Are Urologists Ready for Interpretation of Multiparametric MRI Findings? A Prospective Multicentric Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of the Test Cases
- Case 1: 59 years old, PSA 5.5 ng/mL, no previous biopsies, PIRADS 4, posterior—apical lesion.
- Case 2: 80 years old, PSA 7.2 ng/mL, no previous biopsies, PIRADS 4, posterior—median lesion.
- Case 3: 74 years old, PSA 5.4 ng/mL, no previous biopsies, PIRADS 4, anterior—median lesion.
- Case 4: 77 years old, PSA 5.7 ng/mL, previous negative biopsy, PIRADS 4, anterior—median lesion.
- Case 5: 73 years old, PSA 5.5 ng/mL, no previous biopsies, PIRADS 5, posterior—apical lesion.
- Case 6: 59 years old, PSA 5.2 ng/mL, previous negative biopsy, PIRADS 4, anterior—apical lesion.
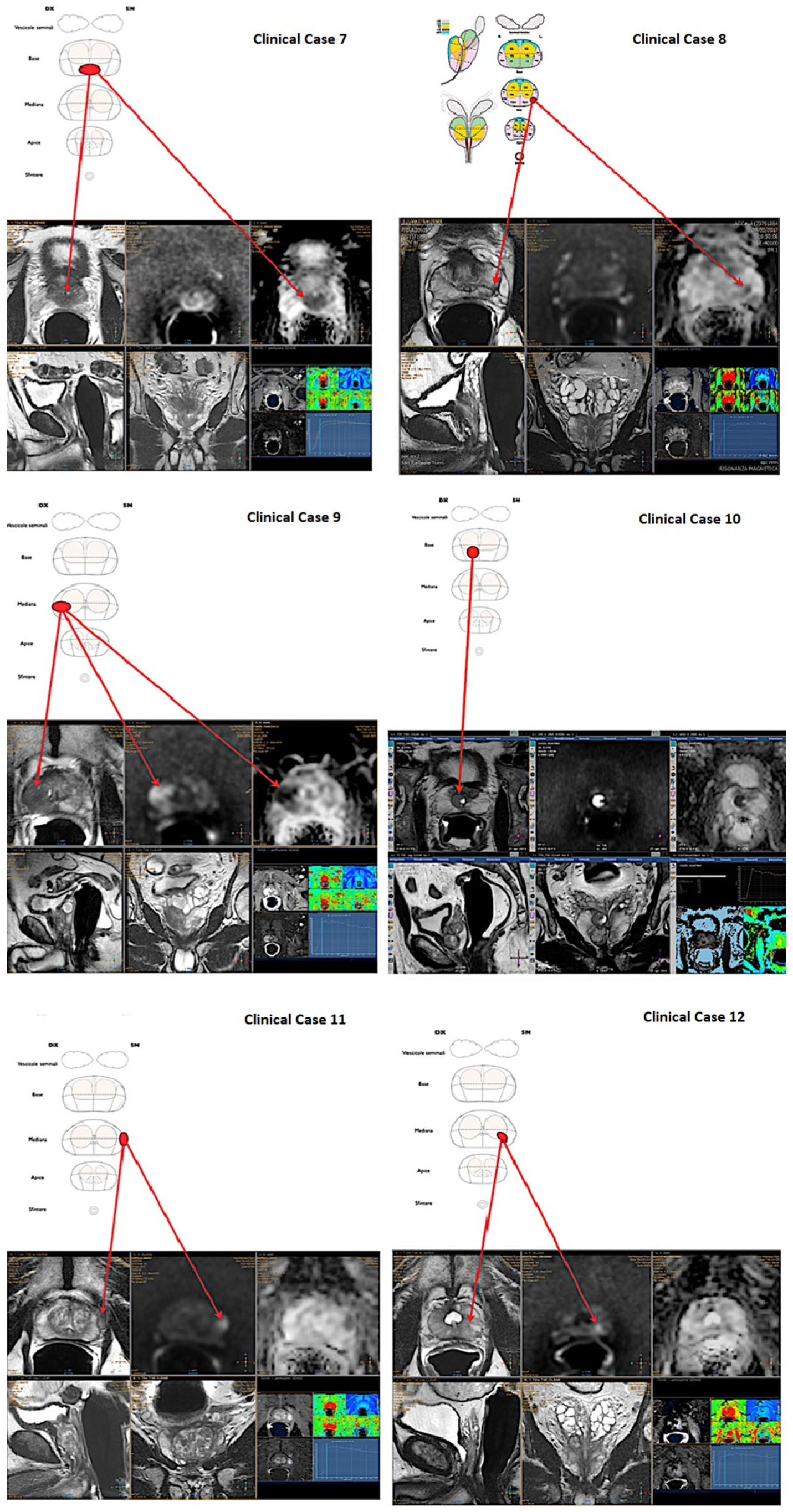
- Case 7: 73 years old, PSA 6.5 ng/mL, previous negative biopsy, PIRADS 5, posterior—basal lesion.
