Reduction in Radiation Exposure of CT Perfusion by Optimized Imaging Timing Using Temporal Information of the Preceding CT Angiography of the Carotid Artery in the Stroke Protocol
Abstract
:1. Introduction
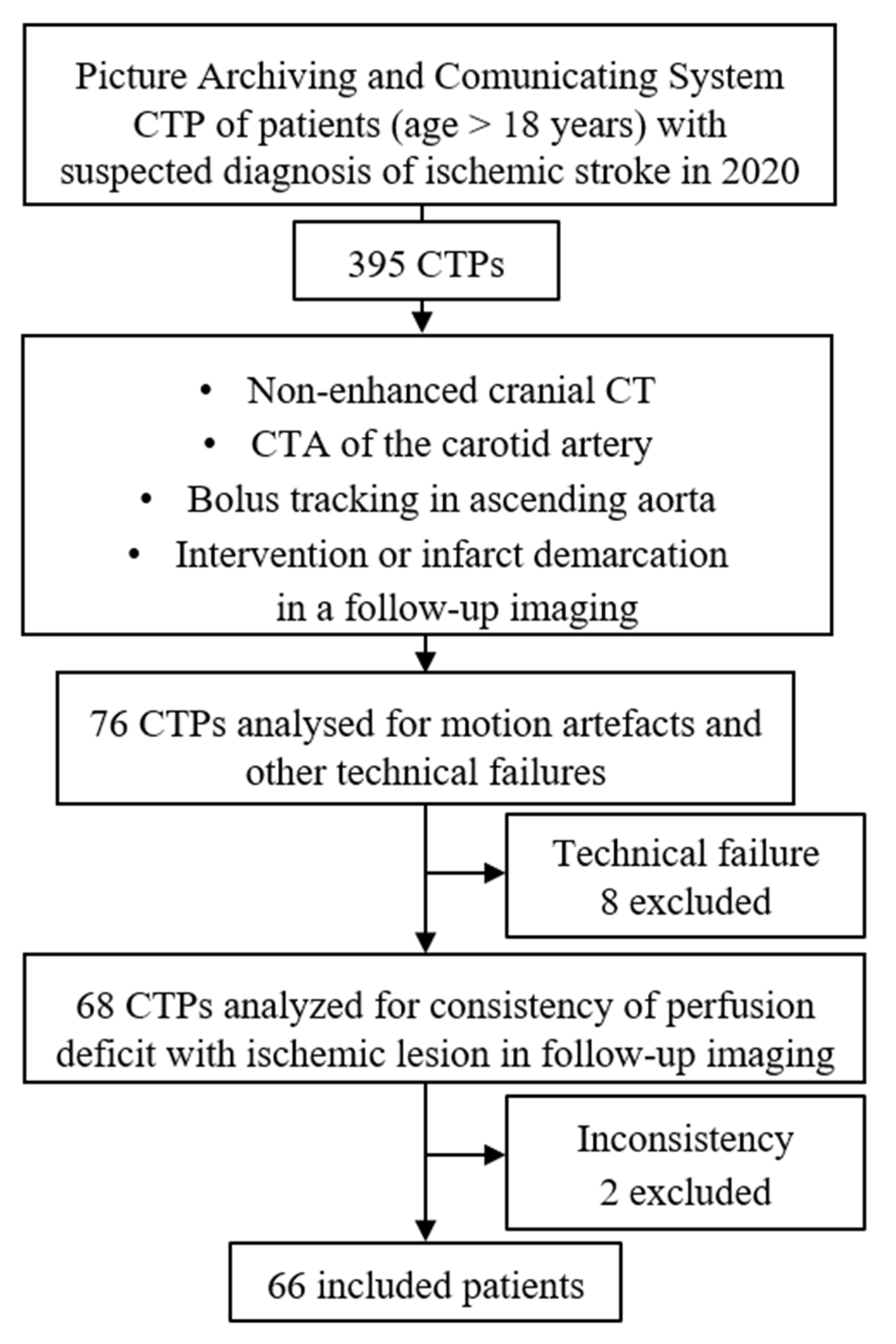
2. Materials and Methods
2.1. Patient Population
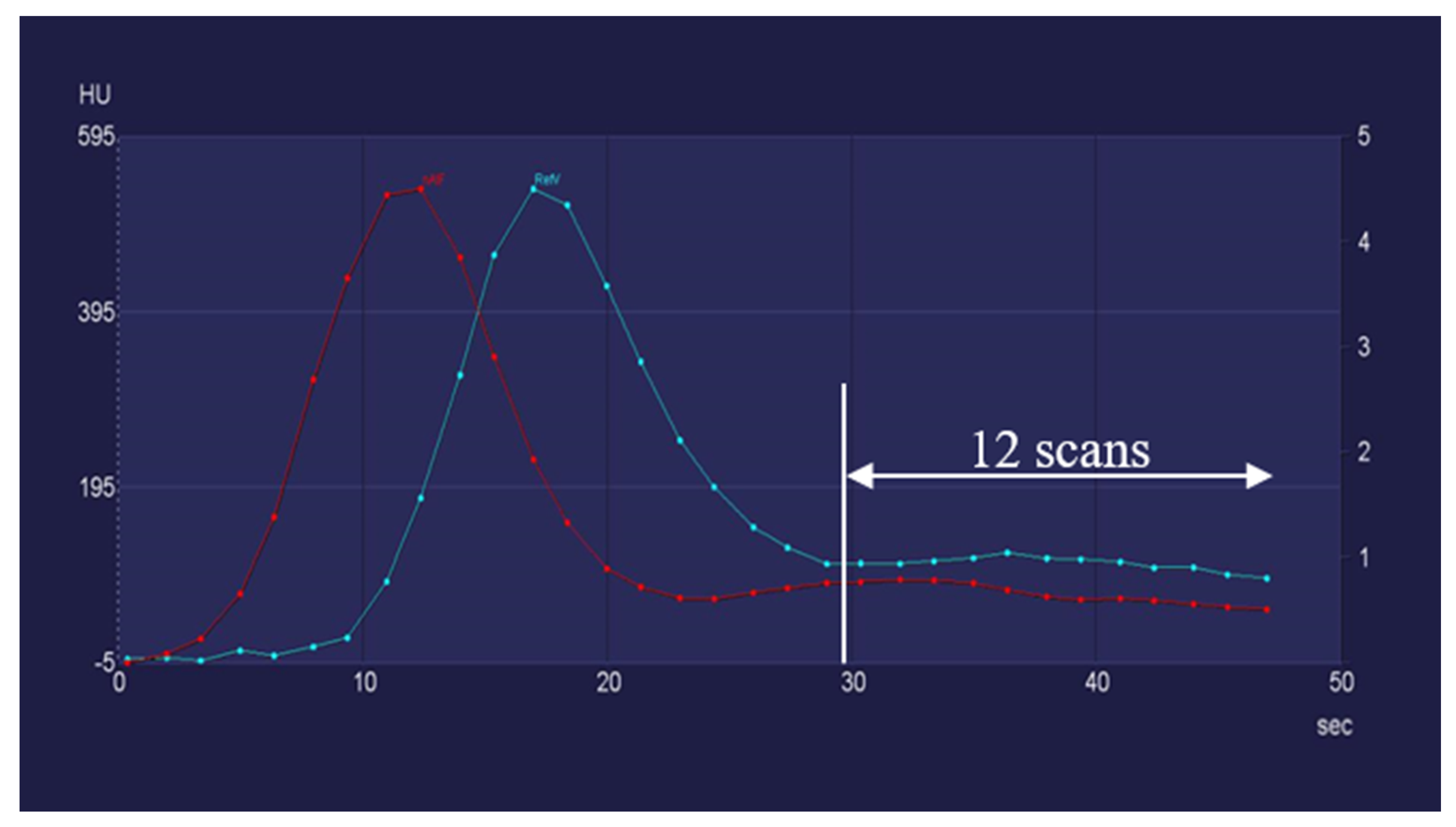
2.2. CT Examination Protocol
2.3. Image Processing and Volumetry
2.4. Study Design
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| CTP | CT perfusion |
| MRI | magnetic resonance imaging |
| PRR | potential recuperation ration |
| oCTP | original CTP |
| sCTP | simulated CTP |
References
- Amiri, H.; Bluhmki, E.; Bendszus, M.; Eschenfelder, C.C.; Donnan, G.A.; Leys, D.; Molina, C.; Ringleb, P.A.; Schellinger, P.D.; Schwab, S.; et al. European Cooperative Acute Stroke Study-4: Extending the time for thrombolysis in emergency neurological deficits ECASS-4: ExTEND. Int. J. Stroke 2016, 11, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Gasparotti, R.; Grassi, M.; Mardighian, D.; Frigerio, M.; Pavia, M.; Liserre, R.; Magoni, M.; Mascaro, L.; Padovani, A.; Pezzini, A. Perfusion CT in Patients with Acute Ischemic Stroke Treated with Intra-Arterial Thrombolysis: Predictive Value of Infarct Core Size on Clinical Outcome. Am. J. Neuroradiol. 2009, 30, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.C.V.; Christensen, S.; Levi, C.; Desmond, P.M.; Donnan, G.; Davis, S.M.; Parsons, M.W. Comparison of Computed Tomography Perfusion and Magnetic Resonance Imaging Perfusion-Diffusion Mismatch in Ischemic Stroke. Stroke 2012, 43, 2648–2653. [Google Scholar] [CrossRef]
- Ribo, M.; Tomasello, A.; Lemus, M.; Rubiera, M.; Vert, C.; Flores, A.; Coscojuela, P.; Pagola, J.; Rodriguez-Luna, D.; Bonet, S.; et al. Maximal Admission Core Lesion Compatible with Favorable Outcome in Acute Stroke Patients Undergoing Endovascular Procedures. Stroke 2015, 46, 2849–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bivard, A.; Levi, C.; Krishnamurthy, V.; McElduff, P.; Miteff, F.; Spratt, N.; Bateman, G.; Donnan, G.; Davis, S.; Parsons, M. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain 2015, 138, 1919–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, A.E.; Afat, S.; Brockmann, M.A.; Nikoubashman, O.; Brockmann, C.; Nikolaou, K.; Wiesmann, M. Radiation dose reduction in perfusion CT imaging of the brain: A review of the literature. J. Neuroradiol. 2016, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Brix, G.; Lechel, U.; Nekolla, E.; Griebel, J.; Becker, C. Radiation protection issues in dynamic contrast-enhanced (perfusion) computed tomography. Eur. J. Radiol. 2015, 84, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Eller, A.; Wiesmüller, M.; Wüst, W.; Heiss, R.; Kopp, M.; Saake, M.; Brand, M.; Uder, M.; May, M. Carotid CTA at the Lowest Tube Voltage (70 kV) in Comparison with Automated Tube Voltage Adaption. Am. J. Neuroradiol. 2019, 40, 1374–1382. [Google Scholar] [CrossRef]
- Zensen, S.; Guberina, N.; Opitz, M.; Köhrmann, M.; Deuschl, C.; Forsting, M.; Wetter, A.; Bos, D. Radiation exposure of computed tomography imaging for the assessment of acute stroke. Neuroradiology 2020, 63, 511–518. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418, Correction in Stroke 2019, 50, e440–e441. [Google Scholar] [CrossRef]
- Dorn, F.; Liebig, T.; Muenzel, D.; Meier, R.; Poppert, H.; Rummeny, E.J.; Huber, A. Order of CT stroke protocol (CTA before or after CTP): Impact on image quality. Neuroradiology 2011, 54, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Abels, B.; Klotz, E.; Tomandl, B.; Kloska, S.; Lell, M. Perfusion CT in Acute Ischemic Stroke: A Qualitative and Quantitative Comparison of Deconvolution and Maximum Slope Approach. Am. J. Neuroradiol. 2010, 31, 1690–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, E.; König, M. Perfusion measurements of the brain: Using dynamic CT for the quantitative assessment of cerebral ischemia in acute stroke. Eur. J. Radiol. 1999, 30, 170–184. [Google Scholar] [CrossRef]
- Koenig, M.; Kraus, M.; Theek, C.; Klotz, E.; Gehlen, W.; Heuser, L. Quantitative Assessment of the Ischemic Brain by Means of Perfusion-Related Parameters Derived from Perfusion CT. Stroke 2001, 32, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, J.; Berkhemer, O.A.; Roos, Y.B.; van Bavel, E.; van Zwam, W.H.; van Oostenbrugge, R.J.; van Walderveen, M.A.; Lingsma, H.F.; van der Lugt, A.; Dippel, D.W.; et al. Value of Computed Tomographic Perfusion–Based Patient Selection for Intra-Arterial Acute Ischemic Stroke Treatment. Stroke 2015, 46, 3375–3382. [Google Scholar] [CrossRef]
- Karwacki, G.M.; Vögele, S.; Blackham, K.A. Dose reduction in perfusion CT in stroke patients by lowering scan frequency does not affect automatically calculated infarct core volumes. J. Neuroradiol. 2019, 46, 351–358. [Google Scholar] [CrossRef]
- Albers, G.W.; Thijs, V.N.; Wechsler, L.; Bs, S.K.; Schlaug, G.; Skalabrin, E.; Bammer, R.; Kakuda, W.; Lansberg, M.G.; Shuaib, A.; et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 2006, 60, 508–517. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Straka, M.; Kemp, S.; Mlynash, M.; Wechsler, L.R.; Jovin, T.G.; Wilder, M.J.; Lutsep, H.L.; Czartoski, T.J.; Bernstein, R.A.; et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol. 2012, 11, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Solitaire™ with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) Trial: Protocol for a Randomized, Controlled, Multicenter Study Comparing the Solitaire Revascularization Device with IV tPA with IV tPA Alone in Acute Ischemic Stroke. Int. J. Stroke 2015, 10, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Palm, F.; Urbanek, C.; Wolf, J.; Buggle, F.; Kleemann, T.; Hennerici, M.; Inselmann, G.; Hagar, M.; Safer, A.; Becher, H.; et al. Etiology, Risk Factors and Sex Differences in Ischemic Stroke in the Ludwigshafen Stroke Study, a Population-Based Stroke Registry. Cerebrovasc. Dis. 2011, 33, 69–75. [Google Scholar] [CrossRef]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef]
- Othman, A.E.; Brockmann, C.; Yang, Z.; Kim, C.; Afat, S.; Pjontek, R.; Nikobashman, O.; Brockmann, M.A.; Kim, J.H.; Wiesmann, M. Effects of radiation dose reduction in Volume Perfusion CT imaging of acute ischemic stroke. Eur. Radiol. 2015, 25, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, M.; Berg, S.; Bohner, G.; Klingebiel, R.; Schöpf, V.; Stoeckelhuber, B.M.; Yousry, I.; Linn, J.; Missler, U. Dose reduction in dynamic perfusion CT of the brain: Effects of the scan frequency on measurements of cerebral blood flow, cerebral blood volume, and mean transit time. Eur. Radiol. 2008, 18, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.M.; van der Schaaf, I.C.; Riordan, A.J.; de Jong, H.W.A.M.; Horsch, A.D.; Eijspaart, D.; Smit, E.J.; Mali, W.P.T.M.; Velthuis, B.K. Radiation dose reduction in cerebral CT perfusion imaging using iterative reconstruction. Eur. Radiol. 2013, 24, 484–493. [Google Scholar] [CrossRef]
- Wintermark, M.; Flanders, A.E.; Velthuis, B.; Meuli, R.; Van Leeuwen, M.; Goldsher, D.; Pineda, C.; Serena, J.; van der Schaaf, I.; Waaijer, A.; et al. Perfusion-CT assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006, 37, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wintermark, M.; Smith, W.S.; Ko, N.U.; Quist, M.; Schnyder, P.; Dillon, W.P. Dynamic Perfusion CT: Optimizing the Temporal Resolution and Contrast Volume for Calculation of Perfusion CT Parameters in Stroke Patients. Am. J. Neuroradiol. 2004, 25, 720–729. [Google Scholar]
- Kasasbeh, A.S.; Christensen, S.; Straka, M.; Mishra, N.; Mlynash, M.; Bammer, R.; Albers, G.W.; Lansberg, M.G. Optimal Computed Tomographic Perfusion Scan Duration for Assessment of Acute Stroke Lesion Volumes. Stroke 2016, 47, 2966–2971. [Google Scholar] [CrossRef] [Green Version]
- Corcuera-Solano, I.; McLellan, A.M.; Doshi, A.H.; Pawha, P.S.; Tanenbaum, L.N. Whole-Brain Adaptive 70-kVp Perfusion Imaging with Variable and Extended Sampling Improves Quality and Consistency While Reducing Dose. Am. J. Neuroradiol. 2014, 35, 2045–2051. [Google Scholar] [CrossRef]




| Simulated CTP30 | Simulated CTP28 | Simulated CTP26 | Simulated CTP24 | Simulated CTP22 | Simulated CTP20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Scans | 30 | 28 | 26 | 24 | 22 | 20 | |||||||
| CTDI | 183.9 mGy | 171.6 mGy | 159.4 mGy | 147.1 mGy | 134.8 mGy | 122.6 mGy | |||||||
| Infarct core (mL) | Mean | 33.01 | p value = 0.1930 | 37.52 | p value = 0.8880 | 36.97 | p value = 0.0564 | 38.58 | p value = 0.0001 * | 39.03 | p value = 0.0024 * | 41.57 | p value < 0.0001 * |
| Standard deviation | 29.48 | 36.65 | 36.34 | 36.79 | 38.07 | 38.19 | |||||||
| Minimum | 8.31 | 6.34 | 6.16 | 6.04 | 3.49 | 6.00 | |||||||
| Maximum | 174.75 | 219.21 | 222.55 | 226.50 | 228.6 | 225.87 | |||||||
| Penumbra (mL) | Mean | 118.86 | p value = 0.1215 | 132.68 | p value = 0.3609 | 131.64 | p value = 0.0508 | 119.82 | p value < 0.0001 * | 109.86 | p value < 0.0001 * | 102.96 | p value < 0.0001 * |
| Standard deviation | 59.86 | 62.37 | 57.85 | 54.23 | 52.55 | 49.29 | |||||||
| Minimum | 46.17 | 43.39 | 43.84 | 40.73 | 11.82 | 2.26 | |||||||
| Maximum | 174.75 | 326.08 | 317.44 | 296.27 | 175.05 | 245.10 | |||||||
| Potential Recuperation Ratio | Mean | 78.0% | p value = 0.7369 | 77.0% | p value = 0.7071 | 78.7% | p value = 0.7805 | 76.6% | p value < 0.0001 * | 74.6% | p value < 0.0001 | 71.2% | p value < 0.0001 * |
| Standard deviation | 13.2% | 16.7% | 13.3% | 13.6% | 14.2% | 17.8% | |||||||
| Minimum | 35.5% | 0.8% | 33.7% | 28.9% | 27.3% | 8.8% | |||||||
| Maximum | 95.4% | 96.0% | 96.5% | 93.6% | 92.9% | 92.8% | |||||||
| sCTP30 | sCTP28 | sCTP26 | sCTP24 | sCTP22 | sCTP20 | ||
|---|---|---|---|---|---|---|---|
| Number of Scans | 30 | 28 | 26 | 24 | 22 | 20 | |
| Percentage of patients with a difference in PRR value of | ≤1% | 84.5% | 58.5% | 55.4% | 24.2% | 15.2% | 10.6% |
| ≤3% | 96.9% | 86.2% | 84.8% | 50.0% | 36.4% | 22.7% | |
| ≤5% | 100.0% | 100.0% | 100.0% | 75.8% | 62.1% | 40.9% | |
| ≤10% | 100.0% | 100.0% | 100.0% | 97.0% | 78.8% | 66.7% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deak, Z.; Schuettoff, L.; Lohse, A.-K.; Fabritius, M.; Reidler, P.; Forbrig, R.; Kunz, W.; Dimitriadis, K.; Ricke, J.; Sabel, B. Reduction in Radiation Exposure of CT Perfusion by Optimized Imaging Timing Using Temporal Information of the Preceding CT Angiography of the Carotid Artery in the Stroke Protocol. Diagnostics 2022, 12, 2853. https://doi.org/10.3390/diagnostics12112853
Deak Z, Schuettoff L, Lohse A-K, Fabritius M, Reidler P, Forbrig R, Kunz W, Dimitriadis K, Ricke J, Sabel B. Reduction in Radiation Exposure of CT Perfusion by Optimized Imaging Timing Using Temporal Information of the Preceding CT Angiography of the Carotid Artery in the Stroke Protocol. Diagnostics. 2022; 12(11):2853. https://doi.org/10.3390/diagnostics12112853
Chicago/Turabian StyleDeak, Zsuzsanna, Lara Schuettoff, Ann-Kathrin Lohse, Matthias Fabritius, Paul Reidler, Robert Forbrig, Wolfgang Kunz, Konstantin Dimitriadis, Jens Ricke, and Bastian Sabel. 2022. "Reduction in Radiation Exposure of CT Perfusion by Optimized Imaging Timing Using Temporal Information of the Preceding CT Angiography of the Carotid Artery in the Stroke Protocol" Diagnostics 12, no. 11: 2853. https://doi.org/10.3390/diagnostics12112853
APA StyleDeak, Z., Schuettoff, L., Lohse, A.-K., Fabritius, M., Reidler, P., Forbrig, R., Kunz, W., Dimitriadis, K., Ricke, J., & Sabel, B. (2022). Reduction in Radiation Exposure of CT Perfusion by Optimized Imaging Timing Using Temporal Information of the Preceding CT Angiography of the Carotid Artery in the Stroke Protocol. Diagnostics, 12(11), 2853. https://doi.org/10.3390/diagnostics12112853







