Role of MRI-Derived Radiomics Features in Determining Degree of Tumor Differentiation of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
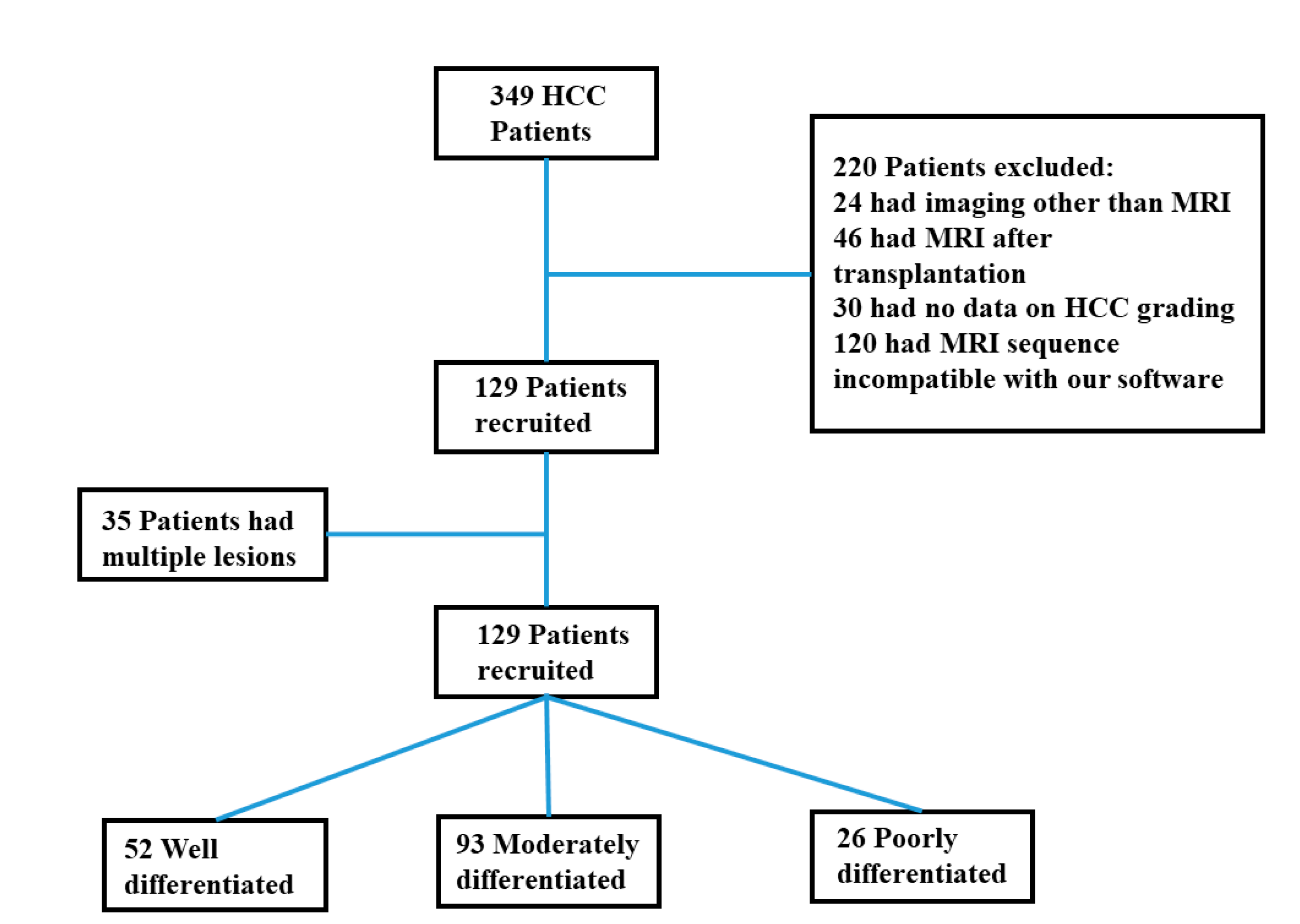
2.1. Study Population
2.2. Histopathology
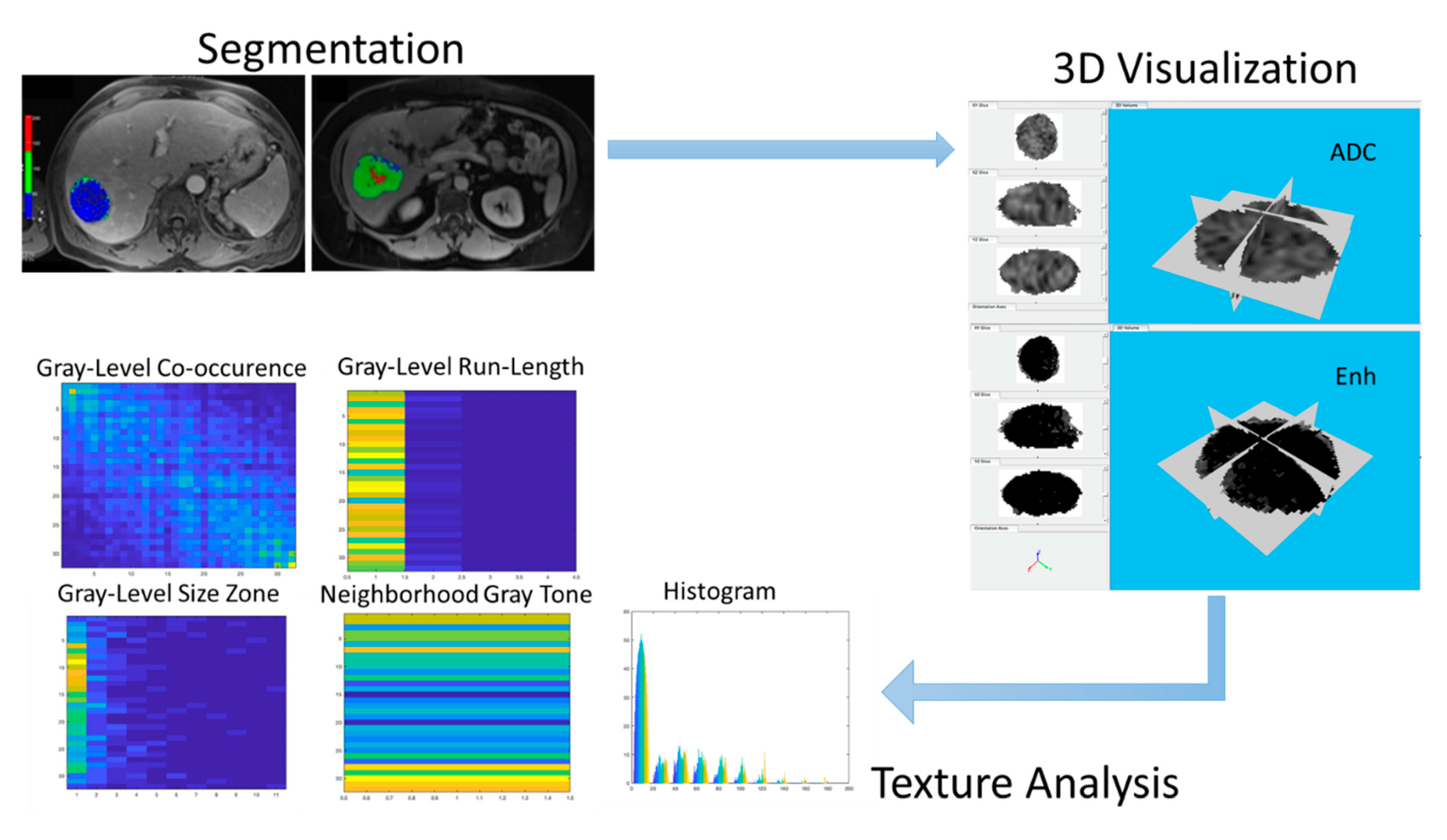
2.3. MRI Technique and Tumor Segmentation
2.4. Feature Extraction
2.5. Grey Level Discretization
2.6. Statistical Analysis
3. Results
3.1. Descriptive Findings
3.2. Radiomics Feature Extraction
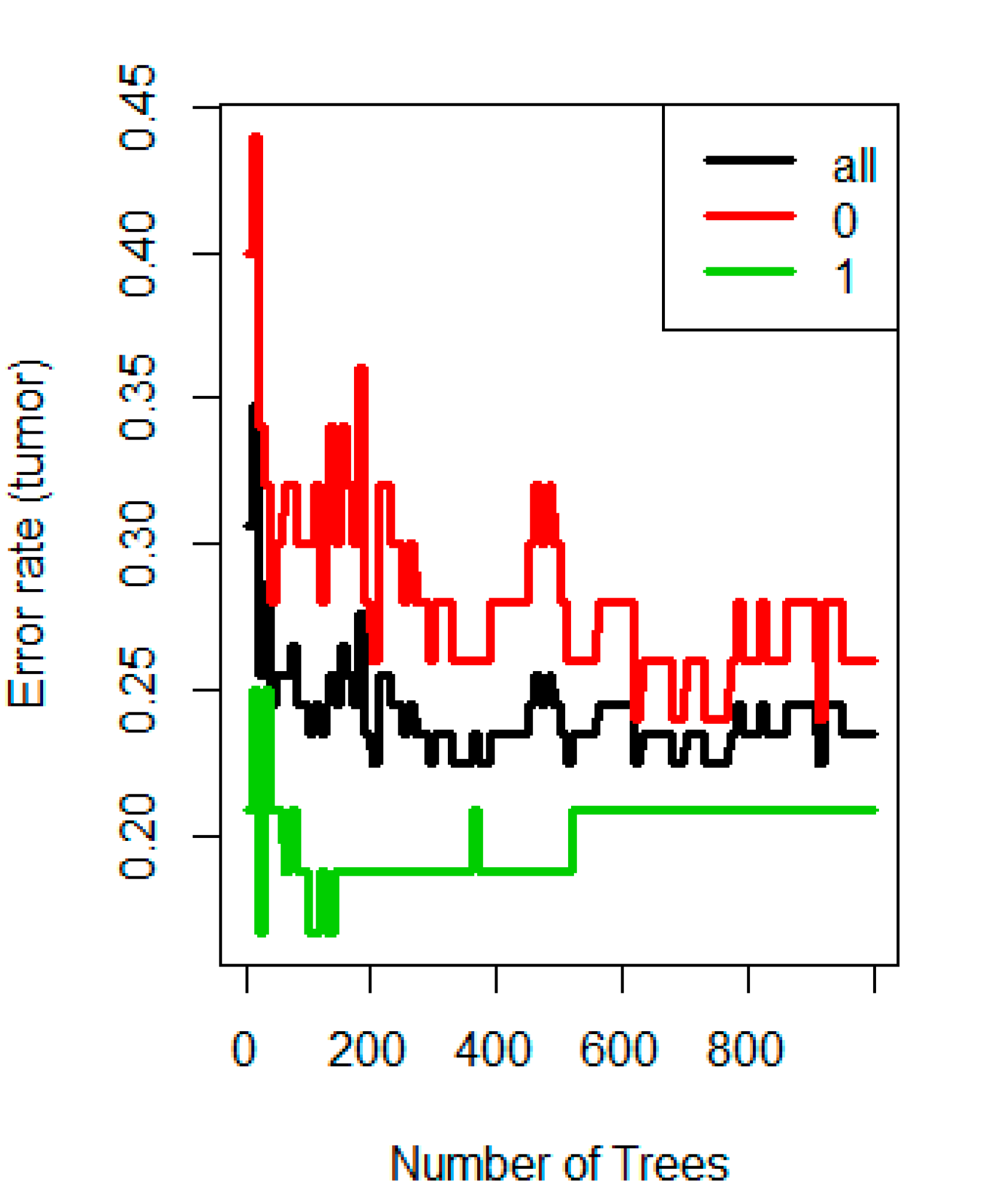
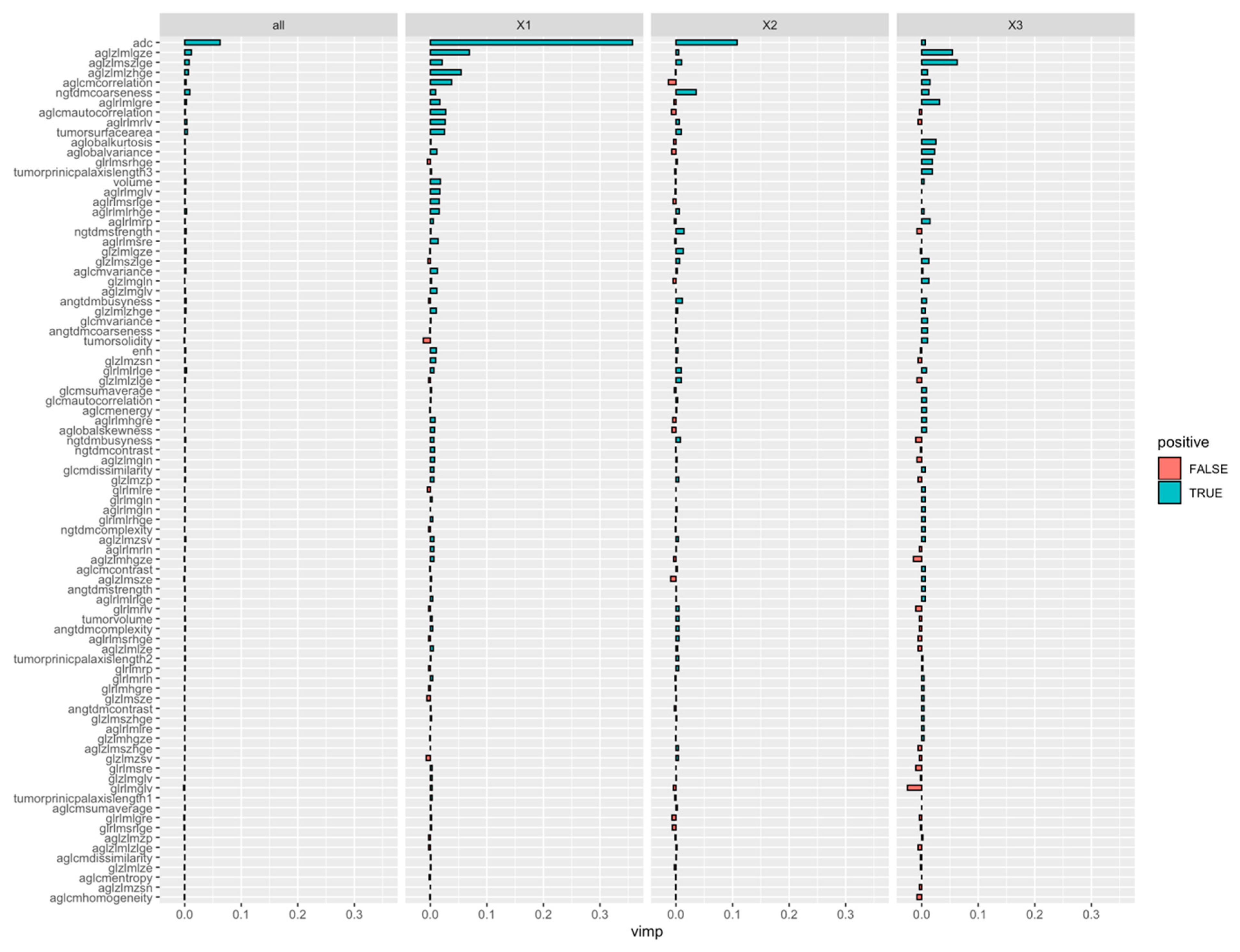
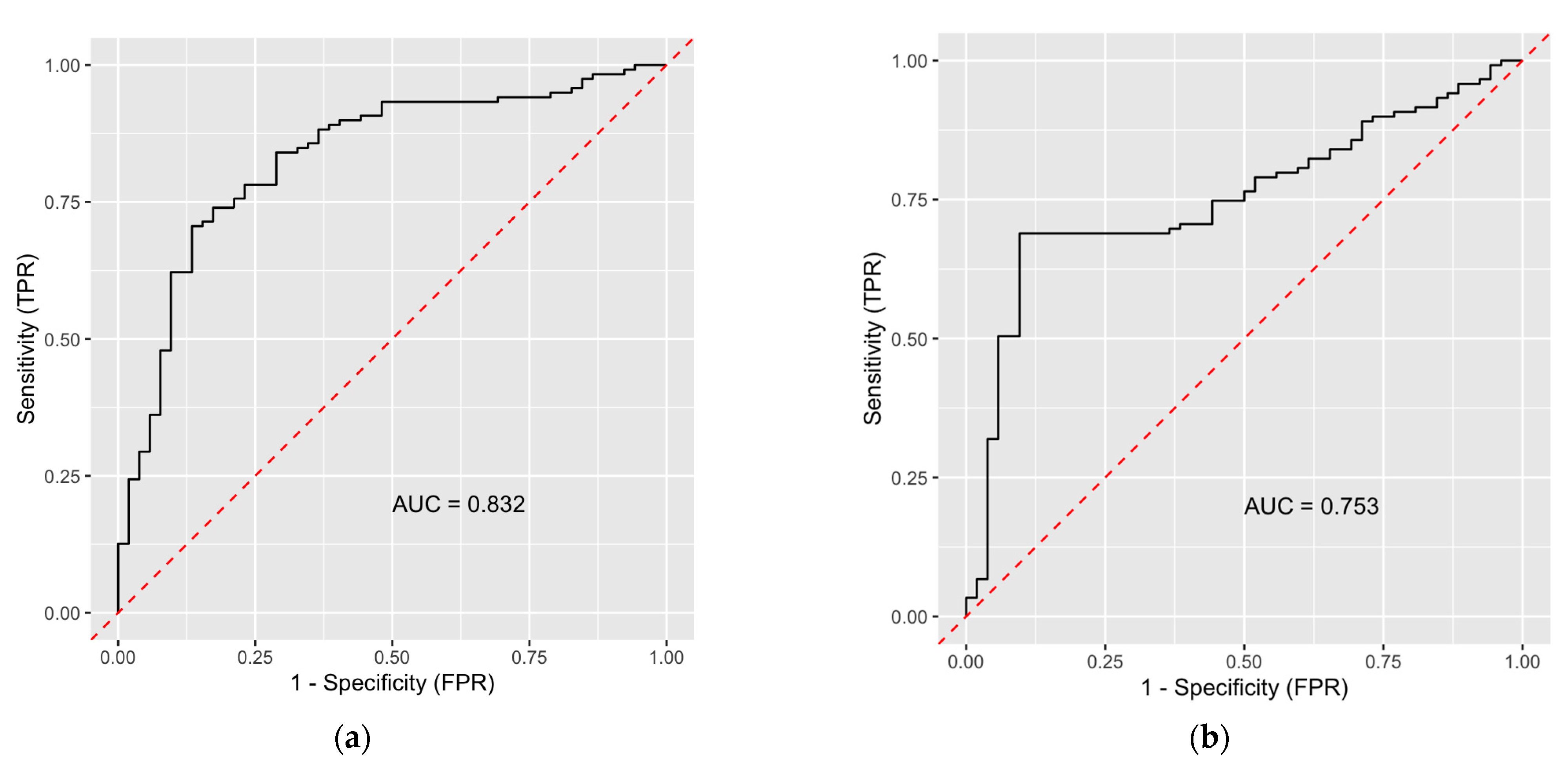
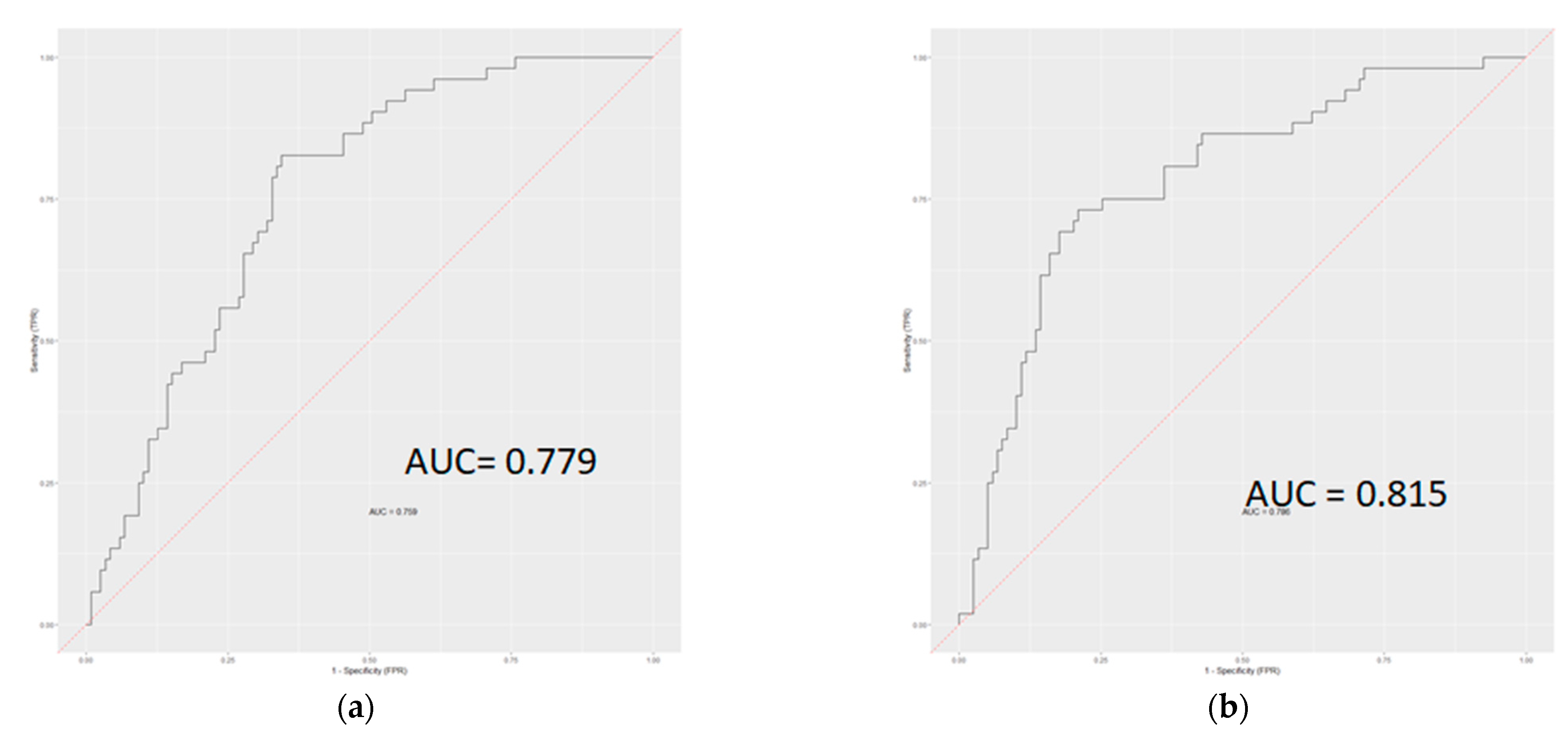
3.3. Classification Ability of Radiomics-Based Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. MRI Technique
Appendix A.2. Segmentation
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Han, D.H.; Choi, G.H.; Kim, K.S.; Choi, J.S.; Park, Y.N.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Han, K.-H. Prognostic significance of the worst grade in hepatocellular carcinoma with heterogeneous histologic grades of differentiation. J. Gastroenterol. Hepatol. 2013, 28, 1384–1390. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Shi, Y.; Wang, F.; Meng, X. Correlations between the minimum and mean apparent diffusion coefficient values of hepatocellular carcinoma and tumor grade. J. Magn. Reson. Imaging 2016, 44, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Kitao, A.; Zen, Y.; Matsui, O.; Gabata, T.; Kobayashi, S.; Koda, W.; Kozaka, K.; Yoneda, N.; Yamashita, T.; Kaneko, S.; et al. Hepatocellular Carcinoma: Signal Intensity at Gadoxetic Acid–enhanced MR Imaging—Correlation with Molecular Transporters and Histopathologic Features. Radiology 2010, 256, 817–826. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Lao, I.-H.; Hsieh, P.-L.; Su, Y.-Y.; Mak, C.-W.; Sun, D.-P.; Sheu, M.-J.; Kuo, H.-T.; Chen, T.-J.; Ho, C.-H.; et al. Gadoxetic acid-enhanced magnetic resonance imaging can predict the pathologic stage of solitary hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2636–2649. [Google Scholar] [CrossRef]
- Colen, R.R.; Wang, J.; Singh, S.K.; Gutman, D.A.; Zinn, P.O. Glioblastoma: Imaging Genomic Mapping Reveals Sex-specific Oncogenic Associations of Cell Death. Radiology 2015, 275, 215–227. [Google Scholar] [CrossRef]