- Case 8: 73 years old, PSA 4.5 ng/mL, previous negative biopsy, PIRADS 4, posterior—median lesion.
- Case 9: 75 years old, PSA 7.6 ng/mL, no previous biopsies, PIRADS 5, posterior—median lesion.
- Case 10: 67 years old, PSA 7.9 ng/mL, no previous biopsies, PIRADS 3, posterior—basal lesion.
- Case 11: 69 years old, PSA 7.8 ng/mL, no previous biopsies, PIRADS 3, anterior—median lesion.
- Case 12: 67 years old, PSA 5.4 ng/mL, no previous biopsies, PIRADS 4, posterior—median lesion.
2.2. Test Administration
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S. Newer imaging modalities in urology. J. Urol. 2015, 31, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Van den Bergh, R.C.N.; Briers, E.; Van der Broek, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU guidelines on Prostate Cancer. 2019. Available online: www.uroweb.org (accessed on 17 September 2022).
- Yoshida, K.; Takahashi, N.; King, B.F.; Kawashima, A.; Harris, P.C.; Cornell, L.D.; Gall, E.C.-L.; Inoue, D.; Mizushima, I.; Kawano, M.; et al. Multiple unilateral subcapsular cortical hemorrhagic cystic disease of the kidney: CT and MRI findings and clinical characteristic. Eur. Radiol. 2019, 29, 4843–4850. [Google Scholar] [CrossRef]
- Engels, R.R.M.; Israël, B.; Padhani, A.R.; Barentsz, J.O. Multiparametric Magnetic Resonance Imaging for the Detection of Clinically Significant Prostate Cancer: What Urologists Need to Know. Part 1: Acquisition. Eur. Urol. 2019, 77, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Israël, B.; van der Leest, M.; Sedelaar, M.; Padhani, A.; Zámecnik, P.; Barentsz, J.O. Multiparametric Magnetic Resonance Imaging for the Detection of Clinically Significant Prostate Cancer: What Urologists Need to Know. Part 2: Interpretation. Eur. Urol. 2019, 77, 469–480. [Google Scholar] [CrossRef]
- Venderink, W.; Bomers, J.G.; Overduin, C.; Padhani, A.; de Lauw, G.R.; Sedelaar, M.J.; Barentsz, J.O. Multiparametric Magnetic Resonance Imaging for the Detection of Clinically Significant Prostate Cancer: What Urologists Need to Know. Part 3: Targeted Biopsy. Eur. Urol. 2019, 77, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Margolis, D.J.A.; McClure, T.D.; Thomas, A.; Finley, D.S.; Reiter, R.E.; Huang, J.; Raman, S.S. Radical prostatectomy: Value of prostate MRI in surgical planning. Abdom. Imaging 2012, 37, 664–674. [Google Scholar] [CrossRef]
- Glass, A.S.; Dall’Era, M.A. Use of Multi-Parametric Magnetic Resonance Imaging in Prostate Cancer Active Surveillance. BJU Int. 2019, 124, 730–737. [Google Scholar] [CrossRef]
