Abstract
Eosinophilic esophagitis (EoE) is a chronic, immune-mediated inflammatory disease, characterized by esophageal dysfunction and intense eosinophil infiltration localized in the esophagus. In recent decades, EoE has become a growing concern as a major cause of dysphagia and food impaction in adolescents and adults. EoE is a clinicopathological disease for which the histological demonstration of esophageal eosinophilia is essential for diagnosis. Therefore, the recognition of the characteristic endoscopic features with subsequent biopsy are critical for early definitive diagnosis and treatment, in order to prevent complications. Accumulating reports have revealed that EoE has several non-specific characteristic endoscopic findings, such as rings, furrows, white exudates, stricture/narrowing, edema, and crepe-paper esophagus. These findings were recently unified under the EoE endoscopic reference score (EREFS), which has been widely used as an objective, standard measurement for endoscopic EoE assessment. However, the diagnostic consistency of those findings among endoscopists is still inadequate, leading to underdiagnosis or misdiagnosis. Some endoscopic findings suggestive of EoE, such as multiple polypoid lesions, caterpillar sign, ankylosaurus back sign, and tug sign/pull sign, will aid the diagnosis. In addition, image-enhanced endoscopy represented by narrow band imaging, endocytoscopy, and artificial intelligence are expected to render endoscopic diagnosis more efficient and less invasive. This review focuses on suggestions for endoscopic assessment and biopsy, including recent advances in optical technology which may improve the diagnosis of EoE.
1. Introduction
Eosinophilic gastrointestinal disorders (EGIDs) are refractory gastrointestinal inflammatory entities that cause chronic eosinophilic inflammation and dysfunction in the gastrointestinal tract based on excessive Th2-dominant immunological responses to dietary antigens [1]. EGIDs are broadly classified into eosinophilic esophagitis (EoE), in which the inflammation is confined to the esophagus, and eosinophilic gastroenteritis (EGE), in which the gastrointestinal tract is widely affected, irrespective of esophageal involvement. EoE has been widely recognized as a major cause of dysphagia and food impaction in adolescents and adults, with a dramatically increasing prevalence worldwide over the past several decades [2,3,4,5].
EoE is diagnosed based on the presence of clinical symptoms and histologically-proven esophageal eosinophilia with a peak of ≥15 eosinophils/hpf (~60 eosinophils/mm2), excluding diseases and conditions inducing secondary esophageal eosinophilia such as EGE, hyper eosinophilic syndrome, drug-induced esophagitis or achalasia [6]. Thus, EoE is a clinicopathological disease that requires histopathological evidence of intense intraepithelial eosinophils for a definitive diagnosis. Therefore, it is crucial for diagnosis that endoscopists master EoE’s peculiar endoscopic findings, such as edema, rings, furrows, white exudates and strictures, which represent a main gateway for its identification [7]. The EoE endoscopic reference score (EREFS) is expected to standardize the endoscopic assessment, and thus improve diagnostic accuracy [8]. However, these findings are not always specific to EoE and vary in prevalence, degree, and distribution within the esophagus. More notably, up to 10% of patients with EoE have a normal-appearing esophagus [7]. This is another challenge, represented by the low diagnostic agreement among and within endoscopists. Therefore, despite the development and revisions of clinical guidelines and consensus, endoscopic findings have not yet been incorporated into the diagnostic criteria for EoE [9,10,11,12]. Several efforts to improve recognition and diagnostic accuracy of endoscopic abnormalities using image enhancement endoscopy (IEE), endocytoscopy, and artificial intelligence (AI) have been recently reported. In this article, we review the basics and the most recent advances and challenges in the endoscopic diagnosis of EoE.
2. Typical Presentation of EoE with Food Impaction
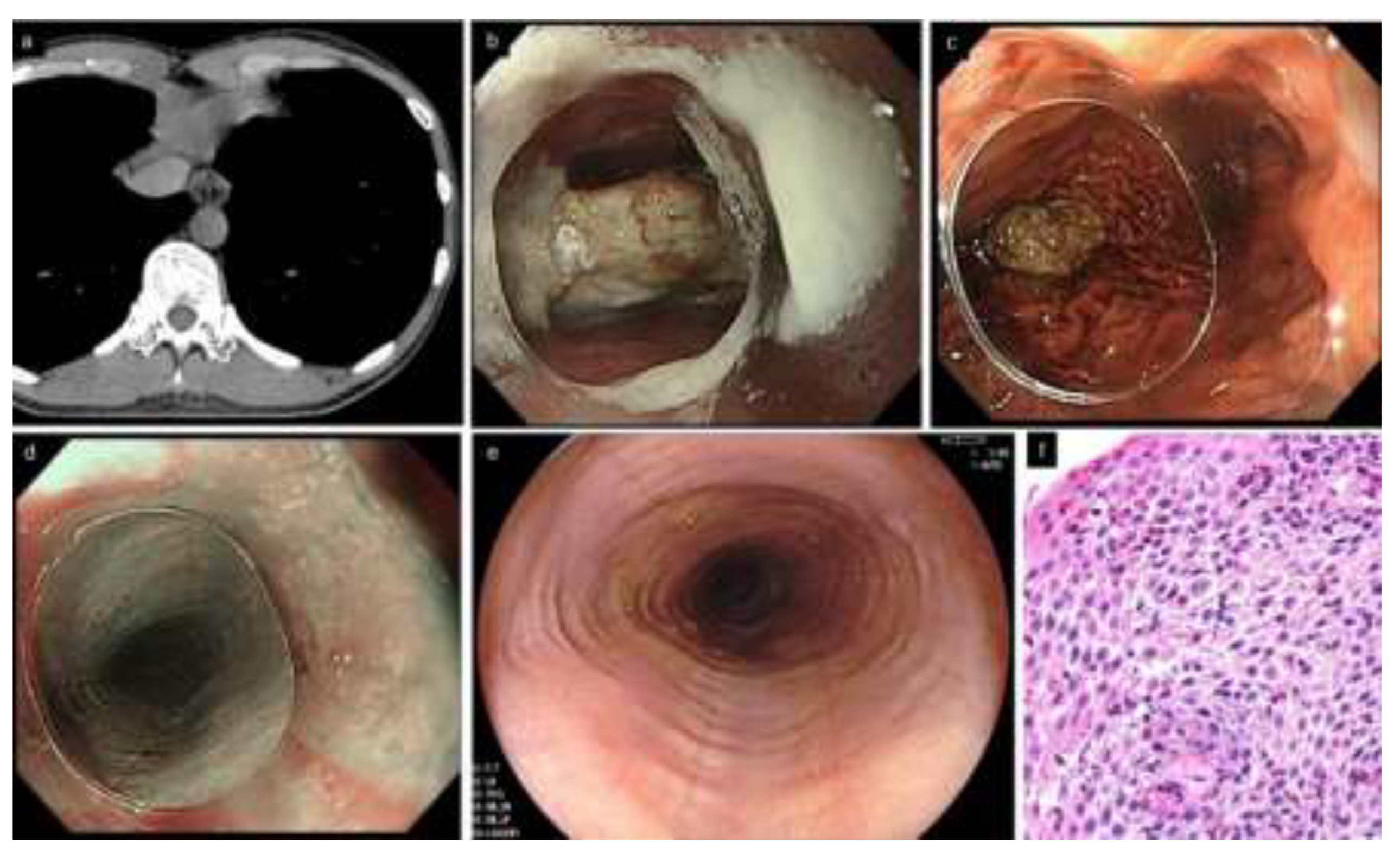
EoE is also referred to as steak house syndrome, as the most commonly impacted foods are meat products [13,14]. Upon urgent endoscopy for a patient with food impaction, adhesion of food residues on the esophageal mucosa, or reflux of gastric contents into the esophagus, may prevent clear observation by the endoscopist, even after impacted food removal from the esophagus; thus, they may potentially miss EoE diagnosis. The food bolus is successfully removed in the majority of patients at the first endoscopy; however, esophageal biopsies fail to be obtained in up to 40% of the patients undergoing endoscopy for removal of impacted food [13,15,16]. This implies that endoscopists may find it difficult to perform esophageal biopsies in addition to the removal of food bolus in the emergency setting. Other reports found that endoscopy within 6 months to 2 years of initial food impaction was performed only in 45–78% of cases, and esophageal biopsies were obtained only in 28–76% of cases [16,17,18]. To avoid missing EoE, it is critical to perform re-endoscopy in patients with food impaction as soon as possible (Figure 1). The American Society for Gastrointestinal Endoscopy consensus statements recommend that biopsy be performed, if possible, at initial food impaction in suspected EoE, avoiding the impaction site which may be potentially injured [19].
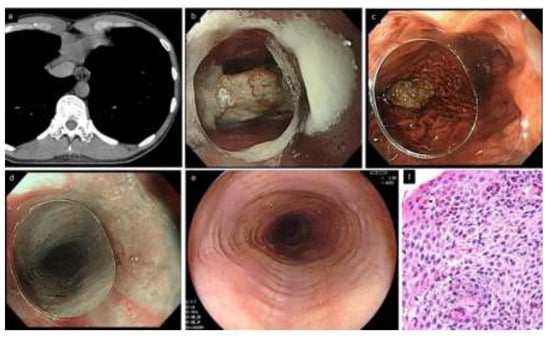
Figure 1.
A typical patient with EoE complicated by food impaction (33 years old, male). (a). Chest CT. Esophageal lumen is dilated and filled with structures that appear to be impacted food. (b,c). Urgent endoscopy. Food bolus (piece of meat) is present in the middle esophagus. Impacted food is removed by letting it drop into the stomach, pushing with an endoscope equipped with a clear distal attachment hood. (d). Subtle rings and furrows are visible by narrow band imaging, suspicious of EoE. (e). Rings and furrows are more distinctly observed in the re-endoscopy after one week without PPI. (f). Esophageal biopsies show a peak of 52 eosinophils/hpf. EoE, eosinophilic esophagitis; PPI, proton pump inhibitor.
Notably, proton pump inhibitors (PPI) used prior to endoscopic examination can mask EoE endoscopically and histologically [20]. Hillman et al. estimated that EoE was missed in 1 of 5 patients with food impaction biopsied while on a PPI [16]. PPIs may also improve acid reflux symptoms, resulting in a reduction in medical visits and an increase in missed EoE cases. Since short-term recurrence of food impaction is uncommon, less than 5% within 1 year, re-endoscopy following discontinuation of medications is recommended [16]. In addition, endoscopists should consider that esophageal mechanical injury, such as esophageal perforation or intramural dissection, may occur, albeit rare, especially in patients complaining of severe pharyngeal and chest pain [21,22]. Since the condition may be exacerbated by performing urgent endoscopy in such patients, computed tomography (CT) and esophagogram should be considered before endoscopy.
3. Disease Concept and Endoscopic Abnormalities
The disease entity of EoE has been distinctly established by the report of Attwood et al. in 1993, in which they described a case series of 12 patients characterized by dysphagia as a chief complaint, young male predominance, no pathological gastro-esophageal reflux, intense, eosinophilic inflammation localized in the esophagus, and normal endoscopy [23]. However, a subsequent large number of endoscopic studies have revealed that EoE displays several non-specific, but abnormal findings [24,25,26]. In many studies around the 1990s to early 2000s, those endoscopic findings have been described by terms as varied as the following: ringed/corrugated esophagus, concentric/fixed rings, esophageal trachealization, feline esophagus, linear/longitudinal furrows, linear fissures, white plaques/exudates, white stipple-like exudates, cobblestone-like pattern, decreased/reduced vascularity, loss of vascularity, edema, stricture/stenosis, narrow/small caliber esophagus, long segment strictures, mucosal fragility, and crepe-paper esophagus [24,25,26,27,28,29,30,31,32,33].
