Retinal Vascularization Abnormalities Studied by Optical Coherence Tomography Angiography (OCTA) in Type 2 Diabetic Patients with Moderate Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Protocol
2.3. Statistical Analysis
3. Results
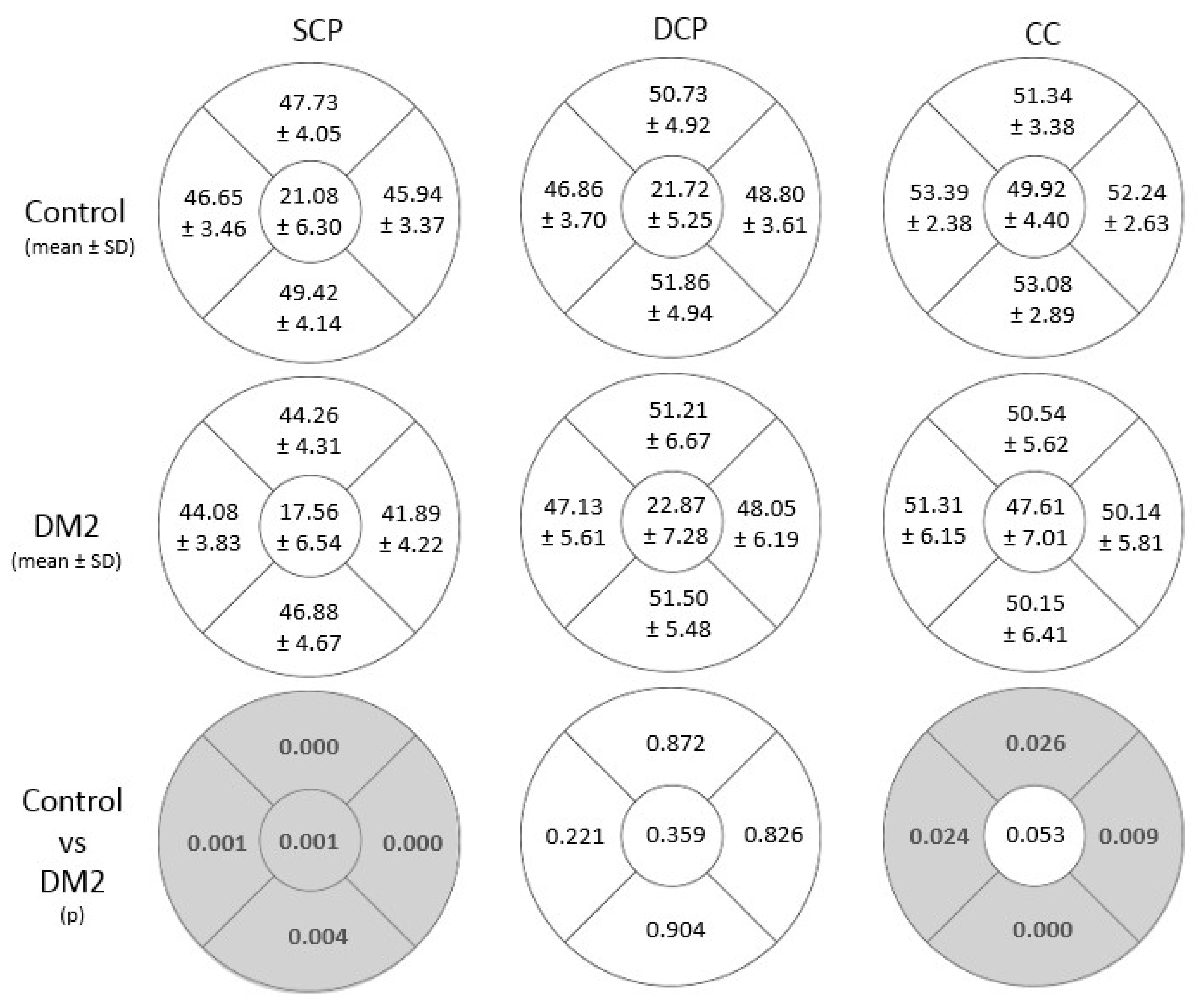
3.1. Vascular Density and Microvascular Changes Studied with OCTA
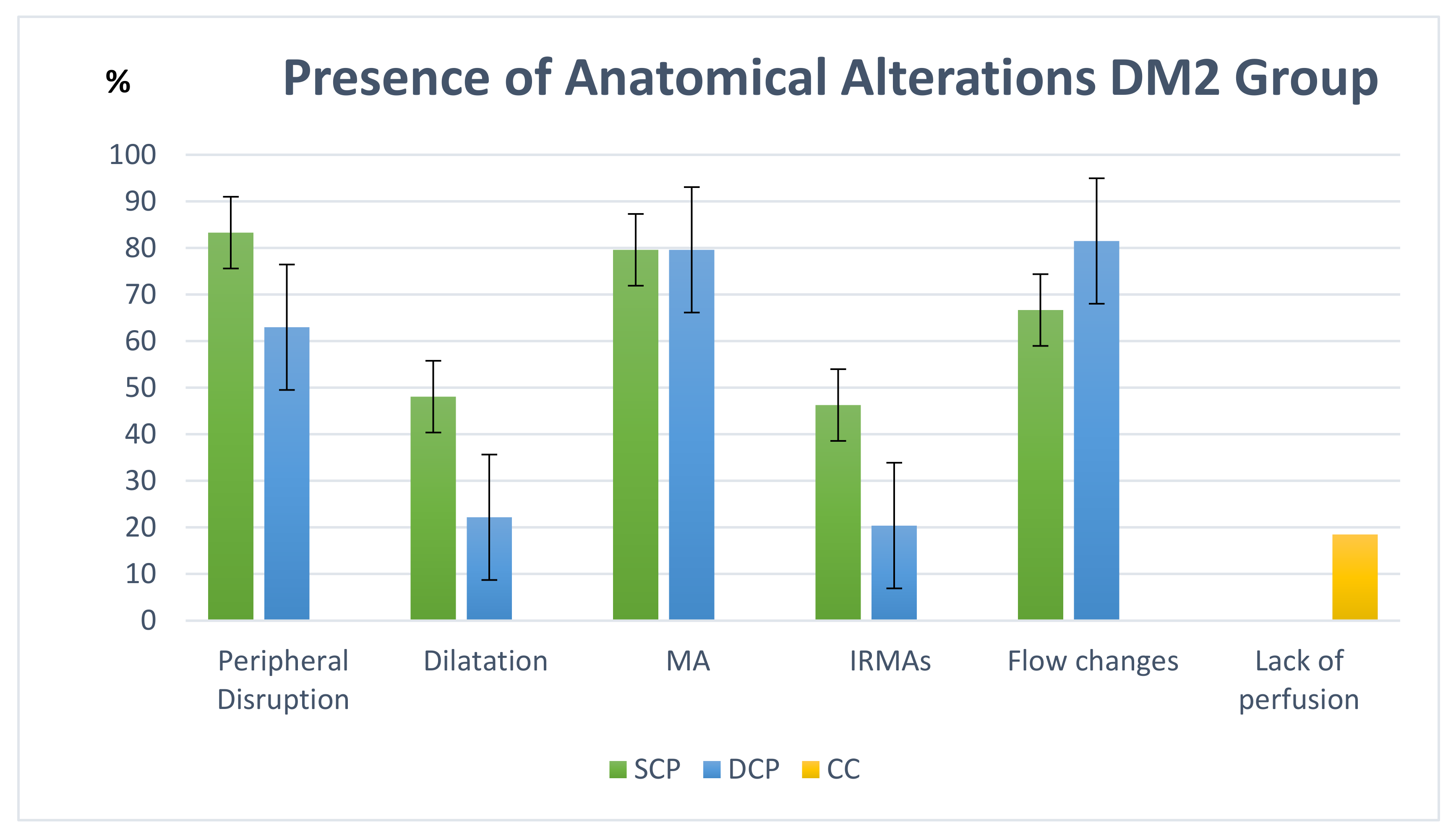
3.2. Anatomo-Functional Correlation Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewing, F.M.; Deary, I.J.; Strachan, M.W.; Frier, B.M. Seeing beyond retinopathy in diabetes: Electrophysiological and psychophysical abnormalities and alterations in vision. Endocr. Rev. 1998, 19, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheloni, R.; Gandolfi, S.A.; Signorelli, C.; Odone, A. Global prevalence of diabetic retinopathy: Protocol for a systematic review and meta-analysis. BMJ Open 2019, 9, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Ronning, K.E.; Karlen, S.J.; Burns, M.E.; Pugh, E.N.; Zawadzki, R.J. In vivo multimodal retinal imaging of disease-related pigmentary changes in retinal pigment epithelium. Sci. Rep. 2021, 11, 16252. [Google Scholar] [CrossRef]
- Pinilla, I.; Sanchez-Cano, A.; Insa, G.; Bartolomé, I.; Perdices, L.; Orduna-Hospital, E. Choroidal differences between spectral and swept-source domain technologies. Curr. Eye Res. 2020, 46, 239–247. [Google Scholar] [CrossRef]
- Cuenca, N.; Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Kutsyr, O.; Albertos-Arranz, H.; Fernández-Sánchez, L.; Martínez-Gil, N.; Noailles, A.; López-Garrido, J.A.; López-Gálvez, M.; et al. Interpretation of OCT and OCTA images from a histological approach: Clinical and experimental implications. Prog. Retin. Eye Res. 2020, 77, 100828. [Google Scholar] [CrossRef]
