Placenta, the Key Witness of COVID-19 Infection in Premature Births
Abstract
:1. Introduction
2. Materials and Methods
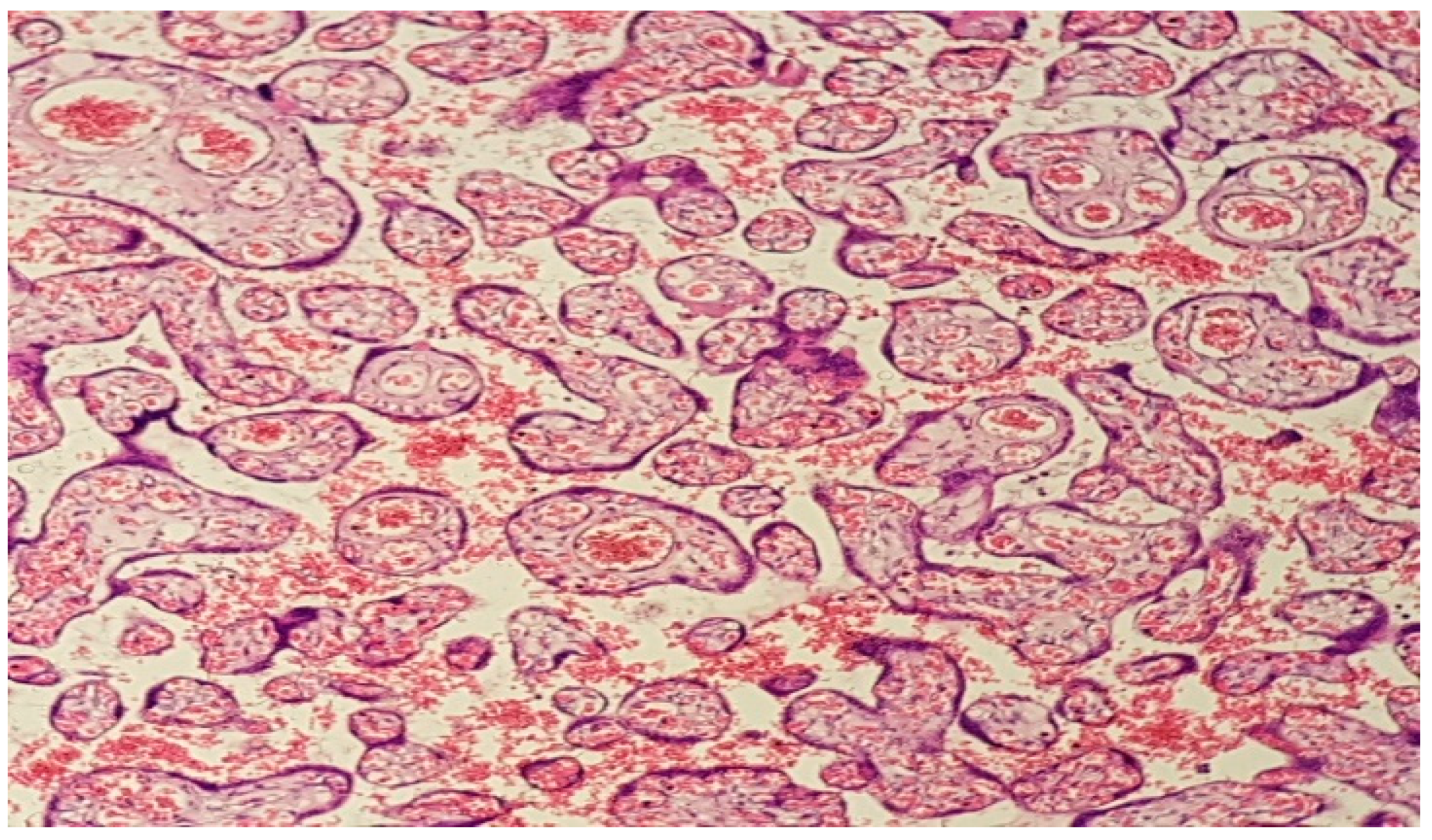
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 Infection With Serious Maternal Morbidity and Mortality From Obstetric Complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef]
- Bhatia, P.; Bhatia, K. Pregnancy and the lungs. Postgrad. Med. J. 2000, 76, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, Y.; Zhao, Y.; Xi, H.; Liu, C.; Qu, F.; Feng, X. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. Eur. J. Clin. Microbiol. 2020, 39, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Zhang, J.; Nakpu, J.; Hamada, H.; Dunk, C.E.; Li, S.; Imperio, G.E.; Nadeem, L.; Kibschull, M.; Lye, P.; et al. Expression of Severe Acute Respiratory Syndrome Coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 2020, 224, 298.e1–298.e8. [Google Scholar] [PubMed]
- Vouga, M.; Favre, G.; Martinez-Perez, O.; Pomar, L.; Acebal, L.F.; Abascal-Saiz, A.; Hernandez, M.R.V.; Hcini, N.; Lambert, V.; Carles, G.; et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci. Rep. 2021, 11, 13898. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Di Mascio, D.; Sen, C.; Saccone, G.; Galindo, A.; Grünebaum, A.; Yoshimatsu, J.; Stanojevic, M.; Kurjak, A.; Chervenak, F.; Suárez, M.J.R.; et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by Coronavirus disease 2019 (COVID-19): A secondary analysis of the WAPM study on COVID-19. J. Périnat. Med. 2020, 48, 950–958. [Google Scholar] [CrossRef]
- D’Antonio, F.; Sen, C.; Di Mascio, D.; Galindo, A.; Villalain, C.; Herraiz, I.; Arisoy, R.; Ovayolu, A.; Eroğlu, H.; Canales, M.G.; et al. Maternal and perinatal outcomes in high compared to low risk pregnancies complicated by severe acute respiratory syndrome coronavirus 2 infection (phase 2): The World Association of Perinatal Medicine working group on coronavirus disease 2019. Am. J. Obstet. Gynecol. MFM 2021, 3, 100329. [Google Scholar] [CrossRef]
- Carbone, L.; Esposito, R.; Raffone, A.; Verrazzo, P.; Carbone, I.F.; Saccone, G. Proposal for radiologic diagnosis and follow-up of COVID-19 in pregnant women. J. Matern. Neonatal Med. 2020, 35, 3002–3003. [Google Scholar] [CrossRef]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA 2020, 323, 2198–2200. [Google Scholar] [CrossRef]
- Bellos, I.; Pandita, A.; Panza, R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 256, 194–204. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Zhang, J.; Zhu, F.; Tang, Y.; Shen, X. A Case of 2019 Novel Coronavirus in a Pregnant Woman with Preterm Delivery. Clin. Infect. Dis. 2020, 71, 844–846. [Google Scholar] [CrossRef]
- Mullins, E.; Evans, D.; Viner, R.M.; O’Brien, P.; Morris, E. Coronavirus in pregnancy and delivery: Rapid review. Ultrasound Obstet. Gynecol. 2020, 55, 586–592. [Google Scholar] [CrossRef]