In a previous meta-analysis, EoE was reported to be endoscopically “normal” in 20% of retrospective studies, but the ratio decreased to only 7% in prospective studies [7]. In other words, more than 90% of EoE have no endoscopic abnormalities. However, the diagnostic operating characteristics of each finding have been shown to be unsatisfactory, with a low sensitivity of 15–67% (edema 54%, rings 67%, exudates 47%, furrows 55%, stricture 15%) and a low positive predictive value of 27–60% (edema 49%, rings 60%, exudates 60%, furrows 60%, stricture 27%) against a high specificity of 82–93% (edema 82%, rings 91%, exudates 93%, furrows 93%, stricture 93%) and high negative predictive value of 85–93% (edema 85%, rings 93%, exudates 89%, furrows 92%, stricture 86%) when analyzed in the prospective studies. In contrast, when analyzing the presence of at least one of those five major findings, sensitivity (91%) and negative predictive value (97%) increased, but specificity (65%) and positive predictive value (39%) decreased. Up to 100 reports analyzed in this meta-analysis have been reported from 1985 to 2011 prior to the publication of the EREFS scoring system; therefore, the assessment of endoscopic abnormalities varies considerably among the studies. The lack of objective and unified terms representing endoscopic abnormalities is deemed to have impacted the diagnostic operating characteristics, in addition to the low awareness of disease, low resolution of endoscopy, disease prevalence, and different settings in which endoscopy was performed.
4. EoE Endoscopic Reference Score (EREFS)
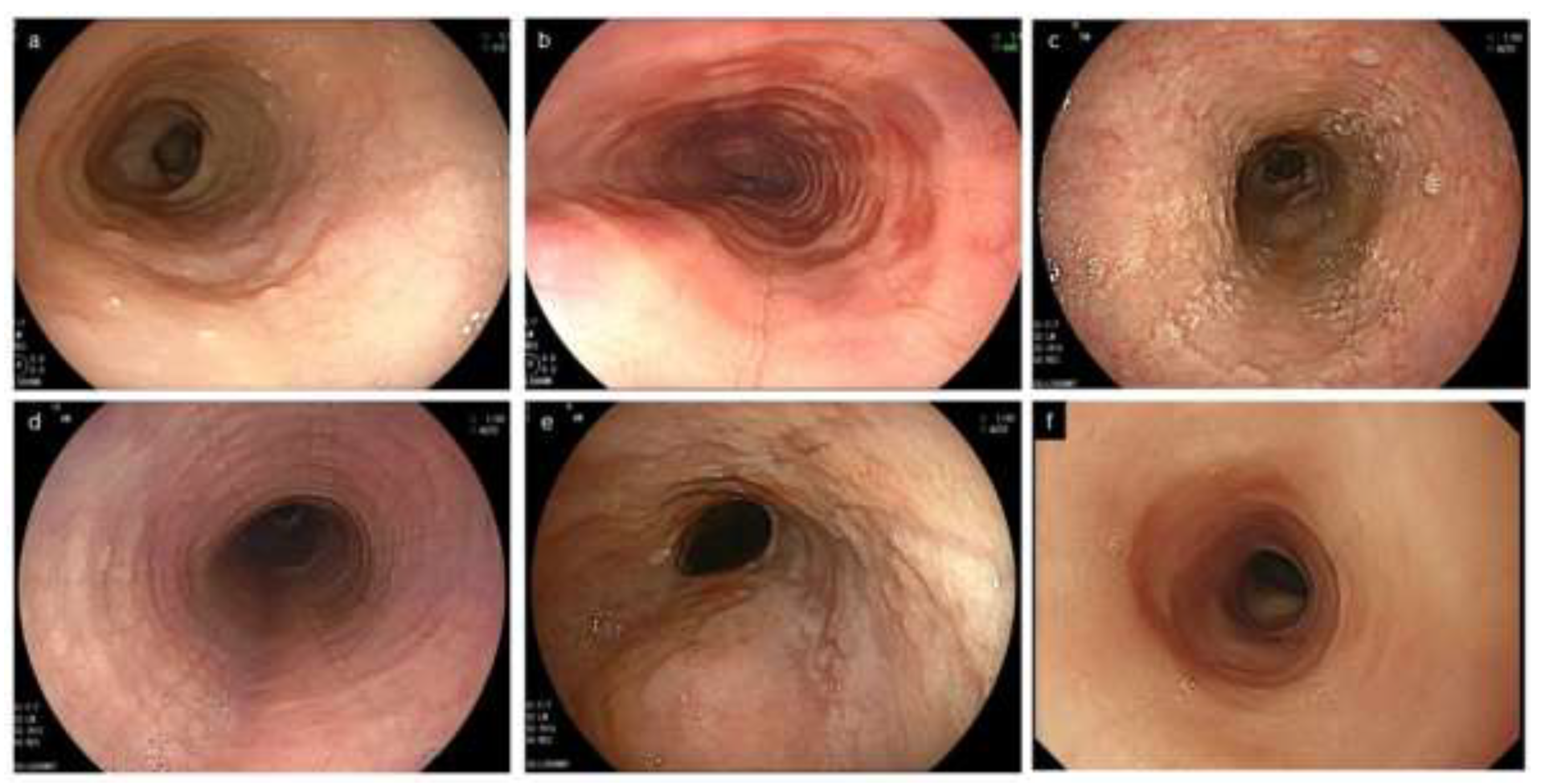
The EoE endoscopic reference score (EREFS) was developed by Hirano et al. in 2013 for objective assessment of endoscopic findings: Edema, Rings, Exudates, Furrows, and Stricture are comprehensively assessed as major features [8]. Soon after, its adjusted version by van Rhijn et al. was reported in 2014 to be useful not only for expert but also for non-expert endoscopists [34] (Figure 2).
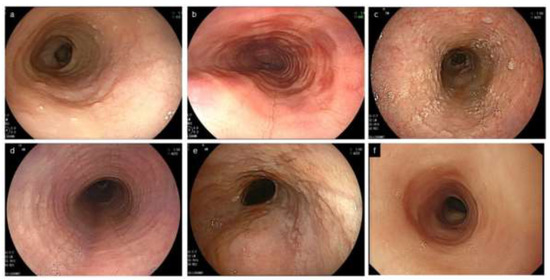
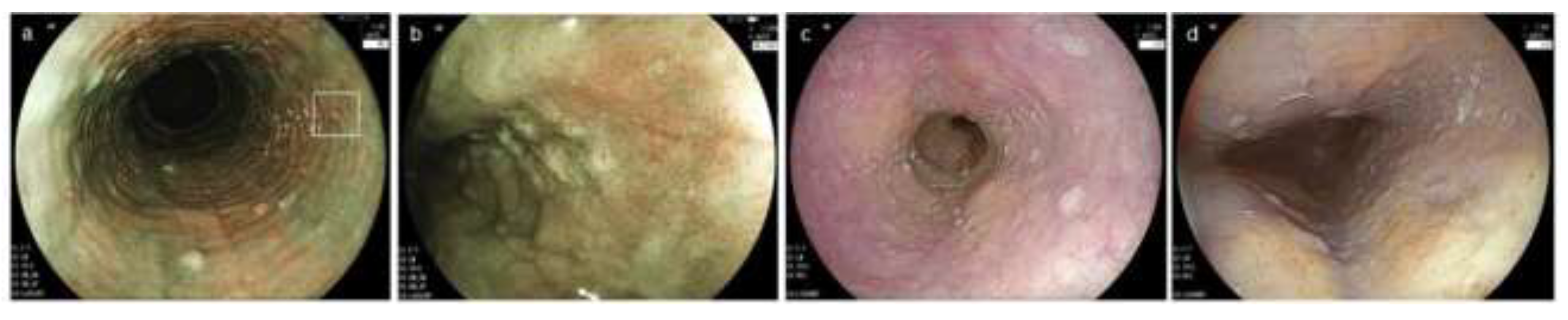
Figure 2.
Characteristic endoscopic findings of EoE. (a). Edema. Decreased vascularity or loss of it is evident. (b). Rings. Multiple concentric rings are almost constitutively present (esophageal trachealization). (c). White exudates. White plaques, difficult to distinguish from Candida, are present. (d). Furrows. Multiple crack-like lines running longitudinally are presented. (e). Stricture. Web-like appearance with proximal esophageal dilatation is displayed. (f). Narrow caliber esophagus. Esophageal narrowing with tubular-appearing lumen is demonstrated. EoE, eosinophilic esophagitis.
The EREFS acronym consists of the initials of the aforementioned five endoscopic findings. Since the first E (Edema) and second E (Exudates) may be confused, the term ERExFS may be preferred [35]. Ma et al. compared the original, simplified, expanded furrows and fully expanded EREFS classification, using paired endoscopic videos from patients with EoE before and after treatment [36]. The original EREFS, the simplified EREFS, and the fully expanded EREFS are totally scored with ranges of 0 to 8, 0 to 7, and 0 to 11, respectively (Table 1). When the distribution and the extent of endoscopic abnormalities are heterogeneous within the esophagus, it is common for EREFS scores to be assessed in the most affected area [19].

Table 1.
The EoE endoscopic reference score (EREFS).
4.1. Major Features of EREFS
Edema, used synonymously with loss of or decreased vascularity, is quite an unspecific finding seen in many other esophageal conditions, including GERD, and has endoscopically lower diagnostic agreement compared with other findings [8,34].
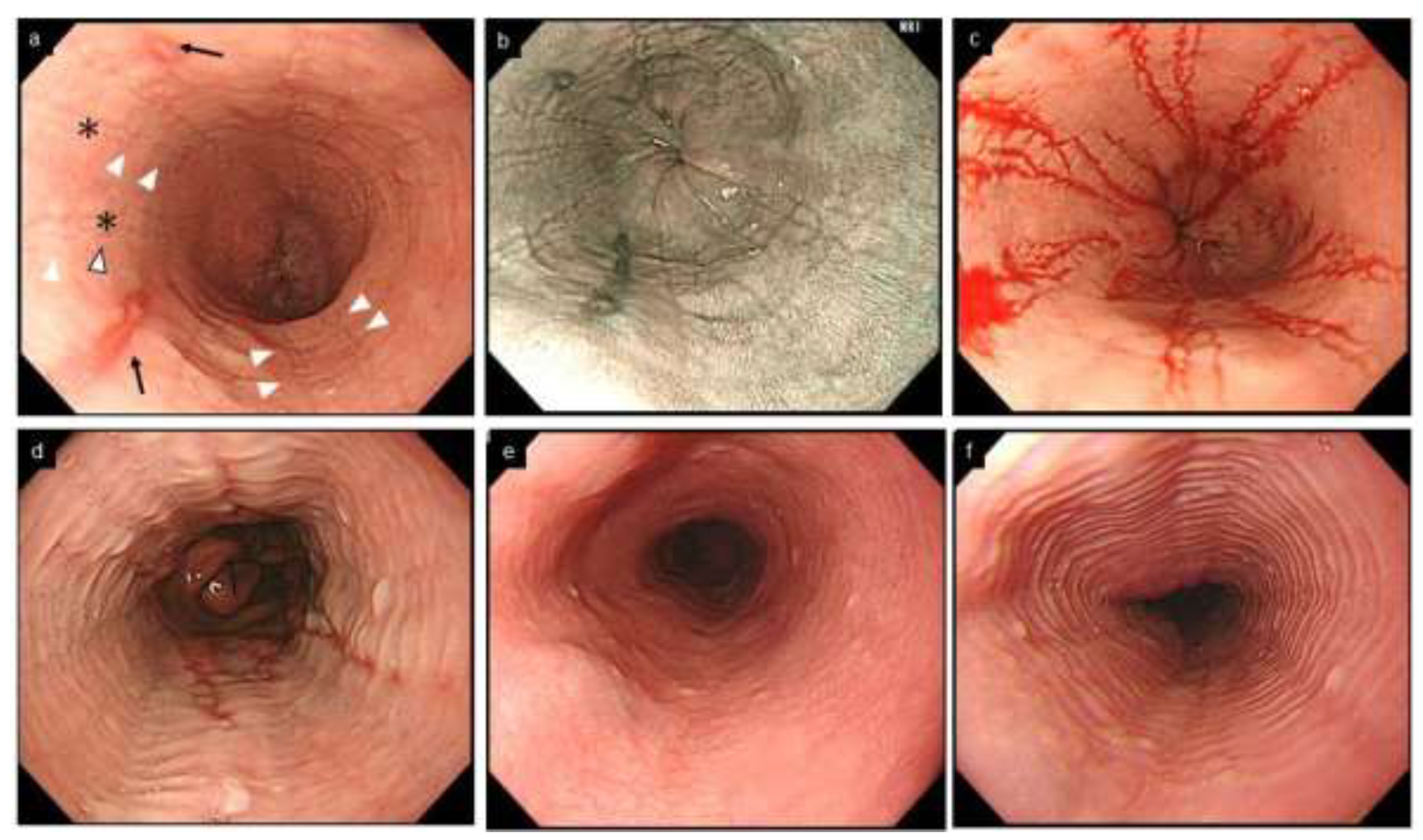
Rings occur as a result of esophageal remodeling with subepithelial fibrosis as well stricture, and correlate with a risk of food impaction attributed to impaired esophageal distensibility [37]. The severity of rings does not correlate with the number of infiltrating eosinophils, and tends to remain after histologic improvement [38,39]. It is known that rings are also observed in up to 10% of non-EoE esophagitis, including GERD [30,40,41] (Figure 3).
