Validation of a Cytological Classification System for the Rapid On-Site Evaluation (Rose) of Pulmonary and Mediastinal Needle Aspirates
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandiera, A.; Arrigoni, G. The impact of pathological analysis on endobronchial ultrasound diagnostic accuracy. Mediastinum 2020, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.C.; Schwartz, L.E.; Baloch, Z.W. Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal 2011, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Suen, K.C.; Abdul-Karim, F.W.; Kaminsky, D.B.; Layfield, L.J.; Miller, T.R.; Spires, S.E. Guidelines of the Papanicolaou Society of Cytopathology for the Examination of Cytologic Specimens Obtained from the Respiratory Tract. Papanicolaou Society of Cytopathology. Task Force on Standards of Practice. Diagn. Cytopathol. 1999, 21, 61–69. [Google Scholar] [CrossRef]
- Oki, M.; Saka, H.; Kitagawa, C.; Kogure, Y.; Murata, N.; Adachi, T.; Ando, M. Rapid On-Site Cytologic Evaluation during Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Diagnosing Lung Cancer: A Randomized Study. Respiration 2013, 85, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Agarwal, R. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the Diagnostic Yield of Transbronchial Needle Aspiration During Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Chest 2018, 153, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Allen, T.C.; Aisner, D.L.; Beasley, M.B.; Cagle, P.T.; Capelozzi, V.L.; Hariri, L.P.; Lantuejoul, S.; Miller, R.; Mino-Kenudson, M.; et al. Rapid On-Site Evaluation of Endobronchial Ultrasound–Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2018, 142, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-F.; Ren, T.; Wang, H.-S.; Feng, Z.-X.; Wang, M.-F. Diagnostic Value of Rapid On-Site Evaluation for CT-Guided Percutaneous Fine Needle Aspiration in the Diagnosis of Pulmonary Occupying Lesions. BioMed Res. Int. 2020, 2020, 9842768. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, A.; Ye, L.; Hong, L. Real-time and accuracy of rapid on-site cytological evaluation of lung cancer. Transl. Cancer Res. 2021, 10, 479–486. [Google Scholar] [CrossRef]
- Gasparini, S.; Ferretti, M.; Secchi, E.B.; Baldelli, S.; Zuccatosta, L.; Gusella, P. Integration of Transbronchial and Percutaneous Approach in the Diagnosis of Peripheral Pulmonary Nodules or Masses. Chest 1995, 108, 131–137. [Google Scholar] [CrossRef]
- Nakajima, T.; Yasufuku, K.; Saegusa, F.; Fujiwara, T.; Sakairi, Y.; Hiroshima, K.; Nakatani, Y.; Yoshino, I. Rapid On-Site Cytologic Evaluation During Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Nodal Staging in Patients With Lung Cancer. Ann. Thorac. Surg. 2013, 95, 1695–1699. [Google Scholar] [CrossRef]
- Khan, K.A.; Narine, N.; Bailey, S.; Holbrook, M.; Shelton, D.; Rana, D. Concordance between Rapid On-Site Cytology Evaluation (ROSE) and Final diagnosis in patients undergoing Endobronchial Ultrasound- guided Transbronchial Needle Aspiration (EBUS-TBNA). Eur. Respiratory Soc. 2020, 56, 2851. [Google Scholar] [CrossRef]