- Sharps, M.C.; Hayes, D.J.; Lee, S.; Zou, Z.; Brady, C.A.; Almoghrabi, Y.; Kerby, A.; Tamber, K.K.; Jones, C.J.; Waldorf, K.M.A.; et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta 2020, 101, 13–29. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Ravishankar, S.; Luo, G.; Redline, R.W. Predictors of High Grade and Other Clinically Significant Placental Findings by Indication for Submission in Singleton Placentas from Term Births. Pediatr. Dev. Pathol. 2020, 23, 274–284. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [Green Version]
- Redline, R.W. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am. J. Obstet. Gynecol. 2005, 192, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Roberts, D.J. Placental pathologic lesions with a significant recurrence risk-what not to miss! APMIS 2018, 126, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, B.B.; Chilukuri, N.; He, H.; Cerda, S.R.; Hong, X.; Wang, G.; Pearson, C.; Burd, I.; Wang, X. Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort. Placenta 2017, 52, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Weiner, E.; Feldstein, O.; Tamayev, L.; Grinstein, E.; Barber, E.; Bar, J.; Schreiber, L.; Kovo, M. Placental histopathological lesions in correlation with neonatal outcome in preeclampsia with and without severe features. Pregnancy Hypertens. 2018, 12, 6–10. [Google Scholar] [CrossRef]
- Aghaamoo, S.; Ghods, K.; Rahmanian, M. Pregnant women with COVID-19: The placental involvement and consequences. J. Mol. Histol. 2021, 52, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1–382.e18. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.M. Maternal vascular malperfusion of the placental bed. APMIS 2018, 126, 551–560. [Google Scholar] [CrossRef]
- Wong, Y.P.; Khong, T.Y.; Tan, G.C. The effects of COVID-19 on placenta and pregnancy: What do we know so far? Diagnostics 2021, 11, 94. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278. [Google Scholar] [CrossRef]
- Debelenko, L.; Katsyv, I.; Chong, A.M.; Peruyero, L.; Szabolcs, M.; Uhlemann, A.-C. Trophoblast damage with acute and chronic intervillositis: Disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum. Pathol. 2021, 109, 69–79. [Google Scholar] [CrossRef]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092–2103. [Google Scholar] [CrossRef]
- Levitan, D.; London, V.; McLaren, R.A.; Mann, J.D.; Cheng, K.; Silver, M.; Balhotra, K.S.; McCalla, S.; Loukeris, K. Histologic and Immunohistochemical Evaluation of 65 Placentas From Women With Polymerase Chain Reaction-Proven Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Arch. Pathol. Lab. Med. 2021, 145, 648–656. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife 2020, 9, e58716. [Google Scholar] [CrossRef]
- Bukowska-Ośko, I.; Popiel, M.; Kowalczyk, P. The immunological role of the placenta in SARS-CoV-2 infection-viral transmission, immune regulation, and lactoferrin activity. Int. J. Mol. Sci. 2021, 22, 5799. [Google Scholar] [CrossRef]




| MATERNAL CHARACTERISTICS | COVID-19 PATIENTS (%) | NON-COVID-19 PATIENTS (%) | p-Value | ||
|---|---|---|---|---|---|
| material age (years-percentage) | 18–30 y.o. | 41 | 18–30 y.o. | 53.8 | 0.164 |
| 31–40 y.o. | 46.2 | 31–40 y.o. | 41 | ||
| 41–45 y.o. | 12.8 | 41–45 y.o. | 5.1 | ||
| BMI (percentage) | <18.5 | 7.7 | <18.5 | 7.7 | 0.749 |
| 18.5–24.9 | 69.2 | 18.5–24.9 | 69.2 | ||