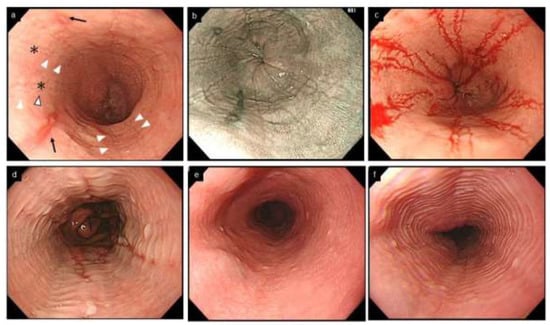
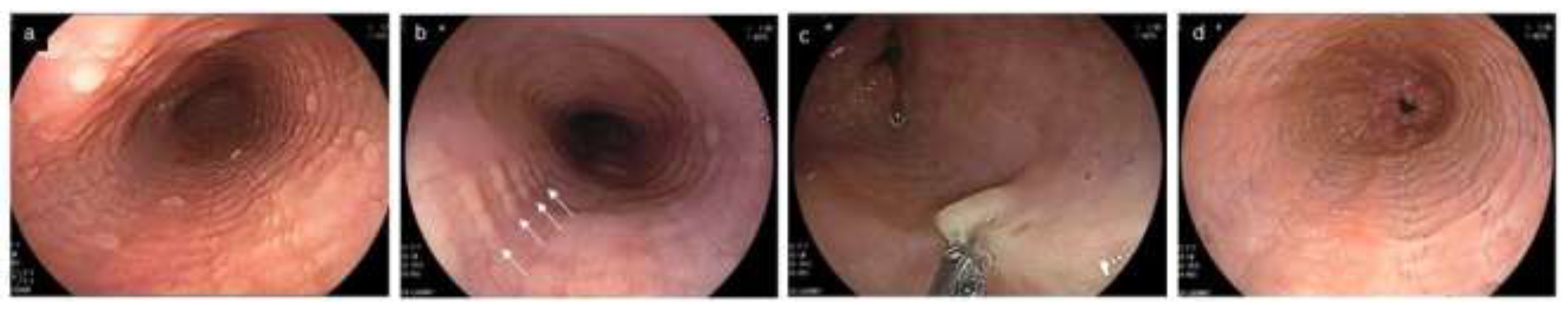
Figure 3.
Esophageal erosion, furrows and rings in GERD and EoE, and feline esophagus in normal esophagus. (a,b). Coexistence of furrows in EoE (arrowhead) and longitudinal erosion in GERD (black arrow). Biopsy should be obtained just above on furrows (asterisk). (c). The visibility of furrows is enhanced after esophageal biopsies with blood pouring on the furrows. (a–c, same patient; a, WLI; b, NBI). (d). Mild rings observed in erosive esophagitis. (e,f). Feline esophagus. Transient and subtle concentric rings are observed in the normal esophagus (e,f, same patient). EoE, eosinophilic esophagitis; GERD, gastroesophageal reflux disease; WLI, white light imaging; NBI, narrow band imaging.
Exudates appear as white plaques or white spots, corresponding to histological eosinophilic microabscesses [42]. Attention should be paid, in the assessment of exudates, to the coexistence of other EoE characteristic findings for the differential diagnosis with candidiasis, as both conditions are typical of dysphagia [43].
Furrows are crack-like fissures longitudinally running in the esophagus, different from the epithelial erosions seen in GERD (Figure 3). Furrows are more easily recognized by decreasing the tension of the esophageal lumen with air deflation, or by employing indigo carmine spray or by blood falling into the fissure after biopsy [44] (Figure 3). EoE and erosive esophagitis may coexist due to their interactional pathophysiology, and EoE is diagnosed if esophageal eosinophilia with a peak of ≥15 eosinophils/hpf is present on biopsies [45].
Stricture with EoE, unlike that in GERD, can occur at a more proximal site of the esophagus [30,32,46]. In clinical practice, the appearance of a stricture may be almost synonymous with the narrow/small caliber esophagus described below.
Considering their relevant pathophysiology, rings and stricture are separately assessed as fibrostenotic changes, apart from the remaining three inflammatory changes edema, exudates, and furrows. [46,47].
The optimal cutoff value for the ERERS score to predict EoE has not been determined. Some reports showed that an EREFS ≥ 2 can predict EoE with relatively high sensitivity and specificity [38,48], while others suggested that it was not sufficient to discriminate between EoE and non-EoE, due to low specificity against high sensitivity [49]. This discrepancy may be due to differences in the endoscopic severity of the EoE group enrolled in each study, and the clinical profiles of the non-EoE comparative groups. Endoscopists should also be aware that endoscopic disease activity by EREFS score does not correlate well with clinical symptoms or histological activity [50,51].
4.2. Minor Features of EREFS
Feline esophagus, narrow caliber esophagus, and crepe paper esophagus were considered in the EREFS as minor features [8].
Feline esophagus is a term derived from the morphological similarity to a cat’s esophagus, in which multiple rings are physiologically present. However, this term now refers to fine and subtle contractile rings observed transiently or intermittently during endoscopy in non-EoE conditions, including normal esophagus [8,52,53] (Figure 3).
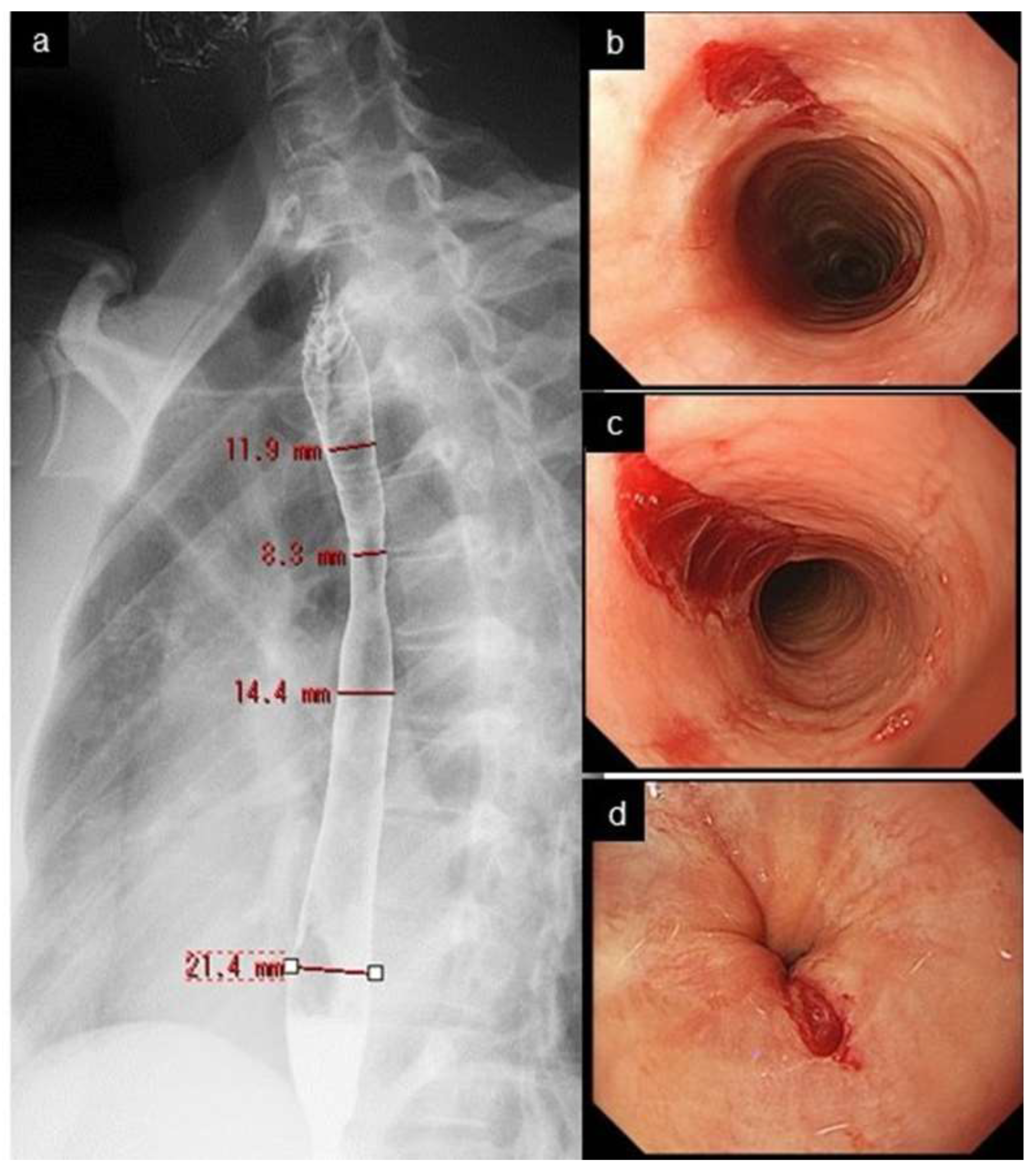
Narrow/small caliber esophagus indicates the diffuse narrowing of the tubular esophagus with a certain longitudinal length, which is different from the strictures seen in GERD or in postoperative anastomotic stricture, presenting as shorter, localized narrowing along with proximal esophageal dilatation. These two conditions are not clearly defined or differentiated from each other [54,55]. Carlson et al. proposed an arbitrary definition: narrow caliber esophagus is defined as an esophageal diameter < 18 mm for > 50% of the length of the organ [55]. Endoscopy had a low sensitivity of 25% in detecting esophageal narrowing, with a radiographic maximal diameter of ≤18 mm [54]. Endoscopists need to know that narrow caliber esophagus is less successfully assessed by endoscopy, compared with a barium esophagogram [56,57] (Figure 2 and Figure 4).
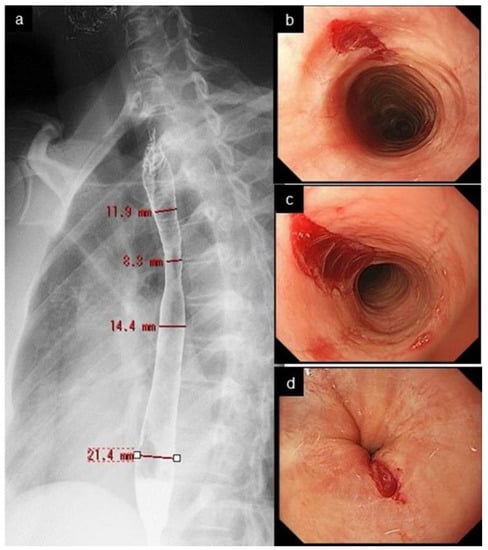
Figure 4.