- Epstein, N.; Hollingsworth, R.; Silvergleid, R. Spinal surgeons need to read patients’ studies to avoid missing pathology. Surg. Neurol. Int. 2015, 6 (Suppl. S10), S313–S317. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Begovic, J.; Pires, A.; Won, E.; Taneja, S.S.; Babb, J.S. Online Interactive Case-Based Instruction in Prostate Magnetic Resonance Imaging Interpretation Using Prostate Imaging and Reporting Data System Version 2: Effect for Novice Readers. Curr. Probl. Diagn. Radiol. 2019, 48, 132–141. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Ambrosi, A.; Giganti, F.; Chau, E.; Kirkham, A.; Punwani, S.; Allen, C.; Emberton, M.; Moore, C.M. A Dedicated Prostate MRI Teaching Course Improves the Ability of the Urologist to Interpret Clinically Significant Prostate Cancer on Multiparametric MRI. Eur. Urol. 2019, 75, 203–204. [Google Scholar] [CrossRef]
- Christidis, D.; McGrath, S.; Leaney, B.; O’Sullivan, R.; Lawrentschuk, N. Interpreting Prostate Multiparametric Magnetic Resonance Imaging: Urologists’ Guide Including Prostate Imaging Reporting and Data System. Urology 2018, 111, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Fan, R.E.; Ghanouni, P.; Sonn, G.A. Teaching Urologists “How to Read Multi-Parametric Prostate MRIs Using PIRADSv2”: Results of an iBook Pilot Study. Urology 2019, 131, 40–45. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Halstuch, D.; Baniel, J.; Lifshitz, D.; Sela, S.; Ber, Y.; Margel, D. Characterizing the learning curve of MRI-US fusion prostate biopsies. Prostate Cancer Prostatic Dis. 2019, 22, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; Pecoraro, M.; Stavrinides, V.; Stabile, A.; Cipollari, S.; Sciarra, A.; Kirkham, A.; Allen, C.; Punwani, S.; Emberton, M.; et al. Interobserver reproducibility of the PRECISE scoring system for prostate MRI on active surveillance: Results from a two-centre pilot study. Eur. Radiol. 2020, 30, 2082–2090. [Google Scholar] [CrossRef]
- Kohestani, K.; Wallström, J.; Dehlfors, N.; Sponga, O.M.; Månsson, M.; Josefsson, A.; Carlsson, S.; Hellström, M.; Hugosson, J. Performance and inter-observer variability of prostate MRI (PI-RADS version 2) outside high-volume centres. Scand. J. Urol. 2019, 53, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N. Interobserver Agreement of PI-RADS v. 2: Not All Features or Observers Are Created Equal. J. Magn. Reason. Imaging 2020, 51, 605–606. [Google Scholar] [CrossRef]
- Mussi, T.C.; Yamauchi, F.I.; Tridente, C.F.; Tachibana, A.; Tonso, V.M.; Recchimuzzi, D.Z.; Leão, L.R.; Luz, D.C.; Martins, T.; Baroni, R.H. Interobserver agreement of PI-RADS v. 2 lexicon among radiologists with different levels of experience. J. Magn. Reason. Imaging 2020, 51, 593–602. [Google Scholar] [CrossRef]
- Brembilla, G.; Takwoingi, Y.; Kasivisvanathan, V. Tackling Interobserver Variability in Multiparametric Magnetic Resonance Imaging (MRI): Is MRI Even Better than We Think for Prostate Cancer Diagnosis? Eur. Urol. 2021, 79, 8–10. [Google Scholar] [CrossRef]
- Giganti, F.; Dinneen, E.; Kasivisvanathan, V.; Haider, A.; Freeman, A.; Kirkham, A.; Punwani, S.; Emberton, M.; Shaw, G.; Moore, C.M.; et al. Inter-reader agreement of the PI-QUAL score for prostate MRI quality in the NeuroSAFE PROOF trial. Eur. Radiol. 2022, 32, 879–889. [Google Scholar] [CrossRef]
- Wei, C.-G.; Zhang, Y.-Y.; Pan, P.; Chen, T.; Yu, H.-C.; Dai, G.-C.; Tu, J.; Yang, S.; Zhao, W.-L.; Shen, J.-K. Diagnostic Accuracy and Interobserver Agreement of PI-RADS Version 2 and Version 2.1 for the Detection of Transition Zone Prostate Cancers. AJR Am. J. Roentgenol. 2021, 216, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Bhayana, R.; O’Shea, A.; Anderson, M.A.; Bradley, W.R.; Gottumukkala, R.V.; Mojtahed, A.; Pierce, T.T.; Harisinghani, M. PI-RADS Versions 2 and 2.1: Interobserver Agreement and Diagnostic Performance in Peripheral and Transition Zone Lesions Among Six Radiologists. AJR Am. J. Roentgenol. 2021, 217, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Giannini, V.; Mazzetti, S.; Cappello, G.; Doronzio, V.; Vassallo, L.; Russo, F.; Giacobbe, A.; Muto, G.; Regge, D. Computer-Aided Diagnosis Improves the Detection of Clinically Significant Prostate Cancer on Multiparametric-MRI: A Multi-Observer Performance Study Involving Inexperienced Readers. Diagnostics 2021, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Akin, O.; Riedl, C.C.; Ishill, N.M.; Moskowitz, C.S.; Zhang, J.; Hricak, H. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur. Radiol. 2010, 20, 995–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]

| (A) Comparison According to Hierarchy | (B) Comparison According to Experience in fPB | (C) Comparison According to Proficiency in mpMRI Reading (≥75% of Correct Identifications) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Residents, n = 36 (49%) 1 | Consultants, n = 37 (51%) 1 | p-Value 2 | Non- Experienced, n = 49 (67%) 1 | Experienced, n = 24 (33%) 1 | p-Value 2 | Non-Proficient, n = 39 (53%) 1 | Proficient, n = 34 (47%) 1 | p-Value 2 | |
| Age | 28 (27, 30) | 50 (39, 58) | <0.001 | 35 (29, 52) | 32 (29, 40) | 0.6 | 31 (28, 50) | 34 (29, 50) | 0.8 |
| Number of PCa diagnosed yearly (per center) | 300 (250, 500) | 300 (250, 500) | 0.5 | 300 (250, 500) | 300 (250, 500) | 0.5 | 500 (275, 500) | 250 (250, 500) | 0.021 |
| Number of RP performed yearly (per center) | 130 (100, 300) | 130 (100, 300) | 0.6 | 130 (100, 300) | 130 (120, 250) | 0.6 | 250 (125, 300) | 120 (100, 300) | 0.058 |
| Number of correct identifications | 8.0 (6.8, 9.0) | 8.0 (6.0, 10.0) | 0.6 | 8.0 (5.0, 9.0) | 9.0 (8.0, 10.0) | 0.004 | 8.0 (5.0, 9.0) | 9.0 (8.0, 10.0) | 0.004 |