- Kele, P.G.; van der Jagt, E.J. Diffusion weighted imaging in the liver. World J. Gastroenterol. 2010, 16, 1567–1576. [Google Scholar] [CrossRef]
- Saito, K.; Kotake, F.; Ito, N.; Ozuki, T.; Mikami, R.; Abe, K.; Shimazaki, Y. Gd-EOB-DTPA Enhanced MRI for Hepatocellular Carcinoma: Quantitative Evaluation of Tumor Enhancement in Hepatobiliary Phase. Magn. Reson. Med. Sci. 2005, 4, 1–9. [Google Scholar] [CrossRef]
- Park, I.K.; Yu, J.-S.; Cho, E.-S.; Kim, J.H.; Chung, J.-J. Apparent diffusion coefficient of hepatocellular carcinoma on diffusion-weighted imaging: Histopathologic tumor grade versus arterial vascularity during dynamic magnetic resonance imaging. PLoS ONE 2018, 13, e0197070. [Google Scholar] [CrossRef]
- Surov, A.; Pech, M.; Omari, J.; Fischbach, F.; Damm, R.; Fischbach, K.; Powerski, M.; Relja, B.; Wienke, A. Diffusion-Weighted Imaging Reflects Tumor Grading and Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2021, 10, 10–24. [Google Scholar] [CrossRef]
- Li, J.; Xue, F.; Xu, X.; Wang, Q.; Zhang, X. Dynamic contrast-enhanced MRI differentiates hepatocellular carcinoma from hepatic metastasis of rectal cancer by extracting pharmacokinetic parameters and radiomic features. Exp. Ther. Med. 2020, 20, 3643–3652. [Google Scholar] [CrossRef]
- Chen, J.; Si, Y.; Zhao, K.; Shi, X.; Bi, W.; Liu, S.-E.; Hua, H. Evaluation of quantitative parameters of dynamic contrast-enhanced magnetic resonance imaging in qualitative diagnosis of hepatic masses. BMC Med. Imaging 2018, 18, 56. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Jiang, B.; Zhao, K.; Zhang, Y.; Xie, X. Clinical application of deep learning and radiomics in hepatic disease imaging: A systematic scoping review. Br. J. Radiol. 2022, 95, 20211136. [Google Scholar] [CrossRef]
- Ren, S.; Qi, Q.; Liu, S.; Duan, S.; Mao, B.; Chang, Z.; Zhang, Y.; Wang, S.; Zhang, L. Preoperative prediction of pathological grading of hepatocellular carcinoma using machine learning-based ultrasomics: A multicenter study. Eur. J. Radiol. 2021, 143, 109891. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, T.; Xu, L.; Zhao, L.; Liu, H.; Gu, L.R.; Wang, D.Z.; Zhang, M. Radiomics Analysis of Contrast-Enhanced CT for Hepatocellular Carcinoma Grading. Front. Oncol. 2021, 11, 660509. [Google Scholar] [CrossRef]