Narrow caliber esophagus and crepe-paper esophagus. Esophagogram shows narrow caliber esophagus from the upper to mid esophagus, with a diameter of 8.3–14.4 mm. (a). Mucosal tear developed at upper (b), mid (c), and lower end (d) of the esophagus after regular passage of peroral endoscope.
Crepe-paper esophagus is an uncommon but pathognomonic finding with EoE, presenting with friable, delicate esophageal mucosa which ulcerates after minor trauma, such as by contact with an endoscope (Figure 4). Of those three minor features, crepe-paper esophagus had the highest diagnostic agreement among endoscopists, and was eventually adopted as a minor feature [8].
5. Diagnostic Agreement of Endoscopic Findings and Diagnosis
In EoE, diagnostic delay without effective therapeutic intervention not only exacerbates the degree of esophageal stricture [58], but also leads to impaired quality of life [59], decreased treatment response [60], repeated esophageal dilatation [61], and an increased risk of critical mechanical esophageal injuries, such as perforation [21] and intramural esophageal dissection [22]. Hence, it is critical to diagnose and treat EoE in the earlier phase [62]. To this end, it is crucial to identify endoscopic abnormalities with a high degree of diagnostic agreement, as a trigger for the endoscopist to perform a biopsy.
Previously reported endoscopic diagnostic agreement of individual components of EREFS, and the overall diagnosis of EoE, are summarized in Table 2. Most studies have focused on EoE images using white light, and a few studies have included non-EoE images in the diagnosis of EoE. Although the results cannot be directly compared because of the differences in observers or image evaluation methods among studies, many reports showed relatively good interobserver agreement regarding furrows and rings with white light imaging (WLI): furrows, moderate to excellent (0.48–0.68); rings, fair to excellent (0.34–0.70). In contrast, interobserver agreement was fair to moderate (0.24–0.5) for edema, fair to substantial (0.21–0.65) for exudates, and fair to moderate (0.31–0.5) for stricture; thus, it was lower than that for furrows and rings, with a variation among the studies. The intraobserver agreement in WLI was generally good, ranging from moderate to substantial (0.43–0.78), except for exudates; they were fair (0.28) in a report by Izumi et al. [63]. These authors also showed unsatisfactory agreement for the overall diagnosis of EoE with an interobserver agreement of 0.34, and an intraobserver agreement of 0.52 in WLI. In a more recent Japanese study, interobserver and intraobserver agreement have been reported to increase to 0.60 and 0.74 for WLI, respectively. This might reflect an increasing awareness of EoE [48]. The comparison between original EREFS and expanded EREFS by Ma et al. does not show a significant difference in the diagnostic agreement regarding each endoscopic finding [36].

Table 2.
Inter- and intraobserver agreement in the endoscopic diagnosis for EoE.
Over the past decade, image enhanced endoscopy (IEE) such as narrow band imaging (NBI) has been widely used in endoscopic diagnosis, especially for gastrointestinal cancerous lesions [64,65,66]. There have been a few reports investigating the usefulness of IEE for the diagnosis of EoE. Prior to the publication of the EREFS scoring system, Peery et al. reported that NBI had no additional effect on WLI in the diagnosis of EoE, when only endoscopists assessments of WLI images were compared with WLI, plus its counterpart NBI images [67]. Gastroenterologists who participated in that study reported that 50% of the images were better viewed with NBI, 7% of the images were better viewed with WLI, and 43% of the images were undecided on. In other reports using NBI combined with magnifying endoscopy, the esophageal mucosa in patients with EoE had a beige color, increases in dot-like intrapapillary capillary loops, and invisibility of cyan submucosal vessels [68,69] (Figure 5). The beige-colored mucosa under NBI has also been found to correspond to an area with histologically active eosinophilic inflammation [70].

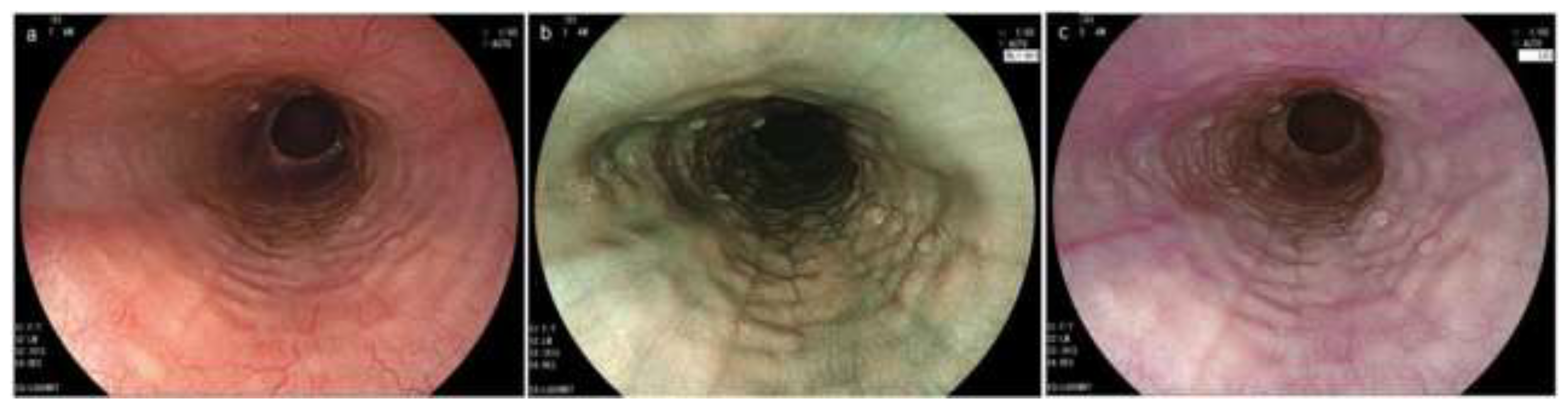
Figure 5.
Endoscopic findings by IEE in EoE. (a). Beige color mucosa (BLI) (b). Magnified image of the area indicated by the white frame in Figure (a). Dot-like intrapapillary capillary loops are seen. Cyan submucosal vessels are invisible (BLI). (c). Yellowish mucosa (LCI, proximal esophagus). (d). Yellowish mucosa (LCI, middle esophagus). IEE, image enhanced endoscopy; EoE, eosinophilic esophagitis; BLI, blue laser imaging; LCI, linked color imaging.
LCI is a newly-developed IEE created by short-wavelength narrow-band laser light combined with white laser light, enabling brighter light in a distant area, and enhancing color differences between red and white [71]. It has been increasingly reported that LCI can improve various esophageal diseases, such as minimal change esophagitis [72,73], erosive esophagitis [74], Barrett’s esophagus, and esophageal adenocarcinoma [75,76,77]. There is a brief report showing that LCI could effectively detect an area of active inflammation, which was observed as yellowish mucosa, compared with cyan or light purple mucosa without active inflammation [78] (Figure 5). We also recently showed that LCI in addition to WLI improved the diagnostic accuracy of the individual endoscopic findings, especially furrows, rings, and stricture, and the overall diagnosis of EoE with “moderate” to “substantial” consistency compared with WLI alone. The improvement in diagnostic accuracy by LCI was more remarkable in cases with milder endoscopic findings than in those with more prominent findings, and in endoscopists with less EoE experience than in those with more EoE experience [48]. It is speculated that LCI potentially improves the visibility of the EoE by increasing the contrast between red and white areas, and illuminating more brightly up to the more distal esophagus (Figure 6). Further investigations are needed to determine whether IEE can improve endoscopic diagnostic agreement for EoE.
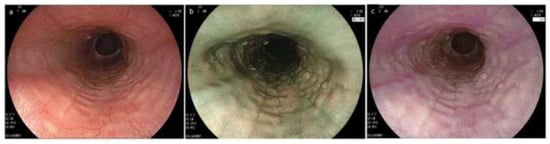
Figure 6.
Endoscopic image sets consisting of WLI, BLI and LCI in EoE. (a) WLI; (b) BLI; (c) LCI (a–c, same patient). Rings and edema in the lower esophagus are more clearly visible in LCI compared to WLI and BLI. EoE, eosinophilic esophagitis; WLI, white light imaging; NBI, narrow band imaging; BLI, blue laser image; LCI, linked color imaging.
6. Other Endoscopic Findings Suggestive of EoE
With an increased awareness of EoE in clinical practice, endoscopic findings suggestive of EoE, other than the characteristic features such as EREFS, have been known to endoscopists. These findings are summarized in Table 3, and their representative images are shown in Figure 7. Several findings, such as multiple polypoid lesions [79,80,81], tug sign/pull sign [82,83], ankylosaurus back sign [84], and caterpillar sign [49] have been reported. In addition, endoscopic abnormalities have been localized in small areas of the lower end of the esophagus, or with patchy distribution in 30–40% of patients [85,86] (Figure 8). Such localized types may be missed, unless the whole esophagus is carefully observed with proper expansion by both air inflation and deep inspiration during endoscopy. Dynamic observation with control of the extensibility of the esophageal wall may help an endoscopist detect milder and localized endoscopic abnormalities (Figure 8). Localized EoE appears to have less symptoms and higher responsiveness to PPI therapy compared with diffuse EoE [87,88], but its etiology and potential progression to diffuse EoE remain to be elucidated. The American Gastroenterological Association has proposed to incorporate into the severity scoring of EoE (in addition to symptoms, complications, and histological features) whether inflammatory features are localized or diffuse, as well as the grade of fibrostenotic features measured by the ability to pass a normal diameter upper endoscope [89].

Table 3.
Characteristics, potential pathology and clinical significance of other endoscopic findings related to EoE.
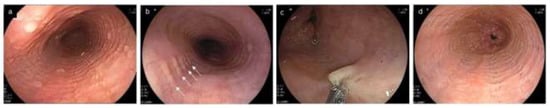
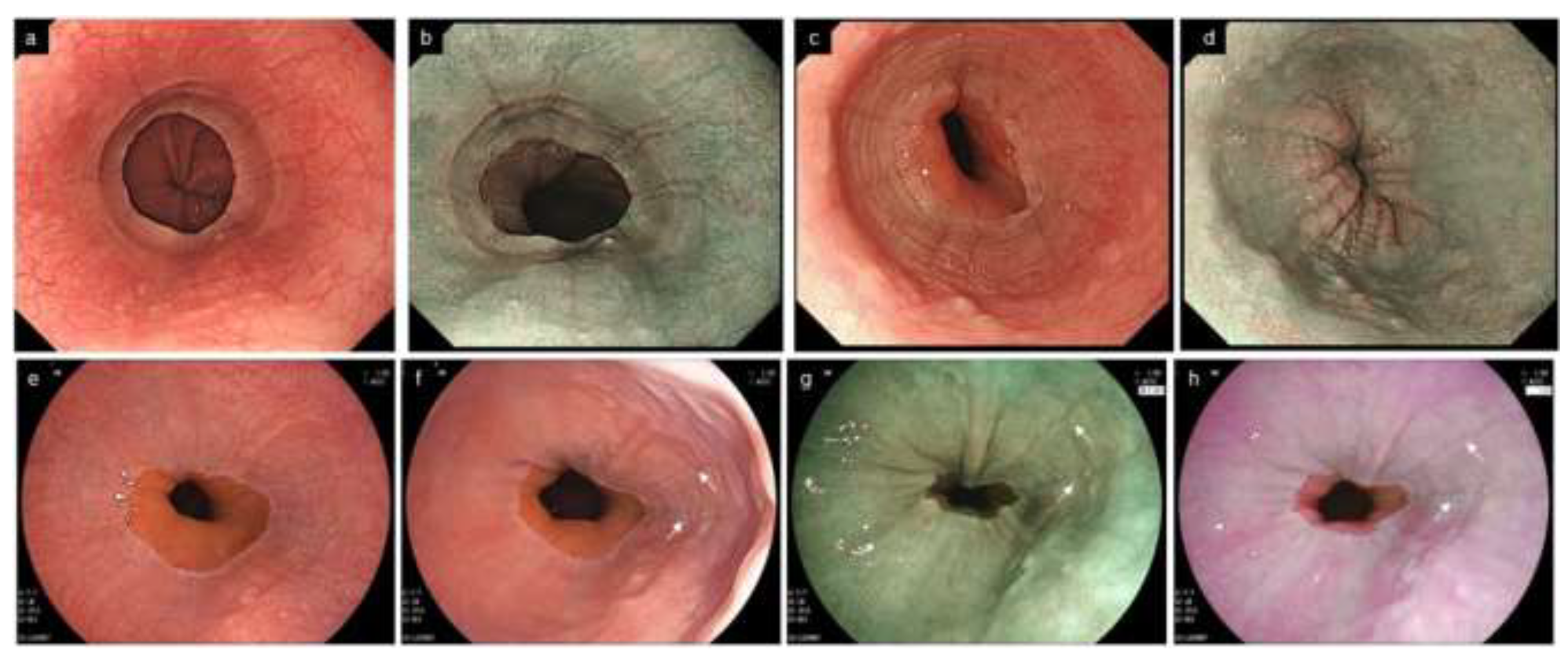
Figure 7.
Other endoscopic findings related to EoE. (a). Multiple polypoid lesions (b). Ankylosaurus back sign (c). Tug sign/pull sign: with pulling by the biopsy forceps, a large amount of esophageal mucosa is lifted as a tent and caught in the forceps opening. (d). Caterpillar sign. EoE, eosinophilic esophagitis.
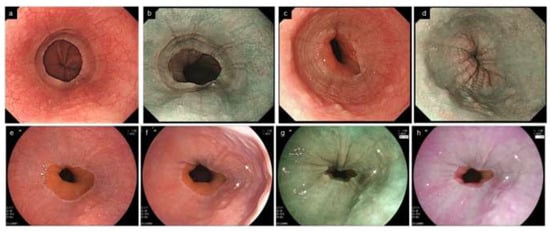
Figure 8.
Localized EoE in the lower end of the esophagus. Endoscopic abnormalities are localized at a small area of 1–2 cm in the lower end of the esophagus. (a,b). Furrows and edema are present in the lower end of the esophagus (same patient, a, WLI; b, NBI). (c,d). Furrows, rings and edema in the lower end of the esophagus (same patient, c, WLI; d, NBI). (e). No abnormal findings appear to be found under full expansion of the esophageal lumen (WLI). (f–h). Reducing the esophageal wall tension increases the visibility of furrows (arrow) and edema. Dynamic observation controlling the extensibility of the esophageal wall increases the visibility of furrows and edema (same patient, f, WLI; g, BLI; h, LCI). EoE, eosinophilic esophagitis; WLI, white light imaging; NBI, narrow band imaging; BLI, blue laser imaging; LCI, linked color imaging.
7. Other New Modalities for Endoscopic Diagnosis
It has been recently reported that endocytoscopy, using super-magnifying technology, can microscopically detect infiltrated intraepithelial eosinophils, characterized by bilobed nuclei [90,91]. Endocytoscopy has not yet been commonly used in the clinical setting owing to the complicated procedures including cell staining and the related cost. Moreover, it can only provide information on cellular and mucosal structures in a very superficial area of the esophagus. However, it may serve as a promising tool for more effectively detecting the esophageal site with abundant eosinophils, and thus decreasing the invasiveness of multiple biopsies.
Artificial intelligence (AI) can endoscopically discriminate EoE from normal esophagus with > 95% accuracy, higher than that of the endoscopist, leading to decreasing diagnostic delay by recommending re-endoscopy with esophageal biopsies, or consultation with a specialist for EoE—especially in cases with non-expert endoscopists [92,93]. Although still images have been mainly used in previous studies, real-time diagnostic assistance during endoscopy, for encouraging biopsies, may become possible if the diagnostic algorithm using video learning is established in the future. AI may also predict non-invasive histological remission with high accuracy, as reported in ulcerative colitis [94,95]. However, some issues remain to be improved in AI diagnosis. In a Japanese report, AI-based diagnosis tended to misdiagnose milder endoscopic abnormalities as normal esophagus (a factor of low sensitivity), and to misdiagnose normal structures such as vertical lines, transient ring or glycogenesis acanthosis as EoE (a factor of low specificity), similar to what was also conducted by endoscopists [93]. Especially in Japan, milder patients with both symptoms and endoscopic findings have been commonly diagnosed in the GI screening for health checkups [96,97]. Despite the recent increasing recognition of endoscopic abnormalities with EoE, it has been shown that up to 10% of EoE patients have a normal-appearing esophagus [98]. In real practice, the endoscopist faces various esophageal diseases and conditions such as erosive esophagitis, esophageal Candida, and neoplastic lesions. Whether high diagnostic accuracy can be maintained even after learning numerous images—that include typical EoE, mild EoE, normal-appearing EoE and other diseases, including those endoscopically similar to EoE—is a future challenge [99]. Eventually, the endoscopist needs to record clear and detailed images for AI to operate on with greater precision.
8. Esophageal Biopsy and Histological Assessment
Multiple biopsies are favorable to diagnose EoE more effectively, due to a heterogeneous distribution of intraepithelial eosinophil infiltration [100,101,102]. It is recommended that ≥2–4 biopsies by ACG (American College of Gastroenterology) guidelines [11] and ≥6 biopsies by UEG (United European Gastroenterology) guidelines [10] should be obtained from more than two different locations. Distribution of eosinophils varies among different endoscopic findings and locations. Furrows and exudates containing more intense eosinophil infiltrations should be preferentially targeted in biopsies [31,96,101]. The biopsy should be selectively obtained from just above the furrows located in valleys, rather than from the mucosa between the valleys, as significantly higher eosinophils are detected in the former [103]. Lines with subtle linear appearances without cracks are not an appropriate target for biopsy due to less eosinophilic infiltration [101]. Rings or stricture, which reflect subepithelial fibrosis, are less favorable targets for biopsy due to a poor correlation with active eosinophilic inflammation [39,51,101]. Histological assessment should include biopsy samples from the distal esophagus, which is more heavily infiltrated with eosinophils than the proximal esophagus [101,104]. Once again, up to 10% of patients exist with an endoscopically normal esophagus [98]. Thus, when EoE is clinically suspected, the endoscopist should obtain esophageal biopsies even if no apparent endoscopic abnormalities are detected. As noted above, endoscopy should be conducted without the use of PPIs whenever possible to avoid underdiagnosis with endoscopic and histologic remission by PPI [16,20].
A peak eosinophil count with ≥15 eosinophils/hpf has been conventionally used as a reliable histological criterion for the definitive diagnosis in EoE [105], which generally indicates an increase in intraepithelial eosinophils. Schoepfer et al. reported that peak intraepithelial eosinophil counts were higher in 63%, identical in 7%, and lower in 30% of patients, compared with the subepithelial layer [106]. Forty percent of EoE patients with <15 intraepithelial eosinophils/hpf had subepithelial peak counts of ≥15, which were thus not diagnosed as EoE, unless biopsy samples included adequate subepithelial tissue. In addition, eosinophilic esophageal myositis (EoEM) with predominant eosinophil infiltration in the muscle layer has been proposed as a subtype of EGIDs, suggesting an association with esophageal motor disorders such as Jackhammer esophagus [107]. Neither endoscopic abnormalities, such as typical EoE, nor increased intraepithelial eosinophils have been found in this condition. For definitive diagnosis, endoscopic ultrasonography-guided fine needle aspiration biopsies and per oral esophageal muscle biopsies need to be performed [94,95]. Thus, when histologically evaluating EoE based on eosinophil count in mucosal biopsies, it is important to note the heterogeneity of eosinophil distribution by biopsy specimen or by horizontal and vertical segments within the esophagus, the diversity of eosinophil counting methods (peak/average number of eosinophils and number of fields of view measured), and the differences in the microscopes used at each institution [108].
Besides absolute counts of eosinophils, EoE has various histological features such as eosinophilic microabscesses, surface laying of eosinophils, eosinophil degranulation, basal cell hyperplasia, lengthening of lamina propria papillae, increased intraepithelial lymphocytes and mast cells, increased intercellular edema, and increased lamina propria fibrosis [42]. Recently, the EoE histologic scoring system (EoE-HSS), which comprehensively evaluates those histological findings including their severity and extent, has been proposed as a more accurate assessment for disease activity or differentiation from GERD [109,110]. Although it has been expected to be useful especially in clinical research or pharmacological trials, it may be too complicated to be used in the real clinical setting, at least in its current version. The more simplified EoE-HSS is desirable. Eosinophil quantification using AI has been recently shown to be helpful in overcoming several challenges including heterogeneous distribution of eosinophils, uncertainty of tissue sampling, or problems with biopsy specimen processing, such as cases where specimens are not cut out vertically [111].
In the absence of gastroenteritis-like symptoms or apparent endoscopic abnormalities in the stomach or the duodenum, gastroduodenal biopsies seem not to be necessary, at least in adult patients because esophageal involvement of eosinophilic gastroenteritis is unlikely [112,113].
9. Conclusions
A variety of factors—differences in endoscopists’ interests and experience, differences in endoscopic equipment performance, the presence of cases with weak or normal appearing esophagus, and the variability in eosinophil infiltration—have influenced diagnostic accuracy in EoE. However, increasing awareness of EoE and its clinical guidelines have led to a decrease in diagnostic delay, endoscopic severity at first diagnosis, and number of endoscopic examinations performed before diagnosis, and consequently more cases are being diagnosed at an earlier stage [114]. In addition to the recent spread of high-resolution endoscopy with IEE, establishing the EREFS scoring system has contributed to the improvement in the diagnostic accuracy of EoE. Newly developed technology, such as endocytoscopy or AI, may support more robust and less invasive definitive diagnosis and management of EoE in real clinical practice.