| Percentage of correct identifications | 67 (56, 75) | 67 (50, 83) | 0.6 | 67 (42, 75) | 75 (67, 83) | 0.004 | 67 (42, 75) | 75 (67, 83) | 0.004 |
| Institution | <0.001 | 0.5 | 0.5 | ||||||
| University hospital | 34 (94%) | 20 (54%) | 35 (71%) | 19 (79%) | 35 (71%) | 19 (79%) | |||
| Non university hospital | 2 (5.6%) | 17 (46%) | 14 (29%) | 5 (21%) | 14 (29%) | 5 (21%) | |||
| Experience in Prostate Biopsy | 0.2 | 0.003 | 0.13 | ||||||
| No | 9 (25%) | 5 (14%) | 14 (29%) | 0 (0%) | 10 (26%) | 4 (12%) | |||
| Yes | 27 (75%) | 32 (86%) | 35 (71%) | 24 (100%) | 29 (74%) | 30 (88%) | |||
| Experience in Fusion Prostate Biopsy | >0.9 | - | 0.016 | ||||||
| No | 24 (67%) | 25 (68%) | - | - | 31 (79%) | 18 (53%) | |||
| Yes | 12 (33%) | 12 (32%) | - | - | 8 (21%) | 16 (47%) | |||
| Involvement in diagnosis and management of PCa | 0.4 | 0.2 | 0.027 | ||||||
| No | 4 (11%) | 2 (5.4%) | 6 (12%) | 0 (0%) | 6 (15%) | 0 (0%) | |||
| Yes | 32 (89%) | 35 (95%) | 43 (88%) | 24 (100%) | 33 (85%) | 34 (100%) | |||
| Prostate cancer multidisciplinary team present | 0.023 | 0.050 | 0.6 | ||||||
| No | 10 (28%) | 20 (54%) | 24 (49%) | 6 (25%) | 17 (44%) | 13 (38%) | |||
| Yes | 26 (72%) | 17 (46%) | 25 (51%) | 18 (75%) | 22 (56%) | 21 (62%) | |||
| Clinical case 1 | 0.2 | 0.027 | 0.007 | ||||||
| Incorrect | 9 (25%) | 5 (14%) | 13 (27%) | 1 (4.2%) | 12 (31%) | 2 (5.9%) | |||
| Correct | 27 (75%) | 32 (86%) | 36 (73%) | 23 (96%) | 27 (69%) | 32 (94%) | |||
| Clinical case 2 | 0.9 | 0.11 | 0.003 | ||||||
| Incorrect | 10 (28%) | 11 (30%) | 17 (35%) | 4 (17%) | 17 (44%) | 4 (12%) | |||
| Correct | 26 (72%) | 26 (70%) | 32 (65%) | 20 (83%) | 22 (56%) | 30 (88%) | |||
| Clinical case 3 | 0.6 | <0.001 | 0.017 | ||||||
| Incorrect | 21 (58%) | 24 (65%) | 38 (78%) | 7 (29%) | 29 (74%) | 16 (47%) | |||
| Correct | 15 (42%) | 13 (35%) | 11 (22%) | 17 (71%) | 10 (26%) | 18 (53%) | |||
| Clinical case 4 | >0.9 | 0.2 | <0.001 | ||||||
| Incorrect | 18 (50%) | 18 (49%) | 27 (55%) | 9 (38%) | 30 (77%) | 6 (18%) | |||
| Correct | 18 (50%) | 19 (51%) | 22 (45%) | 15 (62%) | 9 (23%) | 28 (82%) | |||
| Clinical case 5 | 0.4 | 0.12 | <0.001 | ||||||
| Incorrect | 6 (17%) | 9 (24%) | 13 (27%) | 2 (8.3%) | 14 (36%) | 1 (2.9%) | |||
| Correct | 30 (83%) | 28 (76%) | 36 (73%) | 22 (92%) | 25 (64%) | 33 (97%) | |||
| Clinical case 6 | 0.9 | <0.001 | 0.008 | ||||||
| Incorrect | 21 (58%) | 21 (57%) | 35 (71%) | 7 (29%) | 28 (72%) | 14 (41%) | |||
| Correct | 15 (42%) | 16 (43%) | 14 (29%) | 17 (71%) | 11 (28%) | 20 (59%) | |||
| Clinical case 7 | 0.4 | 0.4 | <0.001 | ||||||
| Incorrect | 13 (36%) | 10 (27%) | 17 (35%) | 6 (25%) | 20 (51%) | 3 (8.8%) | |||
| Correct | 23 (64%) | 27 (73%) | 32 (65%) | 18 (75%) | 19 (49%) | 31 (91%) | |||
| Clinical case 8 | 0.6 | 0.7 | 0.004 | ||||||
