Abstract
Angiomatoid fibrous histiocytoma (AFH) is a rare neoplasm described for the first time by Enzinger in 1979, and classified by World Health Organization 2020 as intermediate malignant potential neoplasm. It mostly occurs in the subcutis and is characterized by varying proportions of epithelioid, ovoid and spindle cells in a nodular and syncytial growth pattern, with some hemorrhagic pseudovascular spaces. In this paper, we report the clinical case of a 62-year-old man who presented with AFH on the right arm, and relapsed three years after first surgical excision. After a further three years, the patient presented with an intramuscular localization of AFH, and 12 months after this, a pulmonary metastasis of AFH was diagnosed. Given the rarity of the spreading of AFH, we performed Fluorescence In Situ Hybridization (FISH) and we detected EWSR1::CREB1 gene fusion.
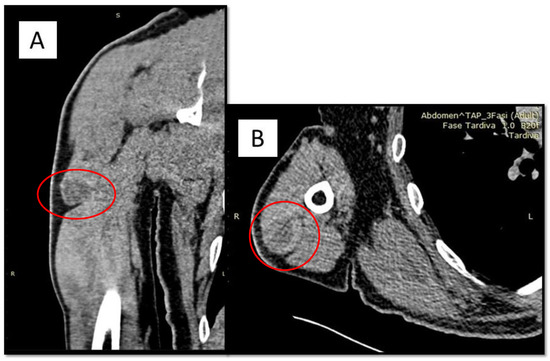
Figure 1.
(A,B) CT findings of oval formation (red circle), 36 × 27 mm of diameter, in the lateral belly of the triceps brachial, located in the proximal third of the muscle, capsulated and well-defined in the surrounding muscle, except for minimal irregularities of the antero-lateral side. A 62-year-old man presented himself to the referral doctor in 2018 for unspecified pain in his left arm. After an orthopedic specialist consultation, it was decided to perform a CT scan with and without contrast medium in an attempt to localize the lesion. The medical history was silent for other pathologies and/or neoplasms. Both total body CT and regional Magnetic Resonance highlighted the presence of an oval formation in the lateral belly of the brachial triceps, measuring 46 × 17 mm, with a mixed content including blood. The lesion seemed encapsulated, with some antero-external irregularities (Figure 1). At pre-operative angiography, intense peripheral vascularization was observed, without any apparent infiltration. Furthermore, PET scan showed areas of incremented radiopharmaceutical accumulation in the belly of the left brachial triceps muscle (SUV max 6.5). It was therefore decided to perform a first cytological aspiration with a fine needle, the results of which were constituted by blood material comprising numerous pigmented histiocyte/macrophage cells. Three months later, a biopsy was performed at the level of the lesion in question which, however, was not conclusive for any diagnosis. Following this report, it was decided to perform surgical removal of the formation and the finding was evaluated by the histopathologist.
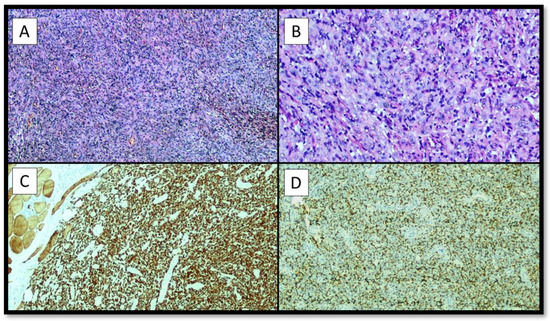
Figure 2.
The histopathological analysis allowed to appreciate a lesion characterized by a peripheral fibrous pseudocapsule, not continuous, and by a large central cystic-haemorrhagic portion, consisting of medium-sized cellular elements (A), partly epitheliomorphs, sometimes with clarified cytoplasm (B), and in some areas spindle, mostly monomorphic, with evident eosinophilic nucleoli, with solid growth (B). A rich inflammatory infiltrate was described, also consisting of foamy histiocytes and hemosiderophages. The mitotic count was 2/10 high power field (HPF) and there were no areas of necrosis. The immunohistochemical reactions were positive for Desmin with a score of +++ (C), CD68 PG-M1 positive with a score of ++/− (D). Reactions for Epithelial Membrane Antigen (EMA), S-100 protein, Miogenin and MyoD1 and CD34 were negative. The fraction of neoplastic proliferation evaluated by Ki67 + was low, around 5–6%. The diagnosis of Angiomatoid Fibrous Histiocytoma (AFH) was then made and the patient was closely followed-up. After less than 6 months, the patient was again subjected to a surgical resection, whose histological sample was analyzed and found to be an intramuscular localization of AFH with free margins. After about 12 months, the patient returned to the referring physician with progressive febrile episodes and asthenia, as well as mild breathing difficulties. A new CT was performed which showed a thickening of about 0.8 cm. An apical resection of the upper lobe of the right lung (LSD) was performed.
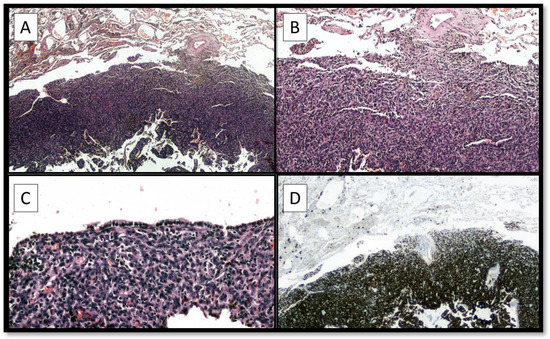
Figure 3.