Author Contributions
Conceptualization, Y.A. and Y.S.; data curation, M.Y., N.M., M.U. and Y.O.; writing—original draft preparation, Y.A.; writing—review and editing, Y.A. and Y.S.; supervision, Y.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gonsalves, N. Eosinophilic Gastrointestinal Disorders. Clin. Rev. Allergy Immunol. 2019, 57, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Whitney-Miller, C.L.; Katzka, D.; Furth, E.E. Eosinophilic Esophagitis: A Retrospective Review of Esophageal Biopsy Specimens from 1992 to 2004 at an Adult Academic Medical Center. Am. J. Clin. Pathol. 2009, 131, 788–792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasad, G.A.; Alexander, J.A.; Schleck, C.D.; Zinsmeister, A.R.; Smyrk, T.C.; Elias, R.M.; Locke, G.R.; Talley, N.J. Epidemiology of Eosinophilic Esophagitis over Three Decades in Olmsted County, Minnesota. Clin. Gastroenterol. Hepatol. 2009, 7, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef]
- Ishimura, N.; Kinoshita, Y. Eosinophilic Esophagitis in Japan: Focus on Response to Acid Suppressive Therapy. J. Gastroenterol. Hepatol. 2018, 33, 1016–1022. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef]
- Kim, H.P.; Vance, R.B.; Shaheen, N.J.; Dellon, E.S. The Prevalence and Diagnostic Utility of Endoscopic Features of Eosinophilic Esophagitis: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 988–996.e5. [Google Scholar] [CrossRef]
- Hirano, I.; Moy, N.; Heckman, M.G.; Thomas, C.S.; Gonsalves, N.; Achem, S.R. Endoscopic Assessment of the Oesophageal Features of Eosinophilic Oesophagitis: Validation of a Novel Classification and Grading System. Gut 2013, 62, 489–495. [Google Scholar] [CrossRef]
- Furuta, G.T.; Liacouras, C.A.; Collins, M.H.; Gupta, S.K.; Justinich, C.; Putnam, P.E.; Bonis, P.; Hassall, E.; Straumann, A.; Rothenberg, M.E. First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic Esophagitis in Children and Adults: A Systematic Review and Consensus Recommendations for Diagnosis and Treatment. Gastroenterology 2007, 133, 1342–1363. [Google Scholar] [CrossRef]
- Liacouras, C.A.; Furuta, G.T.; Hirano, I.; Atkins, D.; Attwood, S.E.; Bonis, P.A.; Burks, A.W.; Chehade, M.; Collins, M.H.; Dellon, E.S.; et al. Eosinophilic Esophagitis: Updated Consensus Recommendations for Children and Adults. J. Allergy Clin. Immunol. 2011, 128, 3–20.e6; quiz 21–22. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gonsalves, N.; Hirano, I.; Furuta, G.T.; Liacouras, C.A.; Katzka, D.A. ACG Clinical Guideline: Evidenced Based Approach to the Diagnosis and Management of Esophageal Eosinophilia and Eosinophilic Esophagitis (EoE). Am. J. Gastroenterol. 2013, 108, 679–692; quiz 693. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Amil Dias, J.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on Eosinophilic Esophagitis: Evidence-Based Statements and Recommendations for Diagnosis and Management in Children and Adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- SenGupta, N.; Tapper, E.B.; Corban, C.; Sommers, T.; Leffler, D.A.; Lembo, A.J. The Clinical Predictors of Aetiology and Complications Among 173 Patients Presenting to the Emergency Department with Oesophageal Food Bolus Impaction from 2004–2014. Aliment. Pharmacol. Ther. 2015, 42, 91–98. [Google Scholar] [CrossRef]
- Byrne, K.R.; Panagiotakis, P.H.; Hilden, K.; Thomas, K.L.; Peterson, K.A.; Fang, J.C. Retrospective Analysis of Esophageal Food Impaction: Differences in Etiology by Age and Gender. Dig. Dis. Sci. 2007, 52, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Sperry, S.L.W.; Crockett, S.D.; Miller, C.B.; Shaheen, N.J.; Dellon, E.S. Esophageal Foreign-Body Impactions: Epidemiology, Time Trends, and the Impact of the Increasing Prevalence of Eosinophilic Esophagitis. Gastrointest. Endosc. 2011, 74, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Hillman, L.; Donohue, S.; Broman, A.T.; Hoversten, P.; Gaumnitz, E.; Lomeli, L. Empiric Proton Pump Inhibitor Therapy After Esophageal Food Impaction May Mask Eosinophilic Esophagitis Diagnosis at Follow-Up. Dis. Esophagus 2021, 34, doab0303. [Google Scholar] [CrossRef]
- Chang, J.W.; Olson, S.; Kim, J.Y.; Dolan, R.; Greenson, J.; Sanders, G.; Rubenstein, J.H. Loss to Follow-Up after Food Impaction among Patients with and without Eosinophilic Esophagitis. Dis. Esophagus 2019, 32, doz056. [Google Scholar] [CrossRef]
- Lenz, C.J.; Leggett, C.; Katzka, D.A.; Larson, J.J.; Enders, F.T.; Alexander, J.A. Food Impaction: Etiology over 35 Years and Association with Eosinophilic Esophagitis. Dis. Esophagus 2019, 32, doy093. [Google Scholar] [CrossRef]
- Aceves, S.S.; Alexander, J.A.; Baron, T.H.; Bredenoord, A.J.; Day, L.; Dellon, E.S.; Falk, G.W.; Furuta, G.T.; Gonsalves, N.; Hirano, I.; et al. Endoscopic Approach to Eosinophilic Esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest. Endosc. 2022, 96, 576–592.e1. [Google Scholar] [CrossRef]
- Odiase, E.; Schwartz, A.; Souza, R.F.; Martin, J.; Konda, V.; Spechler, S.J. New Eosinophilic Esophagitis Concepts Call for Change in Proton Pump Inhibitor Management before Diagnostic Endoscopy. Gastroenterology 2018, 154, 1217–1221.e3. [Google Scholar] [CrossRef]
- Arias-González, L.; Rey-Iborra, E.; Ruiz-Ponce, M.; Laserna-Mendieta, E.J.; Arias, Á.; Lucendo, A.J. Esophageal Perforation in Eosinophilic Esophagitis: A Systematic Review on Clinical Presentation, Management and Outcomes. Dig. Liver Dis. 2020, 52, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Abe, Y.; Sasaki, Y.; Yagi, M.; Mizumoto, N.; Onozato, Y.; Nakamura, S.; Tsuchida, H.; Goto, H.; Ueno, Y. Intramural Esophageal Dissection with Eosinophilic Esophagitis. Clin. J. Gastroenterol. 2022, 15, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.E.A.; Smyrk, T.C.; Demeester, T.R.; Jones, J.B. Esophageal Eosinophilia with Dysphagia. A Distinct Clinicopathologic Syndrome. Dig. Dis. Sci. 1993, 38, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, S.N.; Bergele, C.; Mantides, A. Eosinophilic Esophagitis in Adults: What Is the Clinical Significance? Endoscopy 2006, 38, 515–520. [Google Scholar] [CrossRef]
- Müller, S.; Pühl, S.; Vieth, M.; Stolte, M. Analysis of Symptoms and Endoscopic Findings in 117 Patients with Histological Diagnoses of Eosinophilic Esophagitis. Endoscopy 2007, 39, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.F.; DiBaise, J.K.; Kim, H.J.; De Petris, G.; Crowell, M.D.; Fleischer, D.E.; Sharma, V.K. Patient Characteristics, Clinical, Endoscopic, and Histologic Findings in Adult Eosinophilic Esophagitis: A Case Series and Systematic Review of the Medical Literature. Dis. Esophagus 2007, 20, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.B.; Vargo, J.J.; Goldblum, J.R.; Richter, J.E. The Ringed Esophagus: Histological Features of GERD. Am. J. Gastroenterol. 2001, 96, 984–989. [Google Scholar] [CrossRef]
- Zimmerman, S.L.; Levine, M.S.; Rubesin, S.E.; Mitre, M.C.; Furth, E.E.; Laufer, I.; Katzka, D.A. Idiopathic Eosinophilic Esophagitis in Adults: The Ringed Esophagus. Radiology 2005, 236, 159–165. [Google Scholar] [CrossRef]
- Croese, J.; Fairley, S.K.; Masson, J.W.; Chong, A.K.H.; Whitaker, D.A.; Kanowski, P.A.; Walker, N.I. Clinical and Endoscopic Features of Eosinophilic Esophagitis in Adults. Gastrointest. Endosc. 2003, 58, 516–522. [Google Scholar] [CrossRef]
- Potter, J.W.; Saeian, K.; Staff, D.; Massey, B.T.; Komorowski, R.A.; Shaker, R.; Hogan, W.J. Eosinophilic Esophagitis in Adults: An Emerging Problem with Unique Esophageal Features. Gastrointest. Endosc. 2004, 59, 355–361. [Google Scholar] [CrossRef]
- Straumann, A.; Spichtin, H.P.; Bucher, K.A.; Heer, P.; Simon, H.U. Eosinophilic Esophagitis: Red on Microscopy, White on Endoscopy. Digestion 2004, 70, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Remedios, M.; Campbell, C.; Jones, D.M.; Kerlin, P. Eosinophilic Esophagitis in Adults: Clinical, Endoscopic, Histologic Findings, and Response to Treatment with Fluticasone Propionate. Gastrointest. Endosc. 2006, 63, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Gibbs, W.B.; Fritchie, K.J.; Rubinas, T.C.; Wilson, L.A.; Woosley, J.T.; Shaheen, N.J. Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis from Gastroesophageal Reflux Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1305–1313; quiz 1261. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.D.; Warners, M.J.; Curvers, W.L.; van Lent, A.U.; Bekkali, N.L.; Takkenberg, R.B.; Kloek, J.J.; Bergman, J.J.; Fockens, P.; Bredenoord, A.J. Evaluating the Endoscopic Reference Score for Eosinophilic Esophagitis: Moderate to Substantial Intra- and Interobserver Reliability. Endoscopy 2014, 46, 1049–1055. [Google Scholar] [CrossRef]
- Dellon, E.S. Optimizing the Endoscopic Examination in Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2489–2492.e1. [Google Scholar] [CrossRef]
- Ma, C.; Bredenoord, A.J.; Dellon, E.S.; Alexander, J.A.; Biedermann, L.; Hogan, M.; Guizzetti, L.; Zou, G.; Katzka, D.A.; Chehade, M.; et al. Reliability and Responsiveness of Endoscopic Disease Activity Assessment in Eosinophilic Esophagitis. Gastrointest. Endosc. 2022, 95, 1126–1137.e2. [Google Scholar] [CrossRef]
- Chen, J.W.; Pandolfino, J.E.; Lin, Z.; Ciolino, J.D.; Gonsalves, N.; Kahrilas, P.J.; Hirano, I. Severity of Endoscopically Identified Esophageal Rings Correlates with Reduced Esophageal Distensibility in Eosinophilic Esophagitis. Endoscopy 2016, 48, 794–801. [Google Scholar] [CrossRef]
- Dellon, E.S.; Cotton, C.C.; Gebhart, J.H.; Higgins, L.L.; Beitia, R.; Woosley, J.T.; Shaheen, N.J. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin. Gastroenterol. Hepatol. 2016, 14, 31–39. [Google Scholar] [CrossRef]
- van Rhijn, B.D.; Verheij, J.; Smout, A.J.P.M.; Bredenoord, A.J. The Endoscopic Reference Score Shows Modest Accuracy to Predict Histologic Remission in Adult Patients with Eosinophilic Esophagitis. Neurogastroenterol. Motil. 2016, 28, 1714–1722. [Google Scholar] [CrossRef]
