Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Statistical Analysis
3. Results
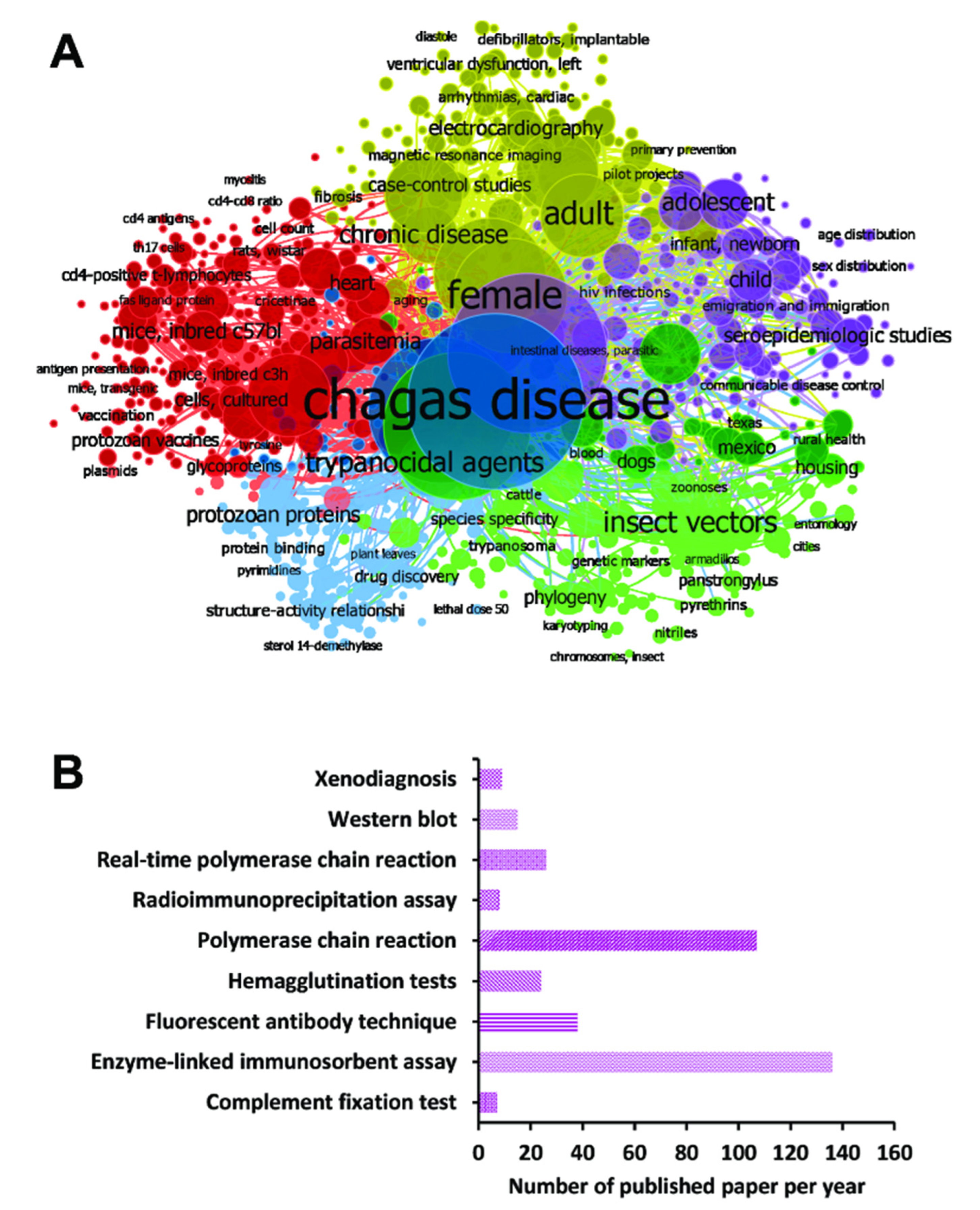
3.1. Search Results and Characteristics of the Selected Studies
3.2. Meta-Analysis of the Diagnostic Techniques for CD
3.2.1. Enzyme-Linked Immunosorbent Assay
3.2.2. Fluorescent Antibody
3.2.3. Hemagglutination Tests
3.2.4. Polymerase Chain Reaction
3.2.5. Real-Time Polymerase Chain Reaction
3.2.6. Other Techniques
3.2.7. Summary ROC Curves (sROC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| AUC | Area Under the Curve |
| AUCFPR | Area Under the Curve Restricted to The False Positive Rates |
| BNZ | Benznidazole |
| CD | Chagas Disease |
| CFT | Complement Fixation Test |
| DOR | Diagnostic Likelihood Ratio |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FDA | Food and Drug Administration |
| HmT | Hemagglutination Test |
| IFAT | Fluorescent Antibody Technique |
| INPLASY | International Platform of Registered Systematic Review and Meta-analysis Protocols |
| LR− | Negative Likelihood Ratio |
| LR+ | Positive Likelihood Ratio |
| MeSH | Medical Subject Headings |
| NCBI | National Center for Biotechnology Information |
| NFX | Nifurtimox |
| PAHO | Pan American Health Organization |
| PCR | Polymerase Chain Reaction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| qPCR | Real-Time Polymerase Chain Reaction |
| RIPA | Radioimmunoprecipitation Assay |
| ROC | Receiver Operating Characteristics |
| sROC | Summary Receiver Operating Characteristics |
| TPPs | Target Product Profiles |
| WB | Western Blot |
| WHO | World Health Organization |
References
- Kratz, J.M. Drug discovery for Chagas disease: A viewpoint. Acta Trop. 2019, 198, 105107. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Punukollu, G.; Gowda, R.M.; Khan, I.A.; Navarro, V.S.; Vasavada, B.C. Clinical aspects of the Chagas’ heart disease. Int. J. Cardiol. 2007, 115, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Martínez-Peinado, N.; Cortes-Serra, N.; Losada-Galvan, I.; Alonso-Vega, C.; Urbina, J.A.; Rodríguez, A.; VandeBerg, J.L.; Pinazo, M.-J.; Gascon, J.; Alonso-Padilla, J. Emerging agents for the treatment of Chagas disease: What is in the preclinical and clinical development pipeline? Expert Opin. Investig. Drugs 2020, 29, 947–959. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Pereiro, A.; Herazo, R.; Chopita, M.; Forsyth, C.; Lenardón, M.; Losada, I.; Torrico, F.; Marchiol, A.; Vera, M. Interventions to bring comprehensive care to people with Chagas disease: Experiences in Bolivia, Argentina and Colombia. Acta Trop. 2020, 203, 105290. [Google Scholar] [CrossRef]
- Irish, A.; Whitman, J.D.; Clark, E.H.; Marcus, R.; Bern, C. Updated estimates and mapping for prevalence of Chagas disease among adults, United States. Emerg. Infect. Dis. 2022, 28, 1313–1320. [Google Scholar] [CrossRef]
- Hotez, P.J.; Dumonteil, E.; Betancourt Cravioto, M.; Bottazzi, M.E.; Tapia-Conyer, R.; Meymandi, S.; Karunakara, U.; Ribeiro, I.; Cohen, R.M.; Pecoul, B. An unfolding tragedy of Chagas disease in North America. PLoS Negl. Trop. Dis. 2013, 7, e2300. [Google Scholar] [CrossRef] [Green Version]
- Villalta, F.; Rachakonda, G. Advances in preclinical approaches to Chagas disease drug discovery. Expert Opin. Drug Discov. 2019, 14, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- Casulli, A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl. Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Rodrigues, G.C.; Supuran, C.T. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin. Drug Discov. 2020, 15, 145–158. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Nunes, M.P.; Teixeira, M.M.; Rocha, M.O.C. Diagnosis and management of Chagas disease and cardiomyopathy. Nat. Rev. Cardiol. 2012, 9, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Nolder, D.; García-Mingo, A.; Moore, D.A.; Chiodini, P.L. Diagnosis and clinical management of Chagas disease: An increasing challenge in non-endemic areas. Res. Rep. Trop. Med. 2022, 13, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Gamboa, L.; López-García, L.; Moreno-Sánchez, F.; Hoyo-Ulloa, I.; Vega-Mémije, M.E.; Mendoza-Bazán, N.; Romero-Valdovinos, M.; Olivo-Díaz, A.; Villalobos, G.; Martínez-Hernández, F. Trypanosoma cruzi infection associated with atypical clinical manifestation during the acute phase of the Chagas disease. Parasites Vectors 2019, 12, 506. [Google Scholar] [CrossRef]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas disease: Chronic Chagas cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef]