- Wu, M.; Tan, H.; Gao, F.; Hai, J.; Ning, P.; Chen, J.; Zhu, S.; Wang, M.; Dou, S.; Shi, D. Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur. Radiol. 2019, 29, 2802–2811. [Google Scholar] [CrossRef]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Grady, L. Random Walks for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2006, 28, 1768–1783. [Google Scholar] [CrossRef] [Green Version]
- Ameli, S.; Shaghaghi, M.; Ghasabeh, M.A.; Pandey, P.; Hazhirkarzar, B.; Ghadimi, M.; Habibabadi, R.R.; Khoshpouri, P.; Pandey, A.; Anders, R.A.; et al. Role of baseline volumetric functional MRI in predicting histopathologic grade and patients’ survival in hepatocellular carcinoma. Eur. Radiol. 2020, 30, 3748–3758. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Chen, X.; Minn, A.J. Random survival forests for high-dimensional data. Stat. Anal. Data Min. ASA Data Sci. J. 2011, 4, 115–132. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Long-Term Survival and Pattern of Recurrence After Resection of Small Hepatocellular Carcinoma in Patients with Preserved Liver Function: Implications for a strategy of salvage transplantation. Ann. Surg. 2002, 235, 373–382. [Google Scholar] [CrossRef]
- Shi, M.; Guo, R.; Lin, X.-J.; Zhang, Y.-Q.; Chen, M.-S.; Zhang, C.-Q.; Lau, W.Y.; Li, J.-Q. Partial Hepatectomy With Wide Versus Narrow Resection Margin for Solitary Hepatocellular Carcinoma: A prospective randomized trial. Ann. Surg. 2007, 245, 36–43. [Google Scholar] [CrossRef]
- Huang, K.; Dong, Z.; Cai, H.; Huang, M.; Peng, Z.; Xu, L.; Jia, Y.; Song, C.; Li, Z.-P.; Feng, S.-T. Imaging biomarkers for well and moderate hepatocellular carcinoma: Preoperative magnetic resonance image and histopathological correlation. BMC Cancer 2019, 19, 364. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, G.; Xie, G.; Zhang, L. Grading of hepatocellular carcinoma based on diffusion weighted images with multiple b-values using convolutional neural networks. Med. Phys. 2019, 46, 3951–3960. [Google Scholar] [CrossRef]
- Defour, L.B.; Mulé, S.; Tenenhaus, A.; Piardi, T.; Sommacale, D.; Hoeffel, C.; Thiéfin, G. Hepatocellular carcinoma: CT texture analysis as a predictor of survival after surgical resection. Eur. Radiol. 2019, 29, 1231–1239. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Liu, Z.; Liang, C. Texture analysis of baseline multiphasic hepatic computed tomography images for the prognosis of single hepatocellular carcinoma after hepatectomy: A retrospective pilot study. Eur. J. Radiol. 2017, 90, 198–204. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Wang, K.; Chen, S.; Wang, G.; Liu, Z.; Liang, C. Malignancy characterization of hepatocellular carcinomas based on texture analysis of contrast-enhanced MR images. J. Magn. Reson. Imaging 2017, 45, 1476–1484. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.M.; Park, J.; Joo, I.; Yoon, J.H.; Lee, D.H.; Ganeshan, B.; Han, J.K. Hepatocellular Carcinoma: Texture Analysis of Preoperative Computed Tomography Images Can Provide Markers of Tumor Grade and Disease-Free Survival. Korean J. Radiol. 2019, 20, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Xu, L.; Wen, X.; Zhang, J.; Yang, P.; Zhang, L.; Xue, X.; Wang, X.; Huang, Q.; Guo, C.; et al. A nomogram based on bi-regional radiomics features from multimodal magnetic resonance imaging for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Quant. Imaging Med. Surg. 2019, 9, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Hectors, S.J.; Lewis, S.; Besa, C.; King, M.J.; Said, D.; Putra, J.; Ward, S.; Higashi, T.; Thung, S.; Yao, S.; et al. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur. Radiol. 2020, 30, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-J.; Byun, J.H.; Kim, J.Y.; Hong, G.-S.; Won, H.J.; Shin, Y.M.; Kim, P.N. Differentiation of small (≤2 cm) hepatocellular carcinomas from small benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted magnetic resonance images. Abdom. Imaging 2015, 40, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Chen, R.-C.; Chou, C.-T.; Lin, C.-Y.; Yu, C.-Y.; Liu, C.-H.; Chou, J.-M.; Hsu, H.-H.; Huang, G.