubin Measurements. JAMA 2020, 323, 1141–1150. [Google Scholar] [CrossRef]
- Harpavat, S.; Garcia-Prats, J.A.; Shneider, B.L. Newborn Bilirubin Screening for Biliary Atresia. N. Engl. J. Med. 2016, 375, 605–606. [Google Scholar] [CrossRef]
- Lien, T.-H.; Chang, M.-H.; Wu, J.-F.; Chen, H.-L.; Lee, H.-C.; Chen, A.-C.; Tiao, M.-M.; Wu, T.-C.; Yang, Y.-J.; Lin, C.-C.; et al. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in taiwan. Hepatology 2011, 53, 202–208. [Google Scholar] [CrossRef]
- Feldman, A.G.; Sokol, R.J. Recent developments in diagnostics and treatment of neonatal cholestasis. Semin. Pediatr. Surg. 2020, 29, 150945. [Google Scholar] [CrossRef]
- Zhou, L.; Shan, Q.; Tian, W.; Wang, Z.; Liang, J.; Xie, X. Ultrasound for the Diagnosis of Biliary Atresia: A Meta-Analysis. AJR Am. J. Roentgenol. 2016, 206, W73–W82. [Google Scholar] [CrossRef]
- Kianifar, H.R.; Tehranian, S.; Shojaei, P.; Adinehpoor, Z.; Sadeghi, R.; Kakhki, V.R.D.; Keshtgar, A.S. Accuracy of hepatobiliary scintigraphy for differentiation of neonatal hepatitis from biliary atresia: Systematic review and meta-analysis of the literature. Pediatr. Radiol. 2013, 43, 905–919. [Google Scholar] [CrossRef]
- Ryeom, H.K.; Choe, B.H.; Kim, J.Y.; Kwon, S.; Ko, C.W.; Kim, H.M.; Lee, S.B.; Kang, D.S. Biliary Atresia: Feasibility of Mangafodipir Trisodium–enhanced MR Cholangiography for Evaluation. Radiology 2005, 235, 250–258. [Google Scholar] [CrossRef]
- Kim, M.-J.; Park, Y.N.; Han, S.J.; Yoon, C.S.; Yoo, H.S.; Hwang, E.H.; Chung, K.S. Biliary Atresia in Neonates and Infants: Triangular Area of High Signal Intensity in the Porta Hepatis at T2-weighted MR Cholangiography with US and Histopathologic Correlation1. Radiology 2000, 215, 395–401. [Google Scholar] [CrossRef]
- Farrant, P.; Meire, H.B.; Mieli-Vergani, G. Ultrasound features of the gall bladder in infants presenting with conjugated hyperbilirubinaemia. Br. J. Radiol. 2000, 73, 1154–1158. [Google Scholar] [CrossRef]
- Humphrey, T.M.; Stringer, M.D. Biliary Atresia: US Diagnosis. Radiology 2007, 244, 845–851. [Google Scholar] [CrossRef]
- Kendrick, A.P.A.T.; Phua, K.B.; Ooi, B.C.; Tan, C.E.L. Biliary atresia: Making the diagnosis by the gallbladder ghost triad. Pediatr. Radiol. 2003, 33, 311–315. [Google Scholar] [CrossRef]
- Ikeda, S.; Sera, Y.; Akagi, M. Serial ultrasonic examination to differentiate biliary atresia from neonatal hepatitis—Special reference to changes in size of the gallbladder. Eur. J. Pediatrics 1989, 148, 396–400. [Google Scholar] [CrossRef]
- McGahan, J.P.; E Phillips, H.; Cox, K.L. Sonography of the normal pediatric gallbladder and biliary tract. Radiology 1982, 144, 873–875. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Wang, W.; Shan, Q.-Y.; Liu, B.-X.; Zheng, Y.-L.; Xu, Z.-F.; Xu, M.; Pan, F.-S.; Lu, M.-D.; Xie, X.-Y. Optimizing the US Diagnosis of Biliary Atresia with a Modified Triangular Cord Thickness and Gallbladder Classification. Radiology 2015, 277, 181–191. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, D.; Jiang, H.; Shan, Q.; Zhang, X.; Xie, X.; Zhou, L. Ultrasound Evaluation of Biliary Atresia Based on Gallbladder Classification: Is 4 Hours of Fasting Necessary? J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2019, 38, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lee, S.-M.; Park, W.-H.; Choi, S.-O. Objective Criteria of Triangular Cord Sign in Biliary Atresia on US Scans. Radiology 2003, 229, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-O.; Park, W.-H.; Lee, H.-J.; Woo, S.-K. ‘Triangular cord’: A sonographic finding applicable in the diagnosis of biliary atresia. J. Pediatr. Surg. 1996, 31, 363–366. [Google Scholar] [CrossRef]
- Park, W.-H.; Choi, S.-O.; Lee, H.-J.; Kim, S.-P.; Zeon, S.-K.; Lee, S.-L. A new diagnostic approach to biliary atresia with emphasis on the ultrasonographic triangular cord sign: Comparison of ultrasonography, hepatobiliary scintigraphy, and liver needle biopsy in the evaluation of infantile cholestasis. J. Pediatr. Surg. 1997, 32, 1555–1559. [Google Scholar] [CrossRef]
- Mittal, V.; Saxena, A.K.; Sodhi, K.S.; Thapa, B.R.; Rao, K.L.N.; Das, A.; Khandelwal, N. Role of Abdominal Sonography in the Preoperative Diagnosis of Extrahepatic Biliary Atresia in Infants Younger Than 90 Days. AJR Am. J. Roentgenol. 2011, 196, W438–W445. [Google Scholar] [CrossRef]
- Kotb, M.A.; Kotb, A.; Sheba, M.F.; Koofy, N.M.E.; El-Karaksy, H.M.; Abdel-Kahlik, M.K.; Abdalla, A.; El-Regal, M.E.; Warda, R.; Mostafa, H.; et al. Evaluation of the Triangular Cord Sign in the Diagnosis of Biliary Atresia. Pediatrics 2001, 108, 416–420. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Jiang, H.; Shan, Q.-Y.; Chen, D.; Lin, X.-N.; Liu, B.-X.; Xie, X.-Y. Liver stiffness measurements with supersonic shear wave elastography in the diagnosis of biliary atresia: A comparative study with grey-scale US. Eur. Radiol. 2017, 27, 3474–3484. [Google Scholar] [CrossRef]
- Weng, Z.; Zhou, L.; Wu, Q.; Zhou, W.; Ma, H.; Fang, Y.; Dang, T.; Liu, M. Enlarged hepatic hilar lymph node: An additional ultrasonographic feature that may be helpful in the diagnosis of biliary atresia. Eur. Radiol. 2019, 29, 6699–6707. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, N.; Zhang, X.; Zhou, W.; Xie, X.; Zhou, L. Ultrasound characteristics combined with gamma-glutamyl transpeptidase for diagnosis of biliary atresia in infants less than 30 days. Pediatr. Surg. Int. 2021, 37, 1175–1182. [Google Scholar] [CrossRef]
- Hwang, S.M.; Jeon, T.Y.; Yoo, S.-Y.; Choe, Y.H.; Lee, S.-K.; Kim, J.H. Early US findings of biliary atresia in infants younger than 30 days. Eur. Radiol. 2018, 28, 1771–1777. [Google Scholar] [CrossRef]
- Koob, M.; Pariente, D.; Habes, D.; Ducot, B.; Adamsbaum, C.; Franchi-Abella, S. The porta hepatis microcyst: An additional sonographic sign for the diagnosis of biliary atresia. Eur. Radiol. 2017, 27, 1812–1821. [Google Scholar] [CrossRef]
- Caponcelli, E.; Knisely, A.S.; Davenport, M. Cystic biliary atresia: An etiologic and prognostic subgroup. J. Pediatr. Surg. 2008, 43, 1619–1624. [Google Scholar] [CrossRef]
- Shan, Q.-Y.; Liu, B.-X.; Zhong, Z.-H.; Chen, H.-D.; Guo, Y.; Xie, X.-Y.; Zhou, W.-Y.; Jiang, H.; Zhou, L.-Y. The Prognosis of Type III Biliary Atresia with Hilar Cyst. Indian J. Pediatr. 2021, 88, 650–655. [Google Scholar] [CrossRef]
- Kim, W.S.; Cheon, J.-E.; Youn, B.J.; Yoo, S.-Y.; Kim, W.Y.; Kim, I.-O.; Yeon, K.M.; Seo, J.K.; Park, K.-W. Hepatic Arterial Diameter Measured with US: Adjunct for US Diagnosis of Biliary Atresia. Radiology 2007, 245, 549–555. [Google Scholar] [CrossRef]
- El-Guindi, M.A.-S.; Sira, M.M.; Sira, A.M.; Salem, T.A.-H.; El-Abd, O.L.; Konsowa, H.A.-S.; El-Azab, D.S.; Allam, A.A.-H. Design and validation of a diagnostic score for biliary atresia. J. Hepatol. 2014, 61, 116–123. [Google Scholar] [CrossRef]
- El-Guindi, M.A.-S.; Sira, M.M.; Konsowa, H.A.-S.; El-Abd, O.L.; Salem, T.A.-H. Value of hepatic subcapsular flow by color Doppler ultrasonography in the diagnosis of biliary atresia. J. Gastroenterol. Hepatol. 2013, 28, 867–872. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, M.-J.; Lee, M.-J.; Yoon, C.S.; Han, S.J.; Oh, J.-T.; Park, Y.N. Biliary Atresia: Color Doppler US Findings in Neonates and Infants. Radiology 2009, 252, 282–289. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, L.; Liao, B.; Cao, Q.; Jiang, H.; Zhou, W.; Wang, G.; Xie, X. Two-Dimensional Shear Wave Elastography Predicts Liver Fibrosis in Jaundiced Infants with Suspected Biliary Atresia: A Prospective Study. Korean J. Radiol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Galina, P.; Alexopoulou, E.; Mentessidou, A.; Mirilas, P.; Zellos, A.; Lykopoulou, L.; Patereli, A.; Salpasaranis, K.; Kelekis, N.L.; Zarifi, M. Diagnostic accuracy of two-dimensional shear wave elastography in detecting hepatic fibrosis in children with autoimmune hepatitis, biliary atresia and other chronic liver diseases. Pediatr. Radiol. 2021, 51, 1358–1368. [Google Scholar] [CrossRef]