- Caupena, C.; Esteban, L.; Jaen, A.; Barreiro, B.; Albero, R.; Perez-Ochoa, F.; De Souza, P.P.; Gibert, O.; Ferrer, C.; Forcada, P.; et al. Concordance Between Rapid On-Site Evaluation and Final Cytologic Diagnosis in Patients Undergoing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Non-Small Cell Lung Cancer Staging. Am. J. Clin. Pathol. 2019, 153, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Fassina, A.; Corradin, M.; Zardo, D.; Cappellesso, R.; Corbetti, F.; Fassan, M. Role and accuracy of rapid on-site evaluation of CT-guided fine needle aspiration cytology of lung nodules. Cytopathology 2010, 22, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, S.; Bravaccini, S.; Tumedei, M.M.; Pironi, F.; Candoli, P.; Puccetti, M. Easily detectable cytomorphological features to evaluate during ROSE for rapid lung cancer diagnosis: From cytology to histology. Oncotarget 2016, 8, 11199–11205. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; Paioli, D.; Scudeller, L.; Casadei, G.P.; Parri, S.F.; Livi, V.; Bondi, A.; Boaron, M.; et al. Rapid On-site Evaluation of Transbronchial Aspirates in the Diagnosis of Hilar and Mediastinal Adenopathy. Chest 2011, 139, 395–401. [Google Scholar] [CrossRef]
- Joseph, M.; Jones, T.; Lutterbie, Y.; Maygarden, S.J.; Feins, R.H.; Haithcock, B.E.; Veeramachaneni, N.K. Rapid On-Site Pathologic Evaluation Does Not Increase the Efficacy of Endobronchial Ultrasonographic Biopsy for Mediastinal Staging. Ann. Thorac. Surg. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Trisolini, R.; Cancellieri, A.; Tinelli, C.; de Biase, D.; Valentini, I.; Casadei, G.; Paioli, D.; Ferrari, F.; Gordini, G.; Patelli, M.; et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015, 148, 1430–1437. [Google Scholar] [CrossRef]
- Bonifazi, M.; Sediari, M.; Ferretti, M.; Poidomani, G.; Tramacere, I.; Mei, F.; Zuccatosta, L.; Gasparini, S. The Role of the Pulmonologist in Rapid On-site Cytologic Evaluation of Transbronchial Needle Aspiration: A prospective study. Chest 2014, 145, 60–65. [Google Scholar] [CrossRef]
- Santosh, T.; Panwar, H.; Ingle, P.; Singh, V.; Bugalia, A.; Hussain, N. FNAC of breast lesions with special reference to IAC standardized reporting and comparative study of cytohistological grading of breast carcinoma. J. Cytol. 2020, 37, 34–39. [Google Scholar] [CrossRef]
- Piana, S.; Frasoldati, A.; Ferrari, M.; Valcavi, R.; Froio, E.; Barbieri, V.; Pedroni, C.; Gardini, G. Is a five-category reporting scheme for thyroid fine needle aspiration cytology accurate? Experience of over 18,000 FNAs reported at the same institution during 1998–2007. Cytopathology 2010, 22, 164–173. [Google Scholar] [CrossRef]
- Hanks, M.; Ryder, S.; Zaitoun, A. The role of cytology in the investigation and management of pancreatobiliary lesions with a transition towards a standardised reporting system: An institutional perspective. Cytopathology 2022, 33, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Muri, M.R.; Trippel, M.; Borner, U.; Weidner, S.; Trepp, R. The Impact of Rapid On-Site Evaluation on the Quality and Diagnostic Value of Thyroid Nodule Fine-Needle Aspirations. Thyroid 2022, 32, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, C.; Eppenberger-Castori, S.; Zechmann, S.; Hanke, J.; Herzog, M.; Prince, S.S.; Christ, E.R.; Ebrahimi, F. Effects of Rapid On-Site Evaluation on Diagnostic Accuracy of Thyroid Fine-Needle Aspiration. Acta Cytol. 