-S. Histological grade of hepatocellular carcinoma correlates with arterial enhancement on gadoxetic acid-enhanced and diffusion-weighted MR images. Gastrointest. Radiol. 2014, 39, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kim, M.-J.; Park, Y.N.; Lee, J.M.; Yoo, S.K.; Rha, S.Y.; Seok, J.Y. Gadoxetate Disodium–Enhanced Hepatobiliary Phase MRI of Hepatocellular Carcinoma: Correlation With Histological Characteristics. Am. J. Roentgenol. 2011, 197, 399–405. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, H.; Chen, J.; Wei, Y.; Cao, L.; Ye, Z.; Li, X.; Ma, L.; Song, B. Hepatocellular carcinoma: Radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging 2019, 19, 22. [Google Scholar] [CrossRef]
- Kim, S.; Shin, J.; Kim, D.-Y.; Choi, G.H.; Kim, M.-J.; Choi, J.-Y. Radiomics on Gadoxetic Acid–Enhanced Magnetic Resonance Imaging for Prediction of Postoperative Early and Late Recurrence of Single Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 3847–3855. [Google Scholar] [CrossRef]
- Corona-Villalobos, C.P.; Halappa, V.G.; Bonekamp, S.; Eng, J.; Reyes, D.; Cosgrove, D.; Rastegar, N.; Pan, L.; Pawlik, T.M.; Kamel, I.R. Functional Magnetic Resonance Imaging Response of Targeted Tumor Burden and Its Impact on Survival in Patients with Hepatocellular Carcinoma. Investig. Radiol. 2015, 50, 283–289. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, P.; Ghasabeh, M.A.; Zarghampour, M.; Khoshpouri, P.; Ameli, S.; Luo, Y.; Kamel, I.R. Baseline Volumetric Multiparametric MRI: Can It Be Used to Predict Survival in Patients with Unresectable Intrahepatic Cholangiocarcinoma Undergoing Transcatheter Arterial Chemoembolization? Radiology 2018, 289, 843–853. [Google Scholar] [CrossRef]
- Zarghampour, M.; Fouladi, D.F.; Pandey, A.; Ghasabeh, M.A.; Pandey, P.; Varzaneh, F.N.; Khoshpouri, P.; Shao, N.; Pan, L.; Grimm, R.; et al. Utility of volumetric contrast-enhanced and diffusion-weighted MRI in differentiating between common primary hypervascular liver tumors. J. Magn. Reson. Imaging 2018, 48, 1080–1090. [Google Scholar] [CrossRef]
| Parameters | T1WI | T2WI | DWI | DKI | T2*WI |
|---|---|---|---|---|---|
| Sequence | FSE | FSE | SS-EPI | SS-EPI | Multiecho GRE |
| Orientation | Oblique axial | Oblique axial, sagittal and coronal | Oblique axial | Oblique axial | Oblique axial |
| Repetition time (msec) | 500 | 5629 | 4000 | 3000 | 100 |
| Echo time (msec) | 75 | 85 | 75 | 75 | 2.7, 6.8, 10.9, 15.1, 19.2, 23.3, 27.4, 31.5, 35.6, 39.7, 43.8, 48.0, 52.1 |
| FOV (mm2) | 380 × 380 | 200 × 200 | 400 × 400 | 360 × 252 | 240 × 192 |
| Matrix (mm2) | 320 × 224 | 448 × 314 | 160 × 128 | 128 × 128 | 192 × 160 |
| Slice Thickness (mm) | 5 | 3 | 3 | 3 | 3 |
| Slice Gap (mm) | 1 | 0 | 0 | 0 | 0 |
| NEX | 2 | 4 | 12 | 2 | 1 |
| b-value (s/mm2) | N/A | N/A | 0, 800 | 0, 1000, 2000 | N/A |
| Bandwidth (kHz) | 62.50 | 31.3 | 250 | 250 | 31.3 |
| Scan time | 1 min 44 s | 4 min 4 s | 2 min 32 s | 5 min 9 s | 1 min 22 s |
| Texture Feature Category | Extracted Features in Each Category |
|---|---|
| Global Features | Mean, Maximum, and Minimum (for both ADC and VE), Tumor Solidity, Surface Area, and Volume |
| Histogram-based Features | Variance, Skewness, and Kurtosis |
| Gray Level Co-occurrence Matrix Features (GLCM) * | Contrast, Correlation, Energy, Variance, Sum average, Dissimilarity, Autocorrelation, Entropy, and Homogeneity |
| Gray Level Run Length Matrix Features (GLRLM) ** | Short-run emphasis (SRE), Long-run Emphasis (LRE), Gray-level non-uniformity (GLN), Run-length non-uniformity (RLN), Run Percentage (RP), Low Gray-level Run Emphasis (LGRE), High Gray-level Run Emphasis (HGRE), Short Run Low Gray-level Emphasis (SRLGE), Short Run High Gray-level Emphasis (SRHGE), Long Run Low Gray-level Emphasis (LRLGE), Long Run High Gray-level Emphasis (LRHGE), Gray-level Variance (GLV), and Run Length Variance (RLV) |
| Gray Level Size Zone Matrix Features (GLSZM) *** | Small Zone Emphasis (SZE), Large Zone Emphasis (LZE), Gray-level non-uniformity (GLN), Zone Size non-uniformity (ZSN), Zone percentage (ZP), Low Gray-level Zone Emphasis (LGZE), High Gray-level Zone Emphasis (HGZE), Small Zone Low Gray-level Emphasis (SZHGE), Large Zone Low Gray-level Emphasis (LZLGE), Large Zone High Gray-level Emphasis (LZHGE), Gray-level Variance (GLV), and Zone Size Variance (RLV) |
| Neighborhood Gray-tone Difference Matrix (NGTDM) **** | Mean, Variance, Kurtosis, Strength, and Skewness |