- Córdova, E.; Maiolo, E.; Corti, M.; Orduña, T. Neurological manifestations of Chagas’ disease. Neurol. Res. 2010, 32, 238–244. [Google Scholar] [CrossRef]
- Sosa-Estani, S.; Segura, E.L. Integrated control of Chagas disease for its elimination as public health problem—A review. Mem. Inst. Oswaldo Cruz 2015, 110, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, E.; Brum-Soares, L.; Reis, R.; Cubides, J.-C. Chagas disease: Review of needs, neglect, and obstacles to treatment access in Latin America. Rev. Soc. Bras. Med. Trop. 2017, 50, 296–300. [Google Scholar] [CrossRef]
- Porrás, A.I.; Yadon, Z.E.; Altcheh, J.; Britto, C.; Chaves, G.C.; Flevaud, L.; Martins-Filho, O.A.; Ribeiro, I.; Schijman, A.G.; Shikanai-Yasuda, M.A.; et al. Target product profile (TPP) for Chagas disease point-of-care diagnosis and assessment of response to treatment. PLoS Negl. Trop. Dis. 2015, 9, e0003697. [Google Scholar] [CrossRef]
- Martinez, S.J.; Romano, P.S.; Engman, D.M. Precision health for Chagas disease: Integrating parasite and host factors to predict outcome of infection and response to therapy. Front. Cell. Infect. Microbiol. 2020, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, V.; Pérez-Silanes, S. Selenium as an interesting option for the treatment of Chagas disease: A review. Eur. J. Med. Chem. 2020, 206, 112673. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C.; Buekens, P. A therapeutic preconceptional vaccine against Chagas disease: A novel indication that could reduce congenital transmission and accelerate vaccine development. PLoS Negl. Trop. Dis. 2019, 13, e0006985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel-Todó, L.; Reche, P.A.; Bigey, P.; Pinazo, M.-J.; Gascón, J.; Alonso-Padilla, J. In silico design of an epitope-based vaccine ensemble for Chagas disease. Front. Immunol. 2019, 10, 2698. [Google Scholar] [CrossRef] [Green Version]
- Lozano, D.; Rojas, L.; Méndez, S.; Casellas, A.; Sanz, S.; Ortiz, L.; Pinazo, M.J.; Abril, M.; Gascón, J.; Torrico, F.; et al. Use of rapid diagnostic tests (RDTs) for conclusive diagnosis of chronic Chagas disease—Field implementation in the Bolivian Chaco region. PLoS Negl. Trop. Dis. 2019, 13, e0007877. [Google Scholar] [CrossRef] [Green Version]
- Moure, Z.; Angheben, A.; Molina, I.; Gobbi, F.; Espasa, M.; Anselmi, M.; Salvador, F.; Tais, S.; Sánchez-Montalvá, A.; Pumarola, T.; et al. Serodiscordance in chronic Chagas disease diagnosis: A real problem in non-endemic countries. Clin. Microbiol. Infect. 2016, 22, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Piron, M.; Fisa, R.; Casamitjana, N.; López-Chejade, P.; Puig, L.; Vergés, M.; Gascón, J.; Gomez i Prat, J.; Portús, M.; Sauleda, S. Development of a real-time PCR Assay for Trypanosoma Cruzi Detection in Blood Samples. Acta Trop. 2007, 103, 195–200. [Google Scholar] [CrossRef]
- Sánchez-Vega, J.T.; Almanza-Mackintoy, A.; Luna-Santillán, A.V.; de La Sancha-Solares, T. A case report of Chagas disease in acute phase diagnosed by xenodiagnosis. Parasitol. Int. 2020, 77, 102121. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; Cortés-Serra, N.; Pinazo, M.J.; Bottazzi, M.E.; Abril, M.; Barreira, F.; Sosa-Estani, S.; Hotez, P.J.; Gascón, J. Strategies to enhance access to diagnosis and treatment for Chagas disease patients in Latin America. Expert Rev. Anti-Infect. Ther. 2019, 17, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Brossas, J.-Y.; Griselda, B.; Bisio, M.; Guihenneuc, J.; Gulin, J.E.N.; Jauréguiberry, S.; Lescure, F.-X.; Fekkar, A.; Mazier, D.; Altcheh, J.; et al. Evaluation of the Chagas western blot IgG assay for the diagnosis of Chagas disease. Pathogens 2021, 10, 1455. [Google Scholar] [CrossRef]