- Rocholz, R.; Corvi, F.; Weichsel, J.; Schmidt, S.; Staurenghi, G. High Resolution Imaging in Microscopy and Ophthalmology. In High Resolution Imaging in Microscopy and Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030166380. [Google Scholar]
- Tey, K.Y.; Teo, K.; Tan, A.C.S.; Devarajan, K.; Tan, B.; Tan, J.; Schmetterer, L.; Ang, M. Optical coherence tomography angiography in diabetic retinopathy: A review of current applications. Eye Vis. 2019, 6, 37. [Google Scholar] [CrossRef]
- Tam, J.; Dhamdhere, K.P.; Tiruveedhula, P.; Lujan, B.J.; Johnson, R.N.; Bearse, M.A.J.; Adams, A.J.; Roorda, A. Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom. Vis. Sci. 2012, 89, E692–E703. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, D.R.; Yi, J.J.; De Koo, L.O.; Ameri, H.; Puliafito, C.A.; Kashani, A.H. Optical Coherence Tomography Angiography of Diabetic Retinopathy in Human Subjects. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 796–805. [Google Scholar] [CrossRef]
- Pappuru, R.K.R.; Ribeiro, L.; Lobo, C.; Alves, D.; Cunha-Vaz, J. Microaneurysm turnover is a predictor of diabetic retinopathy progression. Br. J. Ophthalmol. 2019, 103, 222–226. [Google Scholar] [CrossRef]
- Vujosevic, S.; Toma, C.; Villani, E.; Gatti, V.; Brambilla, M.; Muraca, A.; Ponziani, M.C.; Aimaretti, G.; Nuzzo, A.; Nucci, P.; et al. Early Detection of Microvascular Changes in Patients with Diabetes Mellitus without and with Diabetic Retinopathy: Comparison between Different Swept-Source OCT-A Instruments. J. Diabetes Res. 2019, 2019, 2547216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, T.M.; Marques, J.P.; Soares, M.; Simão, S.; Melo, P.; Martins, A.; Figueira, J.; Murta, J.N.; Silva, R. Macular OCT-angiography parameters to predict the clinical stage of nonproliferative diabetic retinopathy: An exploratory analysis. Eye 2019, 33, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, D.; Anegondi, N.; Gadde, S.G.K.; Srinivasan, P.; Chidambara, L.; Yadav, N.K.; Roy, A.S. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kuonen, A.; Bergin, C.; Ambresin, A. Perifoveal capillary changes in diabetic patients and association between severity and type of diabetes, visual acuity, and enlargement of non-flow area in the retinal capillary plexuses. J. Fr. Ophtalmol. 2021, 44, 367–375. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Akil, H.; Pfau, M.; Sadda, S.R. Swept-Source OCT Angiography Imaging of the Foveal Avascular Zone and Macular Capillary Network Density in Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 3907–3913. [Google Scholar] [CrossRef] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991, 98, 823–833. [Google Scholar] [CrossRef]
- Lupidi, M.; Coscas, G.; Coscas, F.; Fiore, T.; Spaccini, E.; Fruttini, D.; Cagini, C. Retinal Microvasculature in Nonproliferative Diabetic Retinopathy: Automated Quantitative Optical Coherence Tomography Angiography Assessment. Ophthalmic Res. 2017, 58, 131–141. [Google Scholar] [CrossRef]
- Johannesen, S.K.; Viken, J.N.; Vergmann, A.S.; Grauslund, J. Optical coherence tomography angiography and microvascular changes in diabetic retinopathy: A systematic review. Acta Ophthalmol. 2019, 97, 7–14. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Shenazandi, H.; Naseri, D.; Anvari, P.; Kazemi, P.; Aghamohammadi, F.; Alissmail, F.; Alemzadeh, S.A. Foveal Avascular Zone and Vessel Density in Healthy Subjects: An Optical Coherence Tomography Angiography Study. J. Ophthalmic Vis. Res. 2018, 13, 260–265. [Google Scholar] [CrossRef]
- Vieira-Potter, V.J.; Karamichos, D.; Lee, D.J. Ocular Complications of Diabetes and Therapeutic Approaches. BioMed Res. Int. 2016, 2016, 3801570. [Google Scholar] [CrossRef] [Green Version]