| Incorrect | 15 (42%) | 13 (35%) | 18 (37%) | 10 (42%) | 21 (54%) | 7 (21%) | |||
| Correct | 21 (58%) | 24 (65%) | 31 (63%) | 14 (58%) | 18 (46%) | 27 (79%) | |||
| Clinical case 9 | 0.4 | 0.13 | 0.001 | ||||||
| Incorrect | 7 (19%) | 10 (27%) | 14 (29%) | 3 (12%) | 15 (38%) | 2 (5.9%) | |||
| Correct | 29 (81%) | 27 (73%) | 35 (71%) | 21 (88%) | 24 (62%) | 32 (94%) | |||
| Clinical case 10 | 0.001 | >0.9 | <0.001 | ||||||
| Incorrect | 28 (78%) | 15 (41%) | 29 (59%) | 14 (58%) | 30 (77%) | 13 (38%) | |||
| Correct | 8 (22%) | 22 (59%) | 20 (41%) | 10 (42%) | 9 (23%) | 21 (62%) | |||
| Clinical case 11 | 0.6 | 0.2 | <0.001 | ||||||
| Incorrect | 11 (31%) | 9 (24%) | 16 (33%) | 4 (17%) | 19 (49%) | 1 (2.9%) | |||
| Correct | 25 (69%) | 28 (76%) | 33 (67%) | 20 (83%) | 20 (51%) | 33 (97%) | |||
| Clinical case 12 | 0.3 | 0.4 | <0.001 | ||||||
| Incorrect | 12 (33%) | 17 (46%) | 21 (43%) | 8 (33%) | 24 (62%) | 5 (15%) | |||
| Correct | 24 (67%) | 20 (54%) | 28 (57%) | 16 (67%) | 15 (38%) | 29 (85%) | |||
| OR 1 | 95% CI 1 | p-Value | |
|---|---|---|---|
| Role (ref. Resident) | 1.38 | 0.47, 4.20 | 0.5 |
| Institution (ref. University hospital) | 0.73 | 0.20, 2.50 | 0.6 |
| Experience in fPB (ref. No) | 3.40 | 1.24, 9.94 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantica, G.; Suardi, N.; Smelzo, S.; Esperto, F.; Chierigo, F.; Tappero, S.; Borghesi, M.; La Rocca, R.; Oderda, M.; Ennas, M.; et al. Are Urologists Ready for Interpretation of Multiparametric MRI Findings? A Prospective Multicentric Evaluation. Diagnostics 2022, 12, 2656. https://doi.org/10.3390/diagnostics12112656
Mantica G, Suardi N, Smelzo S, Esperto F, Chierigo F, Tappero S, Borghesi M, La Rocca R, Oderda M, Ennas M, et al. Are Urologists Ready for Interpretation of Multiparametric MRI Findings? A Prospective Multicentric Evaluation. Diagnostics. 2022; 12(11):2656. https://doi.org/10.3390/diagnostics12112656
Chicago/Turabian StyleMantica, Guglielmo, Nazareno Suardi, Salvatore Smelzo, Francesco Esperto, Francesco Chierigo, Stefano Tappero, Marco Borghesi, Roberto La Rocca, Marco Oderda, Marco Ennas, and et al. 2022. "Are Urologists Ready for Interpretation of Multiparametric MRI Findings? A Prospective Multicentric Evaluation" Diagnostics 12, no. 11: 2656. https://doi.org/10.3390/diagnostics12112656
APA StyleMantica, G., Suardi, N., Smelzo, S., Esperto, F., Chierigo, F., Tappero, S., Borghesi, M., La Rocca, R., Oderda, M., Ennas, M., Stabile, A., De Cobelli, F., Napolitano, L., Papalia, R., Gontero, P., Introini, C., Briganti, A., Scarpa, R. M., Mirone, V., ... Cardone, G. (2022). Are Urologists Ready for Interpretation of Multiparametric MRI Findings? A Prospective Multicentric Evaluation. Diagnostics, 12(11), 2656. https://doi.org/10.3390/diagnostics12112656