(A,B) The histological analysis highlighted a lesion with morphological characteristics very similar to the previous AFH, although the organ location had changed. In (C) was possible to appreciate the respiratory mucosa that covers a part of AFH. Immunohistochemical investigations were again conducted which were positive for Desmin (D), CD68, with a Ki67 + of about 10–15%. A diagnosis of metastasis in the upper lobe of the right lung of AFH was made and the sample was sent for FISH investigation for diagnostic confirmation. Molecular analyzes revealed the presence of the EWSR1::CREB1 fusion gene. This feature was very important to confirm and start the therapeutic path [1,2,3]. Regarding the differential diagnosis, it is important to differentiate AFH from other types of dermatofibroma, or, in the case of prominent myxoid change, from other myxoid tumors such as low-grade fibromyxoid sarcoma, extraskeletal myxoid chondrosarcoma and myxoid liposarcoma [4]. The case presented is of particular interest for three reasons: (1) AFH metastasis rate described in the literature is around 1–5%, with about 15 cases of AFH lung involvement described [5,6]; (2) from the few published cases of metastatic AFH, it would seem that the presence of the EWSR1::CREB1 fusion gene predisposes more aggressive behavior of the neoplasm [7]; careful follow-up with the possibility of performing a FISH in cases of relapsed AFH is mandatory [1,2,8,9,10,11,12].
Author Contributions
Conceptualization, G.C. and F.R.; methodology, A.C.; software, G.C.; validation, G.C., E.M. and L.R.; formal analysis, G.C., C.L. and E.C.; investigation, G.C., A.C. and F.R.; resources, N.C.; data curation, G.C. and G.I.; writing—original draft preparation, G.C., F.R., A.C. and G.I.; writing—review and editing, G.C., B.M., V.P. and L.R.; visualization, S.M.R.T.; supervision, A.M. and L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from subject involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
In memory of Antonietta Cimmino (A.C.)
Conflicts of Interest
The authors declare no conflict of interest.
References
- Racanelli, D.; Brenca, M.; Baldazzi, D.; Goeman, F.; Casini, B.; De Angelis, B.; Guercio, M.; Milano, G.M.; Tamborini, E.; Busico, A.; et al. Next-Generation Sequencing Approaches for the Identification of Pathognomonic Fusion Transcripts in Sarcomas: The Experience of the Italian ACC Sarcoma Working Group. Front. Oncol. 2020, 10, 489, Erratum in: Front Oncol. 2020, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Fisher, C. Angiomatoid fibrous histiocytoma: The current status of pathology and genetics. Arch. Pathol. Lab. Med. 2015, 139, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Update on the role of trabectedin in the treatment of intractable soft tissue sarcomas. Onco Targets Ther. 2017, 10, 1155–1164. [CrossRef] [PubMed]
- Schaefer, I.M.; Fletcher, C.D. Myxoid variant of so-called angiomatoid “malignant fibrous histiocytoma”: Clinicopathologic characterization in a series of 21 cases. Am. J. Surg. Pathol. 2014, 38, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Maher, O.M.; Prieto, V.G.; Stewart, J.; Herzog, C.E. Characterization of metastatic angiomatoid fibrous histiocytoma. J. Pediatr. Hematol. Oncol. 2015, 37, e268–e271. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Kobayashi, E.; Yoshida, A.; Araki, Y.; Kubota, D.; Tanzawa, Y.; Kawai, A.; Yanagawa, T.; Takagishi, K.; Chuman, H. Angiomatoid fibrous histiocytoma: A series of seven cases including genetically confirmed aggressive cases and a literature review. BMC Musculoskelet. Disord. 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Makise, N.; Shinozaki-Ushiku, A.; Ishibashi, Y.; Ikegami, M.; Kohsaka, S.; Ushiku, T.; Oda, K.; Miyagawa, K.; Aburatani, H.; et al. Scapular Angiomatoid Fibrous Histiocytoma with EWSR1-CREB1 Fusion in an Adult Patient. Case Rep. Orthop. 2021, 2021, 9434222. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Fenouil, T.; Pissaloux, D.; Ameli, R.; Ducray, F.; Meyronet, D.; Honnorat, J. Intracranial non-myxoid angiomatoid fibrous histiocytoma with EWSR1-CREB1 transcript fusion treated with doxorubicin: A case report. Mol. Clin. Oncol. 2021, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Alzahim, M.A.; Abed, A.H.; Mashrah, H.T.; Almahdaly, A.M.; Shaheen, M. Angiomatoid Fibrous Histiocytoma: A Series of Three Cases. Cureus 2021, 13, e16465. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, H.; Bashir, S.; Hassan, U.; Hussain, M.; Mushtaq, S.; Ishtiaq, S. Angiomatoid Fibrous Histiocytoma: A Tumor With Uncertain Behavior and Various Clinicopathological Presentations. Cureus 2022, 14, e28985. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Grinda, T.; Boilève, A.; Levy, A.; Le Pechoux, C.; Haddag, L.; Valent, A.; Lazure, T.; Briand, S.; Honoré, C.; et al. Durable response to crizotinib in metastatic angiomatoid fibrous histiocytoma with EWSR1-CREB1 fusion and ALK overexpression. Ann. Oncol. 2022, 33, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Molecular diagnosis of an atypical case of angiomatoid fibrous histiocytoma based on detection of the EWSR1 gene translocation. J. Dermatol. 2021, 48, e215–e216. [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).