- Khadamy, J.; Abri Aghdam, K.; Falavarjani, K. An update on optical coherence tomography angiography in diabetic retinopathy. J. Ophthalmic Vis. Res. 2018, 13, 487–497. [Google Scholar] [PubMed]
- Sanchez-Cano, A.; Orduna, E.; Segura, F.; Lopez, C.; Cuenca, N.; Abecia, E.; Pinilla, I. Choroidal thickness and volume in healthy young white adults and the relationships between them and axial length, ammetropy and sex. Am. J. Ophthalmol. 2014, 158, 574–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Jiang, C.; Wang, X.; Zhu, L.; Gu, R.; Xu, H.; Jia, Y.; Huang, D.; Sun, X. Macular perfusion in healthy Chinese: An optical coherence tomography angiogram study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3212–3217. [Google Scholar] [CrossRef] [PubMed]
- Coscas, F.; Sellam, A.; Glacet-Bernard, A.; Jung, C.; Goudot, M.; Miere, A.; Souied, E.H. Normative Data for Vascular Density in Superficial and Deep Capillary Plexuses of Healthy Adults Assessed by Optical Coherence Tomography Angiography. InvestIG. Ophthalmol. Vis. Sci. 2016, 57, OCT211–OCT223. [Google Scholar] [CrossRef] [PubMed]
- Iafe, N.A.; Phasukkijwatana, N.; Chen, X.; Sarraf, D. Retinal capillary density and foveal avascular zone area are age-dependent: Quantitative analysis using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5780–5787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.S.; Lim, L.W.; Chow, V.S.; Chay, I.W.; Tan, S.; Cheong, K.X.; Tan, G.T.; Sadda, S.V.R. Optical Coherence Tomography Angiography Evaluation of the Parafoveal Vasculature and Its Relationship With Ocular Factors. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT224–OCT234. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, F.; Fadakar, K.; Berijani, S.; Babeli, A.; Gholizadