Utility of Diagnostic Imaging in the Early Detection and Management of the Fournier Gangrene
Abstract
:1. Introduction
2. Methods
2.1. Vital and Laboratory Parameters
2.2. Radiologic Imaging
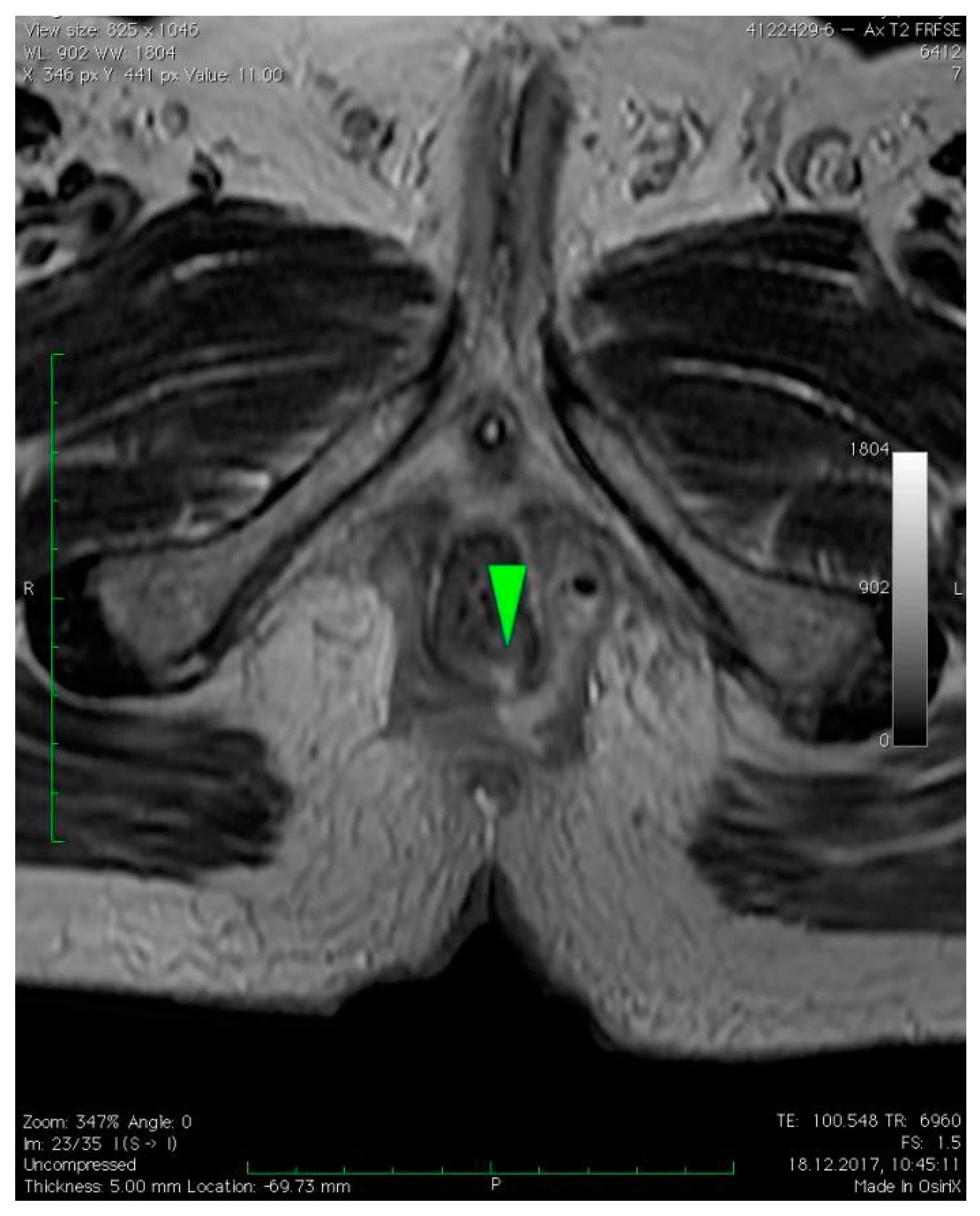
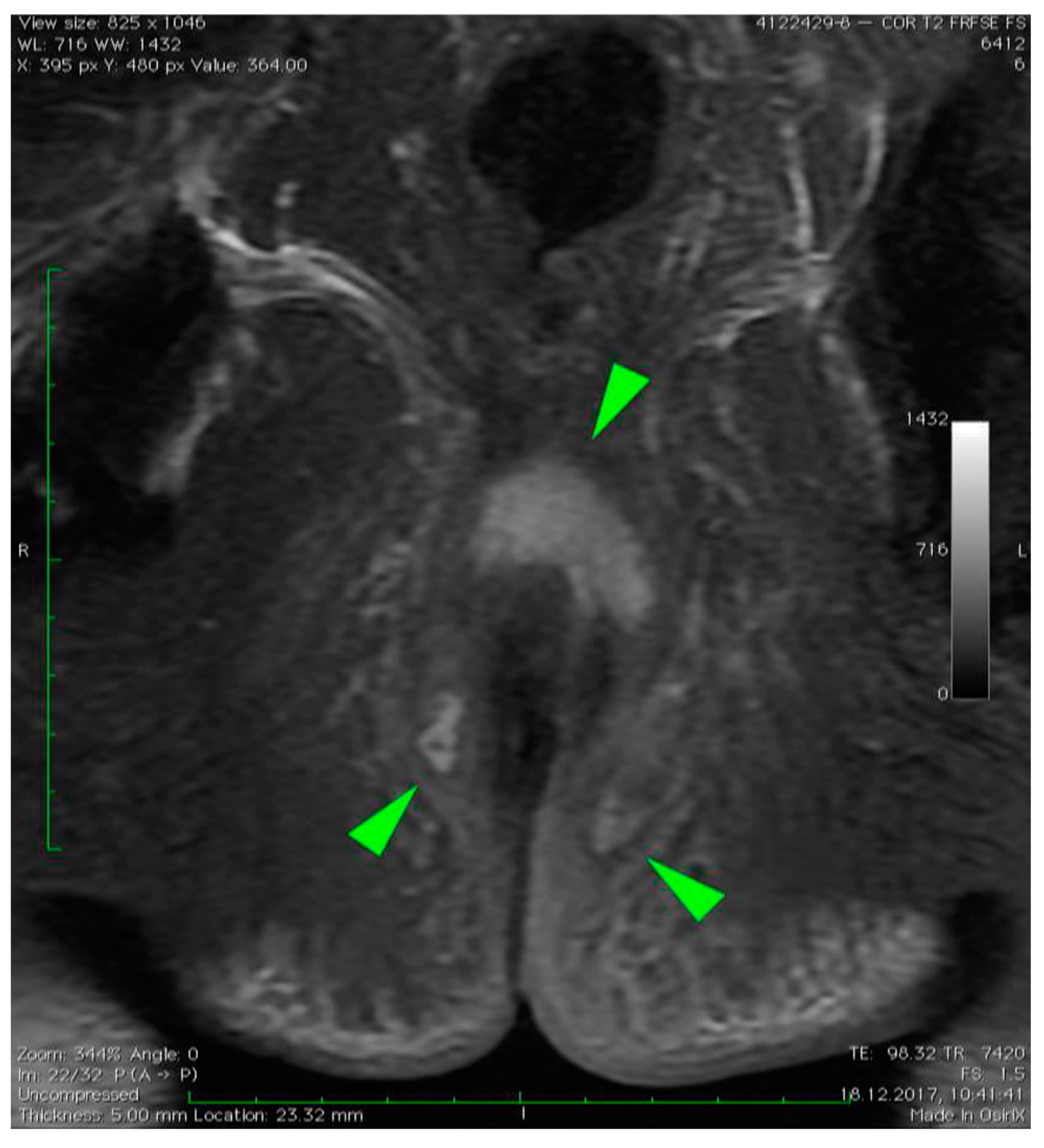
3. Case Presentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masek, M.; Zak, J. Fournierova gangrena [Fournier’s gangrene]. Bratisl. Lek Listy 2001, 102, 46–47. [Google Scholar] [PubMed]
- Levenson, R.B.; Singh, A.K.; Novelline, R.A. Fournier gangrene: Role of imaging. Radiographics 2008, 28, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Thwaini, A.; Khan, A.; Malik, A.; Cherian, J.; Barua, J.; Shergill, I.; Mammen, K. Fournier’s gangrene and its emergency management. Postgrad. Med. J. 2006, 82, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Majeed, M.; Olang, C.A.; Patel, A.; Gorantla, V.R.; Davis, N.; Gluschitz, S. Fournier’s Gangrene Diagnosis and Treatment: A Systematic Review. Cureus 2021, 13, e18948. [Google Scholar] [CrossRef] [PubMed]
- El-Qushayri, A.E.; Khalaf, K.M.; Dahy, A.; Mahmoud, A.R.; Benmelouka, A.Y.; Ghozy, S.; Mahmoud, M.U.; Bin-Jumah, M.; Alkahtani, S.; Abdel-Daim, M.M. Fournier’s gangrene mortality: A 17-year systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 92, 218–225. [Google Scholar] [CrossRef]
- De Miguel Criado, J.; del Salto, L.G.; Rivas, P.F.; del Hoyo, L.F.A.; Velasco, L.G.; de las Vacas, M.I.D.P.; Sanz, A.G.M.; Paradela, M.M.; Moreno, E.F. MR imaging evaluation of perianal fistulas: Spectrum of imaging features. Radiographics 2012, 32, 175–194. [Google Scholar] [CrossRef]
- Safioleas, M.; Stamatakos, M.; Mouzopoulos, G.; Diab, A.; Kontzoglou, K.; Papachristodoulou, A. Fournier’s gangrene: Exists and it is still lethal. Int. Urol. Nephrol. 2006, 38, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Khin, L.W.; Heng, K.S.; Tan, K.C.; Low, C.O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit. Care Med. 2004, 32, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Noegroho, B.S.; Siregar, S.; Mustafa, A.; Rivaldi, M.A. Validation of FGSI Scores in Predicting Fournier Gangrene in Tertiary Hospital. Res. Rep. Urol. 2021, 13, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yilmazlar, T.; Ozturk, E.; Ozguc, H.; Ercan, I.; Vuruskan, H.; Oktay, B. Fournier’s gangrene: An analysis of 80 patients and a novel scoring system. Tech. Coloproctol. 2010, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.A.; Baker, K.S.; Gould, E.S.; Gupta, R. Necrotizing fasciitis and its mimics: What radiologists need to know. AJR Am. J. Roentgenol. 2015, 204, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Shokry, Y.G.; Emad, S.; Halim, M.; Hanna, S.A.Z. MRI findings in Fournier’s gangrene. Eurorad 2013. [Google Scholar] [CrossRef]
- Wongwaisayawan, S.; Krishna, S.; Haroon, M.; Nisha, Y.; Sheikh, A. Fournier gangrene: Pictorial review. Abdom. Radiol. 2020, 45, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.W.; Kopari, N.M.; Pham, T.N.; Evans, H.L. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr. Probl. Surg. 2014, 51, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Kranz, J.; Dräger, D.L.; Schneidewind, L. Neue Aspekte zur Fournierschen Gangrän—Ein Rapid Review [New aspects in Fournier’s gangrene—A rapid review]. Aktuelle Urol. 2021, 52, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kikinis, R.; Pieper, S. 3D Slicer as a tool for interactive brain tumor segmentation. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6982–6984. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Chennamsetty, A.; Khourdaji, I.; Burks, F.; Killinger, K.A. Contemporary diagnosis and management of Fournier’s gangrene. Ther. Adv. Urol. 2015, 7, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Fujita, F.; Tokai, H.; Ito, Y.; Haraguchi, M.; Tajima, Y.; Kanematsu, T. MRI can determine the adequate area for debridement in the case of Fournier’s gangrene. Int. Surg. 2010, 95, 76–79. [Google Scholar] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumisławski, P.; Kołecki, J.; Piotrowska, M.; Kotowski, M.; Szemitko, M.; Sieńko, J. Utility of Diagnostic Imaging in the Early Detection and Management of the Fournier Gangrene. Diagnostics 2022, 12, 2320. https://doi.org/10.3390/diagnostics12102320
Sumisławski P, Kołecki J, Piotrowska M, Kotowski M, Szemitko M, Sieńko J. Utility of Diagnostic Imaging in the Early Detection and Management of the Fournier Gangrene. Diagnostics. 2022; 12(10):2320. https://doi.org/10.3390/diagnostics12102320
Chicago/Turabian StyleSumisławski, Piotr, Janusz Kołecki, Martyna Piotrowska, Maciej Kotowski, Marcin Szemitko, and Jerzy Sieńko. 2022. "Utility of Diagnostic Imaging in the Early Detection and Management of the Fournier Gangrene" Diagnostics 12, no. 10: 2320. https://doi.org/10.3390/diagnostics12102320