- Picado, A.; Angheben, A.; Marchiol, A.; Alarcón de Noya, B.; Flevaud, L.; Pinazo, M.J.; Gállego, M.; Meymandi, S.; Moriana, S. Development of diagnostics for Chagas disease: Where should we put our limited resources? PLoS Negl. Trop. Dis. 2017, 11, e0005148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balouz, V.; Agüero, F.; Buscaglia, C.A. Chagas disease diagnostic applications: Present knowledge and future steps. Adv. Parasitol. 2017, 97, 1–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttenheim, A.M.; Levy, M.Z.; Castillo-Neyra, R.; McGuire, M.; Toledo Vizcarra, A.M.; Mollesaca Riveros, L.M.; Meza, J.; Borrini-Mayori, K.; Naquira, C.; Behrman, J.; et al. A behavioral design approach to improving a Chagas disease vector control campaign in Peru. BMC Public Health 2019, 19, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Br. Med. J. 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Fumagalli, M.A.; Shrivastava, P.; Aguilar-Pineda, J.A.; Nieto-Montesinos, R.; Del-Carpio, G.D.; Peralta-Mestas, A.; Caracela-Zeballos, C.; Valdez-Lazo, G.; Fernandez-Macedo, V.; Pino-Figueroa, A.; et al. Diagnosis of Alzheimer’s disease in developed and developing countries: Systematic review and meta-analysis of diagnostic test accuracy. J. Alzheimers Dis. Rep. 2021, 5, 15–30. [Google Scholar] [CrossRef]
- Hotez, P.; Bottazzi, M.E.; Strub-Wourgaft, N.; Sosa-Estani, S.; Torrico, F.; Pajín, L.; Abril, M.; Sancho, J. A new patient registry for Chagas disease. PLoS Negl. Trop. Dis. 2020, 14, e0008418. [Google Scholar] [CrossRef]
- Kim, S.; Yeganova, L.; Wilbur, W.J. Meshable: Searching PubMed abstracts by utilizing MeSH and MeSH-derived topical terms. Bioinformatics 2016, 32, 3044–3046. [Google Scholar] [CrossRef] [Green Version]
- White, J. PubMed 2.0. Med. Ref. Serv. Q. 2020, 39, 382–387. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef] [Green Version]
- Waltman, L.; Van Eck, N.J. A new methodology for constructing a publication-level classification system of science. J. Assoc. Inf. Sci. Technol. 2012, 63, 2378–2392. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: A practical review for clinical researchers–part II. Statistical methods of meta-analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.R.; Kim, S.-J.; Lee, J. Diagnostic test accuracy: Application and practice using R software. Epidemiol. Health 2019, 41, e2019007. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, J.; Huecker, M.R. Diagnostic testing accuracy: Sensitivity, specificity, predictive values and likelihood ratios. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Huang, Y.; Yin, J.; Samawi, H. Methods improving the estimate of diagnostic odds ratio. Commun. Stat. Simul. Comput. 2018, 47, 353–366. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.S.; Scholten, R.J.P.M.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Requejo, H.I.; Nakamura, P.M.; Vaz, A.J.; Pialarissi, C.S.; Hoshino-Shimizu, S.; Matsumoto, T.K.; Nakamura, H. Diffusion-in-gel enzyme-linked immunosorbent assay (DIG-ELISA) for Chagas’ disease serodiagnosis. Braz. J. Med. Biol. Res. 1991, 24, 471–483. [Google Scholar]
- Solana, M.E.; Katzin, A.M.; Umezawa, E.S.; Miatello, C.S. High specificity of Trypanosoma cruzi epimastigote ribonucleoprotein as antigen in serodiagnosis of Chagas’ disease. J. Clin. Microbiol. 1995, 33, 1456–1460. [Google Scholar] [CrossRef] [Green Version]
- Peralta, J.M.; Teixeira, M.D.G.M.; Shreffler, W.G.; Pereira, J.B.; Burns, J.M.; Sleath, P.R.; Reed, S.G. Serodiagnosis of Chagas’ disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J. Clin. Microbiol. 1994, 32, 971–974. [Google Scholar] [CrossRef] [Green Version]
- Almeida, I.C.; Covas, D.T.; Soussumi, L.M.; Travassos, L.R. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 1997, 37, 850–857. [Google Scholar] [CrossRef]
- Monteón, V.M.; Guzman-Rojas, L.; Negrete-Garcia, C.; Rosales-Encina, J.L.; Reyes-Lopez, P.A. Serodiagnosis of american trypanosomosis by using nonpathogenic trypanosomatid antigen. J. Clin. Microbiol. 1997, 35, 3316–3319. [Google Scholar] [CrossRef] [Green Version]
- Partel, C.D.; Rossi, C.L. A rapid, quantitative enzyme-linked immunosorbent assay (ELISA) for the immunodiagnosis of Chagas’ disease. Immunol. Investig. 1998, 27, 89–96. [Google Scholar] [CrossRef]
- Oelemann, W.M.R.; Teixeira, M.D.G.M.; Veríssimo Da Costa, G.C.; Borges-Pereira, J.; De Castro, J.A.F.; Coura, J.R.; Peralta, J.M. Evaluation of three commercial enzyme-linked immunosorbent assays for diagnosis of Chagas’ disease. J. Clin. Microbiol. 1998, 36, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.T.; Pedrosa, R.C.; Costa-martins, P.; Castello-Branco, L.R.R. Saliva ELISA: A method for the diagnosis of chronic Chagas disease in endemic areas. Acta Trop. 1999, 72, 31–38. [Google Scholar] [CrossRef]
- Houghton, R.L.; Benson, D.R.; Reynolds, L.D.; McNeill, P.D.; Sleath, P.R.; Lodes, M.J.; Skeiky, Y.A.; Leiby, D.A.; Badaro, R.; Reed, S.G. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J. Infect. Dis. 1999, 179, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezawa, E.S.; Bastos, S.F.; Camargo, M.E.; Yamauchi, L.M.; Santos, M.R.; Gonzalez, A.; Zingales, B.; Levin, M.J.; Sousa, O.; Rangel-Aldao, R.; et al. Evaluation of recombinant antigens for serodiagnosis of Chagas’ disease in South and Central America. J. Clin. Microbiol. 1999, 37, 1554–1560. [Google Scholar] [CrossRef] [Green Version]
- Betonico, G.N.; Miranda, E.O.; Silva, D.A.O.; Houghton, R.; Reed, S.G.; Campos-Neto, A.; Mineo, J.R. Evaluation of a synthetic tripeptide as antigen for detection of IgM and IgG antibodies to Trypanosoma cruzi in serum samples from patients with Chagas disease or viral diseases. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 603–606. [Google Scholar] [CrossRef]
- Pereira, V.R.A.; Nakazawa, M.; Furtado, V.C.; Abath, F.G.C.; Gomes, Y.M. Immunodiagnosis of chronic Chagas’ disease using the Tc 46 and Tc 58 antigens. Rev. Soc. Bras. Med. Trop. 2000, 33, 367–370. [Google Scholar] [CrossRef] [Green Version]
- Gomes, Y.M.; Pereira, V.R.; Nakazawa, M.; Rosa, D.S.; Barros, M.d.N.D.; Ferreira, A.G.; Silva, E.D.; Yamada, S.F.; Krieger, M.A.; Goldenberg, S. Serodiagnosis of chronic Chagas infection by using EIE-recombinant-Chagas-biomanguinhos kit. Mem. Inst. Oswaldo Cruz 2001, 96, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Blejer, J.L.; Saguier, M.C.; Salamone, H.J. Antibodies to Trypanosoma cruzi among blood donors in Buenos Aires, Argentina. Int. J. Infect. Dis. 2001, 5, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, M.; Rosa, D.S.; Pereira, V.R.; Moura, M.O.; Furtado, V.C.; Souza, W.V.; Barros, M.; Abath, F.; Gomes, Y.M. Excretory-secretory antigens of Trypanosoma cruzi are potentially useful for serodiagnosis of chronic Chagas’ disease. Clin. Diagn. Lab. Immunol. 2001, 8, 1024–1027. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, B.; Monteón, V.; Reyes, P.A.; Espinoza, B. Standardization of micro-enzyme-linked immunosorbent assay (ELISA) and western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Arch. Med. Res. 2001, 32, 382–388. [Google Scholar] [CrossRef]
- Umezawa, E.S.; Bastos, S.F.; Coura, J.R.; Levin, M.J.; Gonzalez, A.; Rangel-Aldao, R.; Zingales, B.; Luquetti, A.; da Silveira, J.F. An improved serodiagnostic test for Chagas’ disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 2003, 43, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.Á.M.; Verçosa, A.F.A.; Lorena, V.M.B.; Nakazawa, M.; Carvalho, A.B.; Souza, W.V.; Ferreira, A.G.P.; Silva, E.D.; Krieger, M.A.; Goldenberg, S.; et al. Chagas’ disease diagnosis: Comparative analysis of recombinant ELISA with conventional ELISA and the haemagglutination test. Vox. Sang. 2003, 85, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, E.S.; Luquetti, A.O.; Levitus, G.; Ponce, C.; Ponce, E.; Henriquez, D.; Revollo, S.; Espinoza, B.; Sousa, O.; Khan, B.; et al. Serodiagnosis of chronic and acute Chagas’ disease with Trypanosoma cruzi recombinant protein: Results of a collaborative study in six Latin American countries. J. Clin. Microbiol. 2004, 42, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldaña, A.; Samudio, F.; Miranda, A.; Herrera, L.M.; Saavedra, S.P.; Cáceres, L.; Bayard, V.; Calzada, J.E. Predominance of Trypanosoma rangeli infection in children from a Chagas disease endemic area in the west-shore of the Panama canal. Mem. Inst. Oswaldo Cruz 2005, 100, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Vieira Duarte, A.M.; Monteiro de Andrade, H.; Hadad do Monte, S.J.; Coelho Peixoto de Toledo, V.d.P.; Pinto Dabés Guimaraes, T.M. Assessment of chemiluminescence and PCR effectiveness in relation to conventional serological tests for the diagnosis of Chagas’ disease. Rev. Soc. Bras. Med. Trop. 2006, 39, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, J.D.; Guhl, F.; Umezawa, E.S.; Morillo, C.A.; Rosas, F.; Marin-Neto, J.A.; Restrepo, S. Evaluation of adult chronic Chagas’ heart disease diagnosis by molecular and serological methods. J. Clin. Microbiol. 2009, 47, 3945–3951. [Google Scholar] [CrossRef] [Green Version]
- Mateo, H.; Sánchez-Moreno, M.; Marín, C. Enzyme-linked immunosorbent assay with purified Trypanosoma cruzi excreted superoxide dismutase. Clin. Biochem. 2010, 43, 1257–1264. [Google Scholar] [CrossRef]
- Briceño, L.; Rodríguez, E.M.; Medina, M.; Campos, Y.; Mosca, W.; Briceño, A.; León, G. An inexpensive antigen for serodiagnosis of Chagas’ disease. Investig. Clin. 2010, 51, 103–113. [Google Scholar]
- de Marchi, C.R.; Di Noia, J.M.; Frasch, A.C.C.; Neto, V.A.; Almeida, I.C.; Buscaglia, C.A. Evaluation of a recombinant Trypanosoma cruzi mucin-like antigen for serodiagnosis of Chagas’ disease. Clin. Vaccine Immunol. 2011, 18, 1850–1855. [Google Scholar] [CrossRef] [Green Version]
- da Silva Santos, L.; Morais Torres, R.; Machado-de-Assis, G.F.; Bahia, M.T.; Rodrigues Martins, H.; Teixeira-Carvalho, A.; Alves Coelho-dos-Reis, J.G.; Albajar-Viñas, P.; Olindo, M.-F.; de Lana, M. In-house ELISA method to analyze anti-trypanosoma cruzi IgG reactivity for differential diagnosis and evaluation of Chagas disease morbidity. Rev. Soc. Bras. Med. Trop. 2012, 45, 35–44. [Google Scholar] [CrossRef]
- López-Céspedes, Á.; Villagrán, E.; Briceño Álvarez, K.; de Diego, J.A.; Hernández-Montiel, H.L.; Saldaña, C.; Sánchez-Moreno, M.; Marín, C. Trypanosoma cruzi: Seroprevalence detection in suburban population of Santiago de Querétaro (Mexico). Sci. World J. 2012, 2012, 914129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhi, S.A.; Brandariz, S.B.; Lafon, S.O.; Niborski, L.L.; Luquetti, A.O.; Schijman, A.G.; Levin, M.J.; Gómez, K.A. Short report: Evaluation of in-house ELISA using Trypanosoma cruzi lysate and recombinant antigens for diagnosis of chagas disease and discrimination of its clinical forms. Am. J. Trop. Med. Hyg. 2012, 87, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tustin, A.W.; Small, D.S.; Delgado, S.; Castillo Neyra, R.; Verastegui, M.R.; Ancca Juárez, J.M.; Quispe Machaca, V.R.; Gilman, R.H.; Bern, C.; Levy, M.Z. Use of individual-level covariates to improve latent class analysis of Trypanosoma cruzi diagnostic tests. Epidemiol. Methods 2012, 1, 35–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín, C.; Concha-Valdez, F.; Cañas, R.; Gutiérrez-Sánchez, R.; Sánchez-Moreno, M. Anti-Trypanosoma cruzi antibody detection in Eastern Andalusia (Spain). Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.F.; Flórez, O.; Rincón, G.; González, C.I. Comparison of seven diagnostic tests to detect Trypanosoma cruzi infection in patients in chronic phase of Chagas disease. Colomb. Med. 2014, 45, 61–66. [Google Scholar] [CrossRef]
- Dopico, E.; Pimenta Del-rei, R.; Espinoza, B.; Ubillos, I.; Tonin Zanchin, N.I.; Sulleiro, E.; Moure, Z.; Fiorani Celedon, P.A.; Vieira Souza, W.; Domingos da Silva, E.; et al. Immune reactivity to Trypanosoma cruzi chimeric proteins for Chagas disease diagnosis in immigrants living in a non-endemic setting. BMC Infect. Dis. 2019, 19, 251. [Google Scholar] [CrossRef] [Green Version]
- Caballero, E.Z.; Correa, R.; Nascimento, M.S.; Villarreal, A.; Llanes, A.; Kesper, J.R.N. High sensitivity and reproducibility of in-house ELISAs using different genotypes of Trypanosoma cruzi. Parasite Immunol. 2019, 41, e12627. [Google Scholar] [CrossRef]
- Floridia-Yapur, N.; Monje-Rumi, M.; Ragone, P.; Lauthier, J.J.; Tomasini, N.; D’Amato, A.A.; Diosque, P.; Cimino, R.; Gil, J.; Sanchez, D.; et al. TcTASV antigens of Trypanosoma cruzi: Utility for diagnosis and high accuracy as biomarkers of treatment efficacy in pediatric patients. Am. J. Trop. Med. Hyg 2010, 5, 1135–1138. [Google Scholar] [CrossRef]
- Abramo, C.; Fontes, C.J.; Krettli, A.U. Cross-reactivity between antibodies in the sera of individuals with leishmaniasis, toxoplasmosis, and Chagas’ disease and Antigens of the blood-stage forms of plasmodium falciparum determined by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 1995, 53, 202–205. [Google Scholar] [CrossRef]
- Matsumoto, T.K.; Hoshino-Shimizu, S.; Nakamura, P.M.; Andrade, H.F.; Umezawa, E.S. High resolution of Trypanosoma cruzi amastigote antigen in serodiagnosis of different clinical forms of Chagas’ disease. J. Clin. Microbiol. 1993, 31, 1486–1492. [Google Scholar] [CrossRef] [Green Version]
- de Apparecida Levy, A.M.; Boainain, E.; Kloetzel, J.K. In situ indirect fluorescent antibody: A new specific test to detect ongoing chagasic infections. J. Clin. Lab. Anal. 1996, 10, 98–103. [Google Scholar] [CrossRef]