- Parfitt, J.R.; Gregor, J.C.; Suskin, N.G.; Jawa, H.A.; Driman, D.K. Eosinophilic Esophagitis in Adults: Distinguishing Features from Gastroesophageal Reflux Disease: A Study of 41 Patients. Mod. Pathol. 2006, 19, 90–96. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Tanigawa, T.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Watanabe, T.; Arakawa, T. Eosinophilic Esophagitis-Like Endoscopic Findings in Patients with Erosive Esophagitis. Esophagus 2013, 10, 199–204. [Google Scholar] [CrossRef]
- Odze, R.D. Pathology of Eosinophilic Esophagitis: What the Clinician Needs to Know. Am. J. Gastroenterol. 2009, 104, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.M.; Lamana, L.F. Eosinophilic Esophagitis Misdiagnosed Twice as Esophageal Candidiasis. Gastrointest. Endosc. 2010, 71, 394–395; discussion 395. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Sasaki, Y.; Yagi, M.; Yaoita, T.; Nishise, S.; Ueno, Y. Diagnosis and Treatment of Eosinophilic Esophagitis in Clinical Practice. Clin. J. Gastroenterol. 2017, 10, 87–102. [Google Scholar] [CrossRef]
- Spechler, S.J.; Genta, R.M.; Souza, R.F. Thoughts on the Complex Relationship between Gastroesophageal Reflux Disease and Eosinophilic Esophagitis. Am. J. Gastroenterol. 2007, 102, 1301–1306. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Safroneeva, E.; Bussmann, C.; Kuchen, T.; Portmann, S.; Simon, H.U.; Straumann, A. Delay in Diagnosis of Eosinophilic Esophagitis Increases Risk for Stricture Formation in a Time-Dependent Manner. Gastroenterology 2013, 145, 1230–1236.e2. [Google Scholar] [CrossRef]
- Dellon, E.S.; Kim, H.P.; Sperry, S.L.W.; Rybnicek, D.A.; Woosley, J.T.; Shaheen, N.J. A Phenotypic Analysis Shows That Eosinophilic Esophagitis Is a Progressive Fibrostenotic Disease. Gastrointest. Endosc. 2014, 79, 577–585.e4. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Sasaki, Y.; Yagi, M.; Mizumoto, N.; Onozato, Y.; Kon, T.; Shoji, M.; Sakuta, K.; Sakai, T.; Umehara, M.; et al. Linked Color Imaging Improves the Diagnostic Accuracy of Eosinophilic Esophagitis. DEN Open 2023, 3, e146. [Google Scholar] [CrossRef]
- Ayaki, M.; Manabe, N.; Nakamura, J.; Fujita, M.; Kamada, T.; Imamura, K.; Inoue, K.; Haruma, K. The “Caterpillar Sign”: A Novel Endoscopic Indicator of Eosinophilic Esophagitis. Esophagus 2021, 18, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Safroneeva, E.; Straumann, A.; Coslovsky, M.; Zwahlen, M.; Kuehni, C.E.; Panczak, R.; Haas, N.A.; Alexander, J.A.; Dellon, E.S.; Gonsalves, N.; et al. International Eosinophilic Esophagitis Activity Index Study Group. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults with Eosinophilic Esophagitis. Gastroenterology 2016, 150, 581–590.e4. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Barrio-Andrés, J.; Nantes Castillejo, O.; Valdivieso-Cortazar, E.; Pérez-Martínez, I.; Boumidi, A.; Olmos-Jérez, J.A.; Payeras-Llodra, G.; Alcaide-Suarez, N.; Ruiz-Rebollo, L.; et al. The Endoscopic Reference Score Shows Modest Accuracy to Predict Either Clinical or Histological Activity in Adult Patients with Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2017, 45, 300–309. [Google Scholar] [CrossRef]
- Kaplan, M.; Mutlu, E.A.; Jakate, S.; Bruninga, K.; Losurdo, J.; Losurdo, J.; Keshavarzian, A. Endoscopy in Eosinophilic Esophagitis: “Feline” Esophagus and Perforation Risk. Clin. Gastroenterol. Hepatol. 2003, 1, 433–437. [Google Scholar] [CrossRef]
- Furth, E.E.; Rubesin, S.E.; Rose, D. Feline Esophagus. AJR 1995, 164, 900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gentile, N.; Katzka, D.; Ravi, K.; Trenkner, S.; Enders, F.; Killian, J.; Kryzer, L.; Talley, N.J.; Alexander, J. Oesophageal Narrowing Is Common and Frequently Under-Appreciated at Endoscopy in Patients with Oesophageal Eosinophilia. Aliment. Pharmacol. Ther. 2014, 40, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Hirano, I. Narrow-Caliber Esophagus of Eosinophilic Esophagitis: Difficult to Define, Resistant to Remedy. Gastrointest. Endosc. 2016, 83, 1149–1150. [Google Scholar] [CrossRef][Green Version]
- Hirano, I. Clinical Relevance of Esophageal Subepithelial Activity in Eosinophilic Esophagitis. J. Gastroenterol. 2020, 55, 249–260. [Google Scholar] [CrossRef]
- Hernandez, P.V.; Amer, S.; Lam-Himlin, D.M.; DiSantis, D.J.; Menias, C.O.; Horsley-Silva, J.L. Eosinophilic Esophagitis: Imaging Features with Endoscopic and Pathologic Correlation. Abdom. Radiol. 2020, 45, 591–600. [Google Scholar] [CrossRef]
- Lipka, S.; Kumar, A.; Richter, J.E. Impact of Diagnostic Delay and Other Risk Factors on Eosinophilic Esophagitis Phenotype and Esophageal Diameter. J. Clin. Gastroenterol. 2016, 50, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Safroneeva, E.; Coslovsky, M.; Kuehni, C.E.; Zwahlen, M.; Haas, N.A.; Panczak, R.; Taft, T.H.; Hirano, I.; Dellon, E.S.; Gonsalves, N.; et al. International EEsAI Study Group. Eosinophilic Oesophagitis: Relationship of Quality of Life with Clinical, Endoscopic and Histological Activity. Aliment. Pharmacol. Ther. 2015, 42, 1000–1010. [Google Scholar] [CrossRef]
- Eluri, S.; Runge, T.M.; Cotton, C.C.; Burk, C.M.; Wolf, W.A.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. The Extremely Narrow-Caliber Esophagus Is a Treatment-Resistant Subphenotype of Eosinophilic Esophagitis. Gastrointest. Endosc. 2016, 83, 1142–1148. [Google Scholar] [CrossRef]
- Runge, T.M.; Eluri, S.; Cotton, C.C.; Burk, C.M.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype. Am. J. Gastroenterol. 2016, 111, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Koutlas, N.T.; Dellon, E.S. Progression from an Inflammatory to a Fibrostenotic Phenotype in Eosinophilic Esophagitis. Case Rep. Gastroenterol. 2017, 11, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Izumi, D.; Ishimura, N.; Okada, M.; Mikami, H.; Okimoto, E.; Aimi, M.; Mishiro, T.; Oshima, N.; Sato, S.; Ishihara, S.; et al. Poor Inter-observer Agreement on the Endoscopic Diagnosis of Eosinophilic Esophagitis Among Japanese Endoscopists. Esophagus 2017, 14, 309–316. [Google Scholar] [CrossRef]
- Muto, M.; Minashi, K.; Yano, T.; Saito, Y.; Oda, I.; Nonaka, S.; Omori, T.; Sugiura, H.; Goda, K.; Kaise, M.; et al. Early Detection of Superficial Squamous Cell Carcinoma in the Head and Neck Region and Esophagus by Narrow Band Imaging: A Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2010, 28, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, L.; Huang, M.; Jin, Q.; Qin, Y.; Chen, J. The Accuracy of Magnifying Narrow Band Imaging (ME-NBI) in Distinguishing Between Cancerous and Noncancerous Gastric Lesions. Medicine 2018, 97, e9780. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Dohi, O.; Inoue, K.; Yasuda, R.; Murakami, T.; Hirose, R.; Inoue, K.; Naito, Y.; Inada, Y.; Ogiso, K.; et al. Blue Laser Imaging, Blue Light Imaging, and Linked Color Imaging for the Detection and Characterization of Colorectal Tumors. Gut Liver 2019, 13, 140–148. [Google Scholar] [CrossRef]
- Peery, A.F.; Cao, H.; Dominik, R.; Shaheen, N.J.; Dellon, E.S. Variable Reliability of Endoscopic Findings with White-Light and Narrow-Band Imaging for Patients with Suspected Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2011, 9, 475–480. [Google Scholar] [CrossRef]
- Tanaka, K.; Rubio, C.A.; Dlugosz, A.; Truskaite, K.; Befrits, R.; Lindberg, G.; Schmidt, P.T. Narrow-Band Imaging Magnifying Endoscopy in Adult Patients with Eosinophilic Esophagitis/Esophageal Eosinophilia and Lymphocytic Esophagitis. Gastrointest. Endosc. 2013, 78, 659–664. [Google Scholar] [CrossRef]
- Ichiya, T.; Tanaka, K.; Rubio, C.A.; Hammar, U.; Schmidt, P.T. Evaluation of Narrow-Band Imaging Signs in Eosinophilic and Lymphocytic Esophagitis. Endoscopy 2017, 49, 429–437. [Google Scholar] [CrossRef]
- Ayaki, M.; Manabe, N.; Tomida, A.; Tada, N.; Matsunaga, T.; Murota, M.; Fujita, M.; Katsumata, R.; Kobara, H.; Masaki, T.; et al. Beige Mucosa Observable under Narrow-Band Imaging Indicates the Active Sites of Eosinophilic Esophagitis. J. Gastroenterol. Hepatol. 2022, 37, 891–897. [Google Scholar] [CrossRef]
- Shinozaki, S.; Osawa, H.; Hayashi, Y.; Lefor, A.K.; Yamamoto, H. Linked Color Imaging for the Detection of Early Gastrointestinal Neoplasms. Ther. Adv. Gastroenterol. 2019, 12, 1756284819885246. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Min, M.; Dong, T.; Bi, Y.; Tang, A.; Liu, Y. Linked Color Imaging Improves Detection of Minimal Change Esophagitis in Non-Erosive Reflux Esophagitis Patients. Endosc. Int. Open 2018, 6, E1177–E1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Ma, Y.M.; Sun, Q.; Shi, L.L.; Xie, Y.; Zou, X.P. Evaluation of Minimal Change Lesions Using Linked Color Imaging in Patients with Nonerosive Reflux Esophagitis. J. Clin. Gastroenterol. 2022, 56, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Asaoka, D.; Abe, D.; Suzuki, M.; Nakagawa, Y.; Sasaki, H.; Inami, Y.; Ikemura, M.; Utsunomiya, H.; Oki, S.; et al. Linked Color Imaging Improves Visibility of Reflux Esophagitis. BMC Gastroenterol. 2020, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Nagahara, A.; Ishizuka, K.; Okubo, S.; Haga, K.; Suzuki, M.; Nakajima, A.; Komori, H.; Akazawa, Y.; Izumi, K.; et al. Improved Visibility of Barrett’s Esophagus with Linked Color Imaging: Inter- and Intra-Rater Reliability and Quantitative Analysis. Digestion 2018, 97, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Matsumura, T.; Ishikawa, K.; Kaneko, T.; Oura, H.; Ishikawa, T.; Nagashima, A.; Shiratori, W.; Okimoto, K.; Akizue, N.; et al. The Efficacy of Linked Color Imaging in the Endoscopic Diagnosis of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol. Res. Pract. 2020, 2020, 9604345. [Google Scholar] [CrossRef]
- Saito, M.; Koike, T.; Ohara, Y.; Nakagawa, K.; Kanno, T.; Jin, X.; Hatta, W.; Uno, K.; Asano, N.; Imatani, A.; et al. Linked-Color Imaging May Help Improve the Visibility of Superficial Barrett’s Esophageal Adenocarcinoma by Increasing the Color Difference. Intern. Med. 2021, 60, 3351–3358. [Google Scholar] [CrossRef]
- Abe, Y.; Sasaki, Y.; Kon, T.; Ueno, Y. Linked Color Imaging of Eosinophilic Esophagitis. Intern. Med. 2020, 59, 1573–1574. [Google Scholar] [CrossRef]