- Gao, F.; Chen, Y.-Q.; Fang, J.; Gu, S.-L.; Li, L.; Wang, X.-Y. Acoustic Radiation Force Impulse Imaging for Assessing Liver Fibrosis Preoperatively in Infants with Biliary Atresia: Comparison with Liver Fibrosis Biopsy Pathology. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2017, 36, 1571–1578. [Google Scholar] [CrossRef]
- Wang, X.; Qian, L.; Jia, L.; Bellah, R.; Wang, N.; Xin, Y.; Liu, Q. Utility of Shear Wave Elastography for Differentiating Biliary Atresia from Infantile Hepatitis Syndrome. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016, 35, 1475–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Peng, C.; Wang, K.; Wu, D.; Yan, J.; Tu, W.; Chen, Y. The utility of shear wave elastography and serum biomarkers for diagnosing biliary atresia and predicting clinical outcomes. Eur. J. Pediatrics 2021, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.K.; Sun, Y.; Ju, Z.; Liu, S.; Jiang, J.; Koci, M.; Rosenberg, J.; Rubesova, E.; Barth, R.A. Ultrasound shear wave elastography: Does it add value to gray-scale ultrasound imaging in differentiating biliary atresia from other causes of neonatal jaundice? Pediatr. Radiol. 2021, 51, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Thumar, V.; Squires, J.H.; Spicer, P.J.; Robinson, A.L.; Chan, S.S. Ultrasound Elastography Applications in Pediatrics. Ultrasound Q. 2018, 34, 199–205. [Google Scholar] [CrossRef]
- Wu, J.-F.; Lee, C.-S.; Lin, W.-H.; Jeng, Y.-M.; Chen, H.-L.; Ni, Y.-H.; Hsu, H.-Y.; Chang, M.-H. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology 2018, 68, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liao, B.; Zhong, Z.; Zheng, Y.; Liu, B.; Shan, Q.; Xie, X.; Zhou, L. Supersonic shearwave elastography in the assessment of liver fibrosis for postoperative patients with biliary atresia. Sci. Rep. 2016, 6, 31057. [Google Scholar] [CrossRef]
- Leschied, J.R.; Dillman, J.R.; Bilhartz, J.; Heider, A.; Smith, E.A.; Lopez, M.J. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr. Radiol. 2015, 45, 366–375. [Google Scholar] [CrossRef]
- Dillman, J.R.; DiPaola, F.W.; Smith, S.J.; Barth, R.A.; Asai, A.; Lam, S.; Campbell, K.M.; Bezerra, J.A.; Tiao, G.M.; Trout, A. Prospective Assessment of Ultrasound Shear Wave Elastography for Discriminating Biliary Atresia from other Causes of Neonatal Cholestasis. J. Pediatr. 2019, 212, 60–65.e3. [Google Scholar] [CrossRef]
- Hanquinet, S.; Courvoisier, D.S.; Rougemont, A.-L.; Dhouib, A.; Rubbia-Brandt, L.; Wildhaber, B.E.; Merlini, L.; McLin, V.A.; Anooshiravani, M. Contribution of acoustic radiation force impulse (ARFI) elastography to the ultrasound diagnosis of biliary atresia. Pediatr. Radiol. 2015, 45, 1489–1495. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, X.; Pan, Y.; Luo, H. Comparison of the diagnostic value of virtual touch tissue quantification and virtual touch tissue imaging quantification in infants with biliary atresia. Int. J. Clin. Pr. 2021, 75, e13860. [Google Scholar] [CrossRef]
- Boo, Y.; Chang, M.; Jeng, Y.; Peng, S.; Hsu, W.; Lin, W.; Chen, H.; Ni, Y.; Hsu, H.; Wu, J. Diagnostic Performance of Transient Elastography in Biliary Atresia among Infants with Cholestasis. Hepatol. Commun. 2021, 5, 882–890. [Google Scholar] [CrossRef]
- Goldschmidt, I.; Streckenbach, C.; Dingemann, C.; Pfister, E.D.; di Nanni, A.; Zapf, A.; Baumann, U. Application and Limitations of Transient Liver Elastography in Children. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 109–113. [Google Scholar] [CrossRef]
- Duan, X.; Peng, Y.; Liu, W.; Yang, L.; Zhang, J. Does Supersonic Shear Wave Elastography Help Differentiate Biliary Atresia from Other Causes of Cholestatic Hepatitis in Infants Less than 90 Days Old? Compared with Grey-Scale US. BioMed Res. Int. 2019, 2019, 9036362. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, L.Q.; Hu, Y.X.; Xin, Y.; Yang, X.; Wang, X.M. Development and Validation of a Nomogram Incorporating Ultrasonic and Elastic Findings for the Preoperative Diagnosis of Biliary Atresia. Acad. Radiol. 2020, 28, S55–S63. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, D.; Gu, S.; Li, Y.; Pan, W.; Zhang, Y. Three-color risk stratification for improving the diagnostic accuracy for biliary atresia. Eur. Radiol. 2020, 30, 3852–3861. [Google Scholar] [CrossRef]
- Zhou, W.; Li, X.; Zhang, N.; Liao, B.; Xie, X.; Zhang, X.; Wang, G.; Zhou, L. The combination of conventional ultrasound and shear-wave elastography in evaluating the segmental heterogeneity of liver fibrosis in biliary atresia patients after Kasai portoenterostomy. Pediatr. Surg. Int. 2021, 37, 1099–1108. [Google Scholar] [CrossRef]
- Kim, J.R.; Suh, C.H.; Yoon, H.M.; Lee, J.S.; Cho, Y.A.; Jung, A.Y. The diagnostic performance of shear-wave elastography for liver fibrosis in children and adolescents: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2018, 28, 1175–1186. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Chen, S.-L.; Chen, H.-D.; Huang, Y.; Qiu, Y.-X.; Zhong, W.; Xie, X.-Y. Percutaneous US-guided Cholecystocholangiography with Microbubbles for Assessment of Infants with US Findings Equivocal for Biliary Atresia and Gallbladder Longer than 1.5 cm: A Pilot Study. Radiology 2018, 286, 1033–1039. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.C.; Choe, B.-H.; Ryeom, H.K.; Jang, Y.-J.; Kim, H.J.; Park, J.Y.; Cho, S.-M. Efficacy of US-guided Percutaneous Cholecystocholangiography for the Early Exclusion and Type Determination of Biliary Atresia. Radiology 2011, 261, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Treem, W.R.; Grant, E.E.; Barth, K.H.; Kramers, P.W. Ultrasound Guided Percutaneous Cholecystocholangiography for Early Differentiation of Cholestatic Liver Disease in Infants. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 347–352. [Google Scholar] [CrossRef]
- Buda, M.; Wildman-Tobriner, B.; Hoang, J.K.; Thayer, D.; Tessler, F.N.; Middleton, W.D.; Mazurowski, M.A. Management of Thyroid Nodules Seen on US Images: Deep Learning May Match Performance of Radiologists. Radiology 2019, 292, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, Y.; Lv, W.; Liu, L.; Zhou, Q.; Yang, H.; Ren, J.; Liu, G.; Wang, X.; Zhang, X.; et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study. Lancet Digit. Health 2021, 3, e250–e259. [Google Scholar] [CrossRef]
- Shen, Y.; Shamout, F.E.; Oliver, J.R.; Witowski, J.; Kannan, K.; Park, J.; Wu, N.; Huddleston, C.; Wolfson, S.; Millet, A.; et al. Artificial intelligence system reduces false-positive findings in the interpretation of breast ultrasound exams. Nat. Commun. 2021, 12, 5645. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Pei, J.; Zheng, H.; Xie, X.; Yan, L.; Zhang, H.; Han, C.; Gao, X.; Zhang, H.; Zheng, W.; et al. Prospective assessment of breast cancer risk from multimodal multiview ultrasound images via clinically applicable deep learning. Nat. Biomed. Eng. 2021, 5, 522–532. [Google Scholar] [CrossRef]