- Langhi, D.M.; Bordin, J.O.; Castelo, A.; Walter, S.D.; Moraes-Souza, H.; Stumpf, R.J. The application of latent class analysis for diagnostic test validation of chronic Trypanosoma cruzi infection in blood donors. Braz. J. Infect. Dis. 2002, 6, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bergmann Araújo, A.; Aires Berne, M.E. Conventional Serological Performance in Diagnosis of Chagas’ Disease in Southern Brazil. Braz. J. Infect. Dis. 2013, 17, 174–178. [Google Scholar] [CrossRef] [Green Version]
- Blejer, J.L.; Saguier, M.C.; Dinapoli, R.A.; Salamone, H.J. Prevalence of Trypanosoma cruzi antibodies in blood donors. Medicina 1999, 59, 129–132. [Google Scholar] [PubMed]
- Remesar, M.C.; Gamba, C.; Colaianni, I.F.; Puppo, M.; Sartor, P.A.; Murphy, E.L.; Neilands, T.B.; Ridolfi, M.A.; Leguizamón, M.S.; Kuperman, S.; et al. Estimation of sensitivity and specificity of several Trypanosoma cruzi antibody assays in blood donors in Argentina. Transfusion 2010, 49, 2352–2358. [Google Scholar] [CrossRef] [Green Version]
- Egüez, K.E.; Alonso-Padilla, J.; Terán, C.; Chipana, Z.; García, W.; Torrico, F.; Gascon, J.; Lozano-Beltran, D.-F.; Pinazo, M.-J. Rapid diagnostic tests duo as alternative to conventional serological assays for conclusive Chagas disease diagnosis. PLoS Negl. Trop. Dis. 2017, 11, e0005501. [Google Scholar] [CrossRef] [Green Version]
- Barbosa Marcon, G.E.; Durante Andrade, P.; de Albuquerque, D.M.; da Silva Wanderley, J.; de Almeida, E.A.; Guariento, M.E.; Botelho Costa, S.C. (2002). Use of a nested polymerase chain reaction (N-PCR) to detect Trypanosoma cruzi in blood samples from chronic chagasic patients and patients with doubtful serologies. Diagn. Microbiol. Infect. Dis. 2002, 43, 39–43. [Google Scholar] [CrossRef]
- Altcheh, J.; Biancardi, M.; Lapena, A.; Ballering, G.; Freilij, H. Congenital Chagas disease: Experience in the hospital de niños, Ricardo Gutierrez, Buenos Aires, Argentina. Rev. Soc. Bras. Med. Trop. 2005, 38, 41–45. [Google Scholar]
- Fitzwater, S.; Calderon, M.; LaFuente, C.; Galdos-Cardenas, G.; Ferrufino, L.; Verastegui, M.; Gilman, R.H.; Bern, C. Short report: Polymerase chain reaction for chronic Trypanosoma cruzi infection yields higher sensitivity in blood clot than buffy coat or whole blood specimens. Am. J. Trop. Med. Hyg. 2008, 79, 768–770. [Google Scholar] [CrossRef]
- Deborggraeve, S.; Coronado, X.; Solari, A.; Zulantay, I.; Apt, W.; Mertens, P.; Laurent, T.; Leclipteux, T.; Stessens, T.; Dujardin, J.-C.; et al. T. Cruzi OligoC-TesT: A simplified and standardized polymerase chain reaction format for diagnosis of Chagas disease. PLoS Negl. Trop. Dis. 2009, 3, e450. [Google Scholar] [CrossRef] [Green Version]
- Sabino, E.C.; Lee, T.H.; Montalvo, L.; Nguyen, M.L.; Leiby, D.A.; Carrick, D.M.; Otani, M.M.; Vinelli, E.; Wright, D.; Stramer, S.L.; et al. Antibody levels correlate with detection of Trypanosoma cruzi DNA by sensitive polymerase chain reaction assays in seropositive blood donors and possible resolution of infection over time. Transfusion 2013, 53, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilber, S.R.; Alban, S.M.; Gobor, L.; De Oliveira Bescrovaine, J.; Myiazaki, M.I.; Thomaz-Soccol, V. Comparison of conventional serology and PCR methods for the routine diagnosis of Trypanosoma cruzi infection. Rev. Soc. Bras. Med. Trop. 2013, 46, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, C.; Cucunubá, Z.; Flórez, C.; Olivera, M.; Valencia, C.; Zambrano, P.; León, C.; Ramírez, J.D. Molecular diagnosis of Chagas disease in Colombia: Parasitic loads and discrete typing units in patients from acute and chronic phases. PLoS Negl. Trop. Dis. 2016, 10, e0004997. [Google Scholar] [CrossRef]
- Hernández, C.; Teherán, A.; Flórez, C.; Ramírez, J.D. Comparison of parasite loads in serum and blood samples from patients in acute and chronic phases of Chagas disease. Parasitology 2018, 145, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Seiringer, P.; Pritsch, M.; Flores-Chavez, M.; Marchisio, E.; Helfrich, K.; Mengele, C.; Hohnerlein, S.; Bretzel, G.; Löscher, T.; Hoelscher, M.; et al. Comparison of four PCR methods for efficient detection of Trypanosoma cruzi in routine diagnostics. Diagn. Microbiol. Infect. Dis. 2017, 88, 225–232. [Google Scholar] [CrossRef]