| * GLCM elements in row (i) and column (j) represent the frequency in which a given gray level of value (i) is horizontally adjacent to gray-level (j). For the purposes of this study, these calculations were performed in vertical, horizontal, 45°, and 135° directions, which were then averaged together to minimize directional dependence in the samples. ** Rows (i) represent the gray-levels while the columns (j) represent the run-length, or the consecutive number of pixels with a particular gray-level. Elements within the matrix represent the frequency of pixel line segments with a run-length (j) and a gray-level (i). *** Rows (i) represent the gray-levels while the columns (j) represent the 3D zone-size, or the consecutive number of 3D zones with a particular gray-level. Elements within the matrix represent the frequency of zones with a zone-size (j) and a gray-level (i). **** these features provide a histogram of the absolute gradient values in the tissue segment. In this analysis, differences in all pixel values within a tumor segment were analyzed using a 3 × 3 neighborhood |
| Parameter | Total | Degree of Differentiation | p Value Δ | |||
|---|---|---|---|---|---|---|
| Well | Moderate | Poor | ||||
| Age *, years | 62 (57–68) | 61 (57–69) | 62 (58–66) | 63 (57–73) | 0.68 | |
| Sex, n (%) | Male | 102 (79%) | 28 (21.7%) | 60 (46.5%) | 14 (10.8%) | 0.35 |
| Female | 27 (20.9%) | 11 (8.5%) | 12 (9.3%) | 4 (3.1%) | ||
| Race, n (%) | White | 72 (55.8%) | 18 (13.9%) | 40 (31%) | 14 (10.8%) | 0.40 |
| Black | 35 (27.1%) | 12 (9.3%) | 20 (15.5%) | 3 (2.3%) | ||
| Asian | 9 (6.9%) | 4 (3.1%) | 4 (3.1%) | 1 (0.70%) | ||
| Other | 13 (10%) | 5 (3.8%) | 8 (6.2%) | 0 (0%) | ||
| Child-Pugh score, n (%) | Child A | 91 (70.5%) | 31 (24%) | 45 (34.8%) | 15 (11.6%) | 0.12 |
| Child B | 33 (25.5%) | 8 (6.2%) | 22 (17%) | 3 (2.3%) | ||
| Child C | 5 (3.8%) | 0 (0%) | 5 (3.8%) | 0 (0%) | ||
| Patients’ outcome, n (%) | Alive | 71 (55%) | 25 (19.3%) | 39 (30.2%) | 7 (5.4%) | 0.12 |
| Died | 52 (40.3%) | 12 (9.3%) | 29 (22.4%) | 11 (8.5%) | ||
| Lobe, n (%) ¥ | Left | 41 (31.7%) | 16 (12.4%) | 19 (14.7%) | 6 (4.6%) | 0.26 |