- Arnaout, R.; Curran, L.; Zhao, Y.; Levine, J.C.; Chinn, E.; Moon-Grady, A.J. An ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nat. Med. 2021, 27, 882–891. [Google Scholar] [CrossRef]
- Xie, H.; Wang, N.; He, M.; Zhang, L.H.; Cai, H.; Xian, J.; Lin, M.; Zheng, J.; Yang, Y. Using deep-learning algorithms to classify fetal brain ultrasound images as normal or abnormal. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 56, 579–587. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Yu, C.; Liu, J.; Duan, X.; Weng, Z.; Chen, D.; Liang, Q.; Fang, Q.; Zhou, J.; et al. Ensembled deep learning model outperforms human experts in diagnosing biliary atresia from sonographic gallbladder images. Nat. Commun. 2021, 12, 1259. [Google Scholar] [CrossRef]
- Napolitano, M.; Franchi-Abella, S.; Damasio, B.M.; Augdal, T.A.; Avni, F.E.; Bruno, C.; Darge, K.; Ključevšek, D.; Littooij, A.S.; Lobo, L.; et al. Practical approach for the diagnosis of biliary atresia on imaging, part 2: Magnetic resonance cholecystopancreatography, hepatobiliary scintigraphy, percutaneous cholecysto-cholangiography, endoscopic retrograde cholangiopancreatography, percutaneous liver biopsy, risk scores and decisional flowchart. Pediatr. Radiol. 2021, 51, 1545–1554. [Google Scholar] [CrossRef]
- Sung, S.; Jeon, T.Y.; Yoo, S.-Y.; Hwang, S.M.; Choi, Y.H.; Kim, W.S.; Choe, Y.H.; Kim, J.H. Incremental Value of MR Cholangiopancreatography in Diagnosis of Biliary Atresia. PLoS ONE 2016, 11, e0158132. [Google Scholar] [CrossRef]



| US Features | Definition | Positive | Negative | Diagnostic Value of BA when Positive |
|---|---|---|---|---|
| Gallbladder abnormalities | Abnormalities in the length of the gallbladder lumen, the integrity of the mucosal lining, and the degree of contraction of the gallbladder after feeding. | ① The length of the fully filled gallbladder lumen <1.5 cm; ② Lack of smooth and complete echogenic mucosal lining; ③ No contraction of the gallbladder after feeding | ① The length of the fully filled gallbladder lumen >1.5 cm with smooth and complete hyperechogenic mucosal lining; ② Incompletely filled lumen of the unfilled gallbladder with smooth and complete hyperechogenic mucosal lining | Strongly suggest BA |
| Triangular cord sign | The thickness of the echogenic anterior wall of the right portal vein, with or without HA. | >2.0 mm not including HA, or >4.0 mm including HA | ≤2.0 mm not including HA, or ≤4.0 mm including HA | Strongly suggest BA |
| Porta hepatis macro- or microcyst | The cyst in front of the right portal vein at the hepatic portal. | Presence | Absence | Strongly suggest BA |
| Hepatic hilar lymph node | The lymph node located at the porta hepatis, around the hepatoduodenal ligament. | Presence | Absence | Possible BA |
| HA diameter | Measured at the level of right proximal HA running parallel to the right portal vein. | 2.1 mm to 2.5 mm | 1.5 mm to 1.9 mm | Not recommended for diagnosis alone |
| Hepatic subcapsular flow | Vascular structures continued to the liver capsular surface on color Doppler US images. | Presence | Absence | Not recommended for diagnosis alone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Zhou, L. Ultrasound for the Diagnosis of Biliary Atresia: From Conventional Ultrasound to Artificial Intelligence. Diagnostics 2022, 12, 51. https://doi.org/10.3390/diagnostics12010051
Zhou W, Zhou L. Ultrasound for the Diagnosis of Biliary Atresia: From Conventional Ultrasound to Artificial Intelligence. Diagnostics. 2022; 12(1):51. https://doi.org/10.3390/diagnostics12010051
Chicago/Turabian StyleZhou, Wenying, and Luyao Zhou. 2022. "Ultrasound for the Diagnosis of Biliary Atresia: From Conventional Ultrasound to Artificial Intelligence" Diagnostics 12, no. 1: 51. https://doi.org/10.3390/diagnostics12010051