2022, 66, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Kumar, M.; Subramanian, P.; Zubair, A.; Kumar, R.; Thakar, A.; Jain, D.; Mathur, S.R.; Iyer, V.K. Utility of the Milan system for reporting salivary gland cytopathology during rapid on-site evaluation (ROSE) of salivary gland aspirates. Cytopathology 2021, 32, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Kothari, K.; Tummidi, S.; Sood, P.; Agnihotri, M.; Shah, V. Fine-Needle Aspiration Biopsy Cytopathology of Breast Lesions Using the International Academy of Cytology Yokohama System and Rapid On-Site Evaluation: A Single-Institute Experience. Acta Cytol. 2021, 65, 463–477. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non–Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Chen, C.-H.; Cheng, W.-C.; Wu, B.-R.; Chen, C.-Y.; Chen, W.-C.; Hsia, T.-C.; Liao, W.-C.; Tu, C.-Y.; Shih, C.-M.; Hsu, W.-H.; et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: Combination of radial probe endobronchial ultrasound and rapid on-site evaluation. J. Thorac. Dis. 2015, 7, S418–S425. [Google Scholar] [CrossRef]
- Hiroshima, K.; Yoshizawa, A.; Takenaka, A.; Haba, R.; Kawahara, K.; Minami, Y.; Kakinuma, H.; Shibuki, Y.; Miyake, S.; Kajio, K.; et al. Cytology Reporting System for Lung Cancer from the Japan Lung Cancer Society and Japanese Society of Clinical Cytology: An Interobserver Reproducibility Study and Risk of Malignancy Evaluation on Cytology Specimens. Acta Cytol. 2020, 64, 452–462. [Google Scholar] [CrossRef]
- Boler, A.K.; Banu, N.; Bose, K.; Roy, S.; Bandyopadhyay, A. Reproducibility of “the Papanicolaou Society of Cytopathology system for reporting respiratory cytology”—A retrospective analysis of 101 cases of CT -guided FNAC. Diagn. Cytopathol. 2020, 48, 701–705. [Google Scholar] [CrossRef]
- Fanaroff, R.; Legesse, T.B.; Geisinger, K.R. Common Differential Diagnostic Issues in Lung Cytopathology: 3 Case Reports and a Review. AJSP: Rev. Rep. 2021, 26, 155–161. [Google Scholar] [CrossRef]
- Umeda, Y.; Otsuka, M.; Nishikiori, H.; Ikeda, K.; Mori, Y.; Kobayashi, T.; Asai, Y.; Takahashi, Y.; Sudo, Y.; Kodama, K.; et al. Feasibility of rapid on-site cytological evaluation of lung cancer by a trained pulmonologist during bronchoscopy examination. Cytopathology 2019, 30, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Natali, F.; Cancellieri, A.; Giunchi, F.; De Silvestri, A.; Livi, V.; Ferrari, M.; Paioli, D.; Betti, S.; Fiorentino, M.; Trisolini, R. Interobserver agreement between pathologist, pulmonologist and molecular pathologist to estimate the tumour burden in rapid on-site evaluation smears from endosonography and guided bronchoscopy. Cytopathology 2020, 31, 303–309. [Google Scholar] [CrossRef] [PubMed]

| Techniques | Number | Percentage (%) |
|---|---|---|
| EBUS-TBNA (Lymph nodes) | 318 | 13.9 |
| TBNA (Parenchymal lesions) | 994 | 43.5 |
| Percutaneous | 898 | 39.3 |
| Needle aspiration (Central endobronchial lesions) | 22 | 0.9 |
| Conventional TBNA (Lymph nodes) | 50 | 2.1 |
| Total | 2282 |
| Benign Conditions | ||
|---|---|---|
| Number | Percentage (%) | |
| Granulomatous disease | 25 | 1.10 |
| Benign tumors | 43 | 1.88 |
| Reactive lymph node | 131 | 5.74 |
| Inflammation | 142 | 6.22 |
| Other | 2 | 0.09 |
| Malignant lesions | ||
| Number | Percentage (%) | |
| Adenocarcinoma | 566 | 24.80 |
| Squamous cell carcinoma | 116 | 5.08 |
| Small cell lung cancer | 50 | 2.19 |
| Large cell lung cancer | 5 | 0.22 |
| Non-small cell lung cancer, NOS (not otherwise specified) | 443 | 19.41 |
| Lymphoma | 49 | 2.15 |
| Carcinoid | 20 | 0.88 |
| Metastasis | 140 | 6.13 |
| Thymoma | 15 | 0.66 |
| Other cancer | 9 | 0.39 |
| Non diagnostic samples | ||
| 526 | 23.05 | |
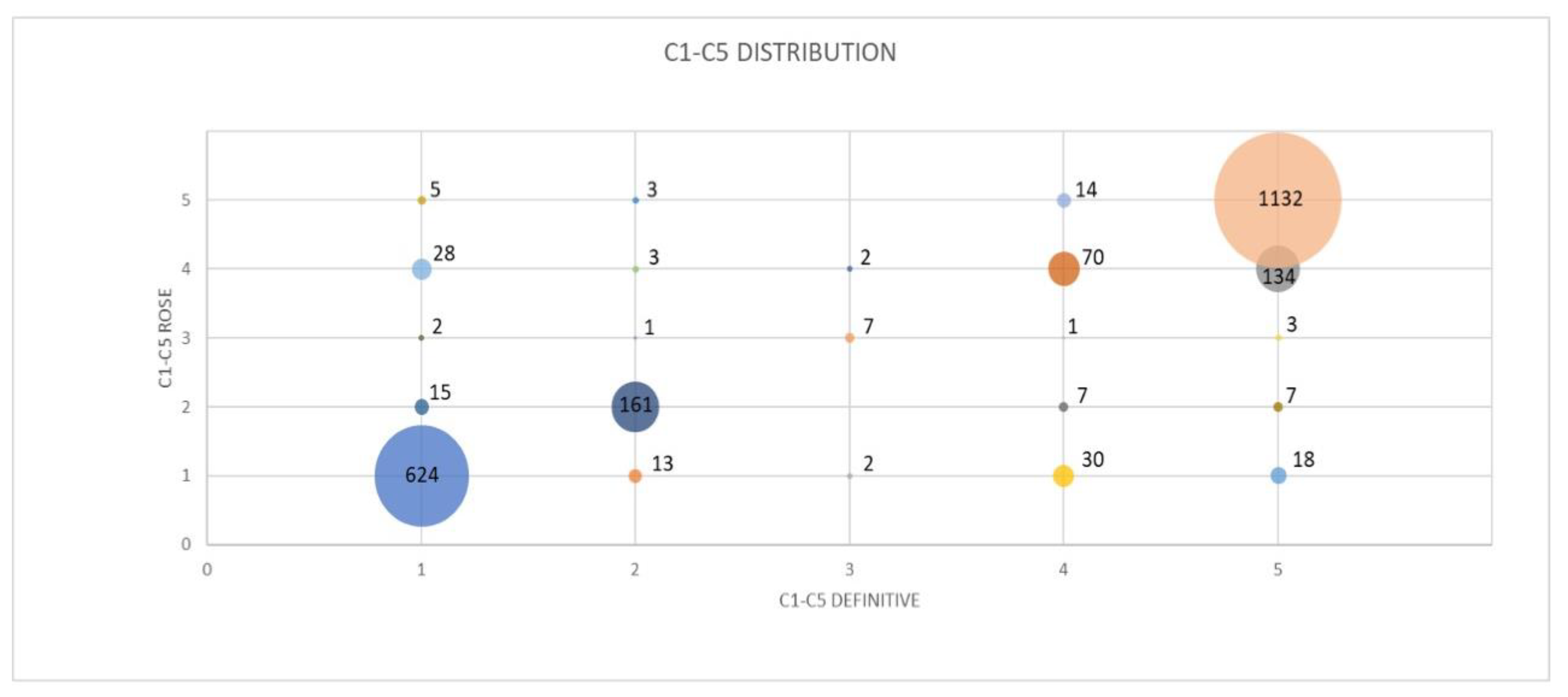
| C1 Definitive | C2 Definitive | C3 Definitive | C4 Definitive | C5 Definitive | Total | |
|---|---|---|---|---|---|---|
| C1 ROSE | 624 (92.58%) | 13 (7.18%) | 2 (18.18%) | 30 (24.59%) | 18 (1.39%) | 687 |
| C2 ROSE | 15 (2.23%) | 161 (88.95%) | 0 (0%) | 7 (5.74%) | 7 (0.54%) | 190 |
| C3 ROSE | 2 (0.30%) | 1 (0.55%) | 7 (63.64%) | 1 (0.82%) | 3 (0.23%) | 14 |
| C4 ROSE | 28 (4.15%) | 3 (1.66%) | 2 (18.18 %) | 70 (57.38%) | 134 (10.36%) | 237 |
| C5 ROSE | 5 (0.74%) | 3 (1.66%) | 0 (0%) | 14 (11.48%) | 1132 (87.48%) | 1154 |
| Total | 674 | 181 | 11 | 122 | 1294 | 2282 |
| Procedure | Cohen’s Kappa | Standard Error | 95% CI |
|---|---|---|---|
| EBUS-TBNA + cTBNA (lymph node) | 0.8618 | 0.0582 | 0.75–0.97 |
| TBNA (lung) | 0.8980 | 0.0273 | 0.84–0.95 |
| Percutaneous needle aspiration | 0.8552 | 0.0291 | 0.80–0.91 |
| Endobronchial needle aspiration | 0.8905 | 0.2025 | 0.49–1.29 |
| Organ | Cohens’ Kappa | Standard Error | 95% CI |
|---|---|---|---|
| Lung | 0.8817 | 0.0211 | 0.84–0.92 |
| Lymph node | 0.8444 | 0.0547 | 0.73–0.95 |
| Anterior mediastinum | 0.8801 | 0.0831 | 0.72–1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccatosta, L.; Rossi, G.; Gasparini, S.; Ferretti, M.; Mei, F.; Sediari, M.; Barbisan, F.; Goteri, G.; Corbo, G.M.; Di Marco Berardino, A. Validation of a Cytological Classification System for the Rapid On-Site Evaluation (Rose) of Pulmonary and Mediastinal Needle Aspirates. Diagnostics 2022, 12, 2777. https://doi.org/10.3390/diagnostics12112777
Zuccatosta L, Rossi G, Gasparini S, Ferretti M, Mei F, Sediari M, Barbisan F, Goteri G, Corbo GM, Di Marco Berardino A. Validation of a Cytological Classification System for the Rapid On-Site Evaluation (Rose) of Pulmonary and Mediastinal Needle Aspirates. Diagnostics. 2022; 12(11):2777. https://doi.org/10.3390/diagnostics12112777
Chicago/Turabian StyleZuccatosta, Lina, Giulio Rossi, Stefano Gasparini, Maurizio Ferretti, Federico Mei, Michele Sediari, Francesca Barbisan, Gaia Goteri, Giuseppe Maria Corbo, and Alessandro Di Marco Berardino. 2022. "Validation of a Cytological Classification System for the Rapid On-Site Evaluation (Rose) of Pulmonary and Mediastinal Needle Aspirates" Diagnostics 12, no. 11: 2777. https://doi.org/10.3390/diagnostics12112777
APA StyleZuccatosta, L., Rossi, G., Gasparini, S., Ferretti, M., Mei, F., Sediari, M., Barbisan, F., Goteri, G., Corbo, G. M., & Di Marco Berardino, A. (2022). Validation of a Cytological Classification System for the Rapid On-Site Evaluation (Rose) of Pulmonary and Mediastinal Needle Aspirates. Diagnostics, 12(11), 2777. https://doi.org/10.3390/diagnostics12112777