- Cura, C.I.; Ramírez, J.C.; Rodríguez, M.; Lopez-Albízu, C.; Irazu, L.; Scollo, K.; Sosa-Estani, S. Comparative study and analytical verification of PCR methods for the diagnosis of congenital Chagas disease. J. Mol. Diagn. 2017, 19, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.F.; Moreira, O.C.; Tenório, P.; Lorena, V.; Lorena-Rezende, I.; Oliveira Júnior, W.; Gomes, Y.; Britto, C. Usefulness of real time PCR to quantify parasite load in serum samples from chronic Chagas disease patients. Parasites Vectors 2015, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Mayta, H.; Romero, Y.K.; Pando, A.; Verastegui, M.; Tinajeros, F.; Bozo, R.; Henderson-Frost, J.; Colanzi, R.; Flores, J.; Lerner, R.; et al. Improved DNA extraction technique from clot for the diagnosis of Chagas disease. PLoS Negl. Trop. Dis. 2019, 13, e0007024. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Herrera, G.; Hernández, C.; Cruz-Saavedra, L.; Muñoz, M.; Flórez, C.; Butcher, R. Evaluation of the analytical and diagnostic performance of a digital droplet polymerase chain reaction (DdPCR) assay to detect Trypanosoma cruzi DNA in blood samples. PLoS Negl. Trop. Dis. 2018, 12, e0007063. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, M.; Zulantay, I.; Apt, W.; Martínez, G.; Rojas, A.; Rodríguez, J. Chronic Chagas disease: PCR-xenodiagnosis without previous microscopic observation is a useful tool to detect viable Trypanosoma cruzi. Biol. Res. 2013, 46, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Ramirez, L.E.; Monteon, V.; Sotelo, J. Diagnosis of American trypanosomiasis (Chagas’ disease) by the new complement fixation test. J. Clin. Microbiol. 1995, 33, 1034–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, M.A.; Brashear, R.J.; Hall, H.J.; Schur, J.D.; Pan, A.A. Detection of antibodies to Trypanosoma cruzi among blood donors in the southwestern and western United States. II. Evaluation of a supplemental enzyme immunoassay and radioimmunoprecipitation assay for confirmation of seroreactivity. Transfusion 1995, 35, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; Seitz, A.; Gilman, R.H.; Lafuente, C.; Galdos-cardenas, G.; Kawai, V.; Lafuente, E.D.; Ferrufino, L.; Bowman, N.M.; Pinedo-Cancino, V.; et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg. 2009, 80, 410–415. [Google Scholar] [CrossRef]
- Reiche, E.M.; Cavazzana Jr, M.; Okamura, H.; Tagata, E.C.; Jankevicius, S.I.; Jankevicius, J.V. Evaluation of the western blot in the confirmatory serologic diagnosis of Chagas’ disease. Am. J. Trop. Med. Hyg. 1998, 59, 750–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, Z.C.; Sousa, O.E.; Marques, W.P.; Saez-Alquezar, A.; Umezawa, E.S. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin. Vaccine Immunol. 2007, 14, 1045–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otani, M.M.; Vinelli, E.; Kirchhoff, L.V.; del Pozo, A.; Sands, A.; Vercauteren, G.; Sabino, E.C. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion 2009, 49, 1076–1082. [Google Scholar] [CrossRef]
- Martins-Melo, F.R.; Castro, M.C.; Werneck, G.L. Levels and trends in Chagas disease-related mortality in Brazil, 2000–2019. Acta Trop. 2021, 220, 105948. [Google Scholar] [CrossRef]
- Adger, W.N.; Crépin, A.-S.; Folke, C.; Ospina, D.; Chapin, F.S.; Segerson, K.; Seto, K.C.; Anderies, J.M.; Barrett, S.; Bennett, E.M.; et al. Urbanization, migration, and adaptation to climate change. One Earth 2020, 3, 396–399. [Google Scholar] [CrossRef]
- Guhl, F.; Ramírez, J.D. Poverty, migration, and Chagas disease. Curr. Trop. Med. Rep. 2021, 8, 52–58. [Google Scholar] [CrossRef]
- Angheben, A.; Buonfrate, D.; Cruciani, M.; Jackson, Y.; Alonso-Padilla, J.; Gascon, J.; Gobbi, F.; Giorli, G.; Anselmi, M.; Bisoffi, Z. Rapid immunochromatographic tests for the diagnosis of chronic Chagas disease in at-risk populations: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007271. [Google Scholar] [CrossRef]
- Olivera, M.J.; Porras Villamil, J.F.; Toquica Gahona, C.C.; Rodríguez Hernández, J.M. Barriers to diagnosis access for Chagas disease in Colombia. J. Parasitol. Res. 2018, 2018, 4940796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, A.M.; Ebell, M.H.; Tarleton, R.L. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl. Trop. Dis. 2012, 6, e1881. [Google Scholar] [CrossRef] [Green Version]