| Right | 84 (65.1%) | 23 (17.8%) | 49 (37.9%) | 12 (9.3%) | ||
| Both lobes | 4 (3.1%) | 0 (0%) | 4 (3.1%) | 0 (0%) | ||
| AFP *, ng/mL | 22.5 (6.8–171.93) | 9.2 (4–94) | 22 (7.8–125) | 256.3 (36.3–9621) | 0.003 | |
| AFP: alpha fetoprotein * All continuous variables are presented by their median and (interquartile ranges) ¥ Total number of lesions is more than the number of patients. More than 1 lesion was identified in 35 patients. Δ p Values of Kruskal–Wallis test reported for continuous variables (age, AFP), and P values of chi square test reported for categorical parameters (sex, race, Child–Pugh score, patients’ outcome, lobe) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameli, S.; Venkatesh, B.A.; Shaghaghi, M.; Ghadimi, M.; Hazhirkarzar, B.; Rezvani Habibabadi, R.; Aliyari Ghasabeh, M.; Khoshpouri, P.; Pandey, A.; Pandey, P.; et al. Role of MRI-Derived Radiomics Features in Determining Degree of Tumor Differentiation of Hepatocellular Carcinoma. Diagnostics 2022, 12, 2386. https://doi.org/10.3390/diagnostics12102386
Ameli S, Venkatesh BA, Shaghaghi M, Ghadimi M, Hazhirkarzar B, Rezvani Habibabadi R, Aliyari Ghasabeh M, Khoshpouri P, Pandey A, Pandey P, et al. Role of MRI-Derived Radiomics Features in Determining Degree of Tumor Differentiation of Hepatocellular Carcinoma. Diagnostics. 2022; 12(10):2386. https://doi.org/10.3390/diagnostics12102386
Chicago/Turabian StyleAmeli, Sanaz, Bharath Ambale Venkatesh, Mohammadreza Shaghaghi, Maryam Ghadimi, Bita Hazhirkarzar, Roya Rezvani Habibabadi, Mounes Aliyari Ghasabeh, Pegah Khoshpouri, Ankur Pandey, Pallavi Pandey, and et al. 2022. "Role of MRI-Derived Radiomics Features in Determining Degree of Tumor Differentiation of Hepatocellular Carcinoma" Diagnostics 12, no. 10: 2386. https://doi.org/10.3390/diagnostics12102386
APA StyleAmeli, S., Venkatesh, B. A., Shaghaghi, M., Ghadimi, M., Hazhirkarzar, B., Rezvani Habibabadi, R., Aliyari Ghasabeh, M., Khoshpouri, P., Pandey, A., Pandey, P., Pan, L., Grimm, R., & Kamel, I. R. (2022). Role of MRI-Derived Radiomics Features in Determining Degree of Tumor Differentiation of Hepatocellular Carcinoma. Diagnostics, 12(10), 2386. https://doi.org/10.3390/diagnostics12102386