| 25–30 | 12.8 | 25–30 | 17.9 | ||
| >30 | 10.3 | >30 | 5.1 | ||
| gesta (number-percentage) | 1 | 33.3 | 1 | 43.6 | 0.581 |
| 2 | 23.1 | 2 | 17.9 | ||
| ≥3 | 43.6 | ≥3 | 38.5 | ||
| para (number-percentage) | 1 | 51.3 | 1 | 59 | 0.696 |
| 2 | 28.2 | 2 | 28.2 | ||
| ≥3 | 20.5 | ≥3 | 12.8 | ||
| monitored pregnancy (percentage) | Yes | 69.2 | Yes | 41 | 0.012 |
| No | 30.8 | No | 59 | ||
| gestational age of distribution (weeks-percentage) | <28 w | 7.7 | <28 w | 17.9 | 0.112 |
| 28–31 w | 12.8 | 28–31 w | 17.9 | ||
| 32–36 w | 79.5 | 32–36 w | 64.1 | ||
| premature rupture of membranes (percentage) | YES | 23.1 | YES | 51.3 | 0.010 |
| No | 76.9 | No | 48.7 | ||
| birth type (vaginal vs. caesarean-percentage) | Vaginal birth | 23.1 | Vaginal birth | 94.9 | <0.001 |
| Caesarean birth | 76.9 | Caesarean birth | 5.1 | ||
| p-Value | |||
|---|---|---|---|
| symptomatic (percentage) | Yes | 53.8 | 0.000 |
| No | 46.2 | ||
| oxygen therapy (percentage) | Yes | 23.1 | 0.002 |
| No | 76.9 | ||
| intubation (percentage) | Yes | 17.9 | 0.006 |
| No | 82.1 | ||
| associated pathologies (percentage) | Yes | 30.8 | 0.001 |
| No | 69.2 | ||
| apgar scores (percentage) | <5 | 10.3 | 0.000 |
| 5–8 | 61.6 | ||
| 9 | 23.1 | ||
| 10 | 5.1 | ||
| COVID-19 PATIENTS | NON-COVID-19 PATIENTS | p-Value | |
|---|---|---|---|
| placental infarct (percentage) | 64.1 | 30.8 | 0.003 |
| perivillous fibrin deposits (percentage) | 59 | 46.2 | 0.263 |
| decidual artheriopathy (percentage) | 66.7 | 23.1 | 0.000 |
| intervillous thrombi (percentage) | 53.8 | 38.5 | 0.177 |
| chorangiosis (percentage) | 17.9 | 10.3 | 0.335 |
| accelerated villous maturation (percentage) | 23.1 | 28.2 | 0.610 |
| inflammatory infiltrate (percentage) | 69.2 | 46.2 | 0.040 |
| no histopathological abnormalities (percentage) | 5.1 | 10.3 | 0.384 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobei, T.-I.; Sima, R.-M.; Gorecki, G.-P.; Poenaru, M.-O.; Olaru, O.-G.; Bobirca, A.; Cirstoveanu, C.; Chicea, R.; Topirceanu-Andreoiu, O.-M.; Ples, L. Placenta, the Key Witness of COVID-19 Infection in Premature Births. Diagnostics 2022, 12, 2323. https://doi.org/10.3390/diagnostics12102323
Bobei T-I, Sima R-M, Gorecki G-P, Poenaru M-O, Olaru O-G, Bobirca A, Cirstoveanu C, Chicea R, Topirceanu-Andreoiu O-M, Ples L. Placenta, the Key Witness of COVID-19 Infection in Premature Births. Diagnostics. 2022; 12(10):2323. https://doi.org/10.3390/diagnostics12102323
Chicago/Turabian StyleBobei, Tina-Ioana, Romina-Marina Sima, Gabriel-Petre Gorecki, Mircea-Octavian Poenaru, Octavian-Gabriel Olaru, Anca Bobirca, Catalin Cirstoveanu, Radu Chicea, Oana-Maria Topirceanu-Andreoiu, and Liana Ples. 2022. "Placenta, the Key Witness of COVID-19 Infection in Premature Births" Diagnostics 12, no. 10: 2323. https://doi.org/10.3390/diagnostics12102323
APA StyleBobei, T.-I., Sima, R.-M., Gorecki, G.-P., Poenaru, M.-O., Olaru, O.-G., Bobirca, A., Cirstoveanu, C., Chicea, R., Topirceanu-Andreoiu, O.-M., & Ples, L. (2022). Placenta, the Key Witness of COVID-19 Infection in Premature Births. Diagnostics, 12(10), 2323. https://doi.org/10.3390/diagnostics12102323