- Organización Panamericana de la Salud. Síntesis de evidencia: Guía para el diagnóstico y el tratamiento de la enfermedad de Chagas. Rev. Panam. Salud Publica 2020, 44, e28. [Google Scholar] [CrossRef]
- Schijman, A.G.; Alonso-Padilla, J.; Longhi, S.A.; Picado, A. Parasitological, serological and molecular diagnosis of acute and chronic Chagas disease: From field to laboratory. Mem. Inst. Oswaldo Cruz 2022, 117, e200444. [Google Scholar] [CrossRef] [PubMed]
- Schijman, A.G. Molecular diagnosis of Trypanosoma cruzi. Acta Trop. 2018, 184, 59–66. [Google Scholar] [CrossRef] [PubMed]
- D’Ávila, D.A.; Galvão, L.M.C.; Sousa, G.R.; Britto, C.; Moreira, O.C.; Chiari, E. Monitoring the parasite load in chronic Chagas disease patients: Comparison between blood culture and quantitative real time PCR. PLoS ONE 2018, 13, e0208133. [Google Scholar] [CrossRef]
- Parrado, R.; Ramirez, J.C.; de la Barra, A.; Alonso-Vega, C.; Juiz, N.; Ortiz, L.; Illanes, D.; Torrico, F.; Gascon, J.; Alves, F.; et al. Usefulness of serial blood sampling and PCR replicates for treatment monitoring of patients with chronic Chagas disease. Antimicrob. Agents Chemother. 2019, 63, e01191-18. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, E.; Lares, M.; Viettri, M.; Medina, M. Comparación entre técnicas inmunológicas y moleculares para el diagnóstico de la enfermedad de Chagas. Enferm. Infecc. Microbiol. Clin. 2013, 31, 277–282. [Google Scholar] [CrossRef]
- Duffy, T.; Cura, C.I.; Ramirez, J.C.; Abate, T.; Cayo, N.M.; Parrado, R.; Diaz Bello, Z.; Velazquez, E.; Muñoz-Calderon, A.; Juiz, N.A.; et al. Analytical performance of a multiplex Real-Time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl. Trop. Dis. 2013, 7, e2000. [Google Scholar] [CrossRef]
- Chan-Pérez, J.I.; Torres-Acosta, J.F.J.; Ortega-Pacheco, A.; Hernández-Cortazar, I.B.; Cigarroa-Toledo, N.; Jiménez-Coello, M. Combined Use of Real-Time PCR and serological techniques for improved surveillance of chronic and acute American Trypanosomiasis in dogs and their owners from an endemic rural area of neotropical Mexico. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100081. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil, P.E.; de Castro, L.; Hasslocher-Moreno, A.M.; Sangenis, L.H.; Braga, J.U. ELISA versus PCR for diagnosis of chronic Chagas disease: Systematic review and meta-analysis. BMC Infect. Dis. 2010, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, H.N.; McAuliffe, I.; Aderohunmu, T.; Wiegand, R.E.; Montgomery, S.P.; Bradbury, R.S.; Handali, S. Evaluation of the performance of ortho T. cruzi ELISA test system for the detection of antibodies to Trypanosoma cruzi. J. Clin. Microbiol. 2022, 60, e00134-22. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.M.T.; Matos, C.S.; Carvalho, A.M.R.S.; Chaves, A.T.; Medeiros, F.A.C.; Barbosa, R.; Marcelino, A.P.; dos Santos Emidio, K.; Coelho, E.A.F.; Duarte, M.C.; et al. Immunogenomic screening approach to identify new antigens for the serological diagnosis of chronic Chagas’ disease. Appl. Microbiol. Biotechnol. 2018, 102, 6069–6080. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Ganeshkumar, S. Systematic reviews and meta-analysis: Understanding the best evidence in primary healthcare. J. Fam. Med. Prim. Care 2013, 2, 9. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candia-Puma, M.A.; Machaca-Luque, L.Y.; Roque-Pumahuanca, B.M.; Galdino, A.S.; Giunchetti, R.C.; Coelho, E.A.F.; Chávez-Fumagalli, M.A. Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2752. https://doi.org/10.3390/diagnostics12112752
Candia-Puma MA, Machaca-Luque LY, Roque-Pumahuanca BM, Galdino AS, Giunchetti RC, Coelho EAF, Chávez-Fumagalli MA. Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(11):2752. https://doi.org/10.3390/diagnostics12112752
Chicago/Turabian StyleCandia-Puma, Mayron Antonio, Laura Yesenia Machaca-Luque, Brychs Milagros Roque-Pumahuanca, Alexsandro Sobreira Galdino, Rodolfo Cordeiro Giunchetti, Eduardo Antonio Ferraz Coelho, and Miguel Angel Chávez-Fumagalli. 2022. "Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 11: 2752. https://doi.org/10.3390/diagnostics12112752
APA StyleCandia-Puma, M. A., Machaca-Luque, L. Y., Roque-Pumahuanca, B. M., Galdino, A. S., Giunchetti, R. C., Coelho, E. A. F., & Chávez-Fumagalli, M. A. (2022). Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis. Diagnostics, 12(11), 2752. https://doi.org/10.3390/diagnostics12112752