- Mulder, D.J.; Gander, S.; Hurlbut, D.J.; Soboleski, D.A.; Smith, R.G.; Justinich, C.J. Multiple Squamous Hyperplastic-Fibrous Inflammatory Polyps of the Oesophagus: A New Feature of Eosinophilic Oesophagitis? J. Clin. Pathol. 2009, 62, 845–846. [Google Scholar] [CrossRef]
- Goyal, A.; Poulik, J.; Chang, C.H.; El-Baba, M. Esophageal Polyp in a Boy with Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 541. [Google Scholar] [CrossRef]
- Gill, J.A.; Shutter, J.; Brady, P. A Rare Endoscopic Feature of Eosinophilic Esophagitis. Endoscopy 2011, 43, E17. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.J.; Robinson, C.L.; Veerappan, G.R.; Summers, T.A.; Maydonovitch, C.L.; Wong, R.K. The Tug Sign: An Endoscopic Feature of Eosinophilic Esophagitis. Am. J. Gastroenterol. 2013, 108, 1938–1939. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Gebhart, J.H.; Higgins, L.L.; Hathorn, K.E.; Woosley, J.T.; Shaheen, N.J. The Esophageal Biopsy “Pull” Sign: A Highly Specific and Treatment-Responsive Endoscopic Finding in Eosinophilic Esophagitis (with Video). Gastrointest. Endosc. 2016, 83, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, N.; Sumi, S.; Okada, M.; Izumi, D.; Mikami, H.; Okimoto, E.; Ishikawa, N.; Tamagawa, Y.; Mishiro, T.; Oshima, N.; et al. Ankylosaurus Back Sign: Novel Endoscopic Finding in Esophageal Eosinophilia Patients Indicating Proton Pump Inhibitor Response. Endosc. Int. Open 2018, 6, E165–E172. [Google Scholar] [CrossRef]
- Kon, T.; Abe, Y.; Sasaki, Y.; Kikuchi, R.; Uchiyama, S.; Kusaka, G.; Yaoita, T.; Yagi, M.; Shoji, M.; Onozato, Y.; et al. Clinical Features of Esophageal Eosinophilia According to Endoscopic Phenotypes. Intern. Med. 2020, 59, 2971–2979. [Google Scholar] [CrossRef]
- Suzuki, Y.; Iizuka, T.; Hosoi, A.; Kikuchi, D.; Okamura, T.; Ochiai, Y.; Hayasaka, J.; Dan, N.; Mitsunaga, Y.; Tanaka, M.; et al. Clinicopathological Differences between Eosinophilic Esophagitis and Asymptomatic Esophageal Eosinophilia. Intern. Med. 2022, 61, 1319–1327. [Google Scholar] [CrossRef]
- Abe, Y.; Iijima, K.; Ohara, S.; Koike, T.; Kikuchi, R.; Kato, K.; Shibuya, D.; Inomata, Y.; Oikawa, K.; Ueno, Y. Localized Esophageal Eosinophilia: Is It an Early Manifestation of Eosinophilic Esophagitis or a Subtype of Gastroesophageal Reflux Disease? Dig. Endosc. 2014, 26, 337–343. [Google Scholar] [CrossRef]
- Sawada, A.; Hashimoto, A.; Uemura, R.; Otani, K.; Tanaka, F.; Nagami, Y.; Yamagami, H.; Tanigawa, T.; Watanabe, T.; Fujiwara, Y. Association between Endoscopic Findings of Eosinophilic Esophagitis and Responsiveness to Proton Pump Inhibitors. Endosc. Int. Open 2019, 7, E433–E439. [Google Scholar] [CrossRef]
- Dellon, E.S.; Khoury, P.; Muir, A.B.; Liacouras, C.A.; Safroneeva, E.; Atkins, D.; Collins, M.H.; Gonsalves, N.; Falk, G.W.; Spergel, J.M.; et al. A Clinical Severity Index for Eosinophilic Esophagitis: Development, Consensus, and Future Directions. Gastroenterology 2022, 163, 59–76. [Google Scholar] [CrossRef]
- Kumagai, Y.; Takubo, K.; Kawada, K.; Higashi, M.; Ishiguro, T.; Sobajima, J.; Fukuchi, M.; Ishibashi, K.; Mochiki, E.; Aida, J.; et al. Endocytoscopic Observation of Various Types of Esophagitis. Esophagus 2016, 13, 200–207. [Google Scholar] [CrossRef]
- Shimamura, Y.; Goda, K.; Hirooka, S.; Inoue, H. Observation of Bilobed Nucleus Sign by Endocytoscopy in Eosinophilic Esophagitis. Gastrointest. Endosc. 2021, 93, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Römmele, C.; Mendel, R.; Barrett, C.; Kiesl, H.; Rauber, D.; Rückert, T.; Kraus, L.; Heinkele, J.; Dhillon, C.; Grosser, B.; et al. An Artificial Intelligence Algorithm Is Highly Accurate for Detecting Endoscopic Features of Eosinophilic Esophagitis. Sci. Rep. 2022, 12, 11115. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, E.; Ishimura, N.; Adachi, K.; Kinoshita, Y.; Ishihara, S.; Tada, T. Application of Convolutional Neural Networks for Diagnosis of Eosinophilic Esophagitis Based on Endoscopic Imaging. J. Clin. Med. 2022, 11, 2529. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kudo, S.-E.; Mori, Y.; Misawa, M.; Ogata, N.; Sasanuma, S.; Wakamura, K.; Oda, M.; Mori, K.; Ohtsuka, K. Fully Automated Diagnostic System with Artificial Intelligence Using Endocytoscopy to Identify the Presence of Histologic Inflammation Associated with Ulcerative Colitis (with Video). Gastrointest. Endosc. 2019, 89, 408–415. [Google Scholar] [CrossRef]
- Takenaka, K.; Ohtsuka, K.; Fujii, T.; Negi, M.; Suzuki, K.; Shimizu, H.; Oshima, S.; Akiyama, S.; Motobayashi, M.; Nagahori, M.; et al. Development and Validation of a Deep Neural Network for Accurate Evaluation of Endoscopic Images from Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 2150–2157. [Google Scholar] [CrossRef]
- Adachi, K.; Mishiro, T.; Tanaka, S.; Kinoshita, Y. Suitable Biopsy Site for Detection of Esophageal Eosinophilia in Eosinophilic Esophagitis Suspected Cases. Dig. Endosc. 2016, 28, 139–144. [Google Scholar] [CrossRef]
- Imamura, K.; Haruma, K.; Matsumoto, H.; Maruyama, Y.; Ayaki, M.; Tazaki, S.; Hisamoto, N.; Manabe, N.; Kamada, T.; Kawamoto, H. Clinical and Endoscopic Characteristics of Eosinophilic Esophagitis in Japan: A Case-Control Study. Asia Pac. Allergy 2020, 10, e16. [Google Scholar] [CrossRef]
- Eluri, S.; Corder, S.R.; Kim, E.; Tappata, M.; Reed, C.C.; Shaheen, N.J.; Dellon, E.S. Clinical Features and Time Trends Associated with an Endoscopically Normal Esophagus in Active Eosinophilic Esophagitis. Endoscopy 2021, 53, 886–892. [Google Scholar] [CrossRef]
- Guimarães, P.; Keller, A.; Fehlmann, T.; Lammert, F.; Casper, M. Deep Learning-Based Detection of Eosinophilic Esophagitis. Endoscopy 2022, 54, 299–304. [Google Scholar] [CrossRef]
- Saffari, H.; Peterson, K.A.; Fang, J.C.; Teman, C.; Gleich, G.J.; Pease, L.F. Patchy Eosinophil Distributions in an Esophagectomy Specimen from a Patient with Eosinophilic Esophagitis: Implications for Endoscopic Biopsy. J. Allergy Clin. Immunol. 2012, 130, 798–800. [Google Scholar] [CrossRef]
- Salek, J.; Clayton, F.; Vinson, L.; Saffari, H.; Pease, L.F.; Boynton, K.; Fang, J.; Cox, K.; Peterson, K.A. Endoscopic Appearance and Location Dictate Diagnostic Yield of Biopsies in Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2015, 41, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Dellon, S.; Speck, O.; Woodward, K.; Covey, S.; Rusin, S.; Shaheen, J.; Woosley, T. Distribution and Variability of Esophageal Eosinophilia in Patients Undergoing Upper Endoscopy. Mod. Pathol. 2015, 28, 383. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, E.; Ishimura, N.; Okada, M.; Izumi, D.; Mikami, H.; Aimi, M.; Tanimura, T.; Mishiro, T.; Oshima, N.; Ishikawa, N.; et al. Specific Locations of Linear Furrows in Patients with Esophageal Eosinophilia. Dig. Endosc. 2017, 29, 49–56. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hashimoto, A.; Uemura, R.; Sawada, A.; Otani, K.; Tanaka, F.; Yamagami, H.; Tanigawa, T.; Watanabe, T.; Kabata, D.; et al. Optimal Biopsy Protocol to Evaluate Histological Effectiveness of Proton Pump Inhibitor Therapy in Patients with Eosinophilic Esophagitis. Digestion 2019, 100, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rusin, S.; Covey, S.; Perjar, I.; Hollyfield, J.; Speck, O.; Woodward, K.; Woosley, J.T.; Dellon, E.S. Determination of Esophageal Eosinophil Counts and Other Histologic Features of Eosinophilic Esophagitis by Pathology Trainees Is Highly Accurate. Hum. Pathol. 2017, 62, 50–55. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Simko, A.; Bussmann, C.; Safroneeva, E.; Zwahlen, M.; Greuter, T.; Biedermann, L.; Vavricka, S.; Godat, S.; Reinhard, A.; et al. Eosinophilic Esophagitis: Relationship of Subepithelial Eosinophilic Inflammation with Epithelial Histology, Endoscopy, Blood Eosinophils, and Symptoms. Am. J. Gastroenterol. 2018, 113, 348–357. [Google Scholar] [CrossRef]
- Sato, H.; Nakajima, N.; Takahashi, K.; Hasegawa, G.; Mizuno, K.I.; Hashimoto, S.; Ikarashi, S.; Hayashi, K.; Honda, Y.; Yokoyama, J.; et al. Proposed Criteria to Differentiate Heterogeneous Eosinophilic Gastrointestinal Disorders of the Esophagus, Including Eosinophilic Esophageal Myositis. World J. Gastroenterol. 2017, 23, 2414–2423. [Google Scholar] [CrossRef]
- Dellon, E.S.; Aderoju, A.; Woosley, J.T.; Sandler, R.S.; Shaheen, N.J. Variability in Diagnostic Criteria for Eosinophilic Esophagitis: A Systematic Review. Am. J. Gastroenterol. 2007, 102, 2300–2313. [Google Scholar] [CrossRef]
- Collins, M.H.; Martin, L.J.; Alexander, E.S.; Todd Boyd, J.; Sheridan, R.; He, H.; Pentiuk, S.; Putnam, P.E.; Abonia, J.P.; Mukkada, V.A.; et al. Newly Developed and Validated Eosinophilic Esophagitis Histology Scoring System and Evidence That It Outperforms Peak Eosinophil Count for Disease Diagnosis and Monitoring. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Lin, B.; Rabinowitz, S.; Haseeb, M.A.; Gupta, R. Usefulness of the Eosinophilic Esophagitis Histologic Scoring System in Distinguishing Active Eosinophilic Esophagitis from Remission and Gastroesophageal Reflux Disease. Gastroenterol. Res. 2021, 14, 220–226. [Google Scholar] [CrossRef]
- Smith, E.R.; Shah, J. Applications of Artificial Intelligence to Eosinophilic Esophagitis. Gastroenterol. Insights 2022, 13, 218–227. [Google Scholar] [CrossRef]
- de Rooij, W.E.; Haasnoot, M.L.; Lei, A.; Warners, M.J.; Mookhoek, A.; Bredenoord, A.J. Utility of Gastric and Duodenal Biopsy Sampling in Adult Eosinophilic Esophagitis Patients to Rule Out Other Gastrointestinal Disorders. Scand. J. Gastroenterol. 2021, 56, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, B.; Zalewski, A.; Gregory, D.; Yang, G.-Y.; Ho, N.; Gonsalves, N.; Hirano, I. Low Prevalence of Extraesophageal Gastrointestinal Pathology in Patients with Eosinophilic Esophagitis. Dig. Dis. Sci. 2022, 67, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Laserna-Mendieta, E.J.; Casabona, S.; Savarino, E.; Pérez-Fernández, M.T.; Ghisa, M.; Pérez-Martínez, I.; Guagnozzi, D.; Perelló, A.; Guardiola-Arévalo, A.; et al. Accurate and Timely Diagnosis of Eosinophilic Esophagitis Improves over Time in Europe. An Analysis of the EoE CONNECT Registry. United Eur. Gastroenterol. J. 2022, 10, 507–517. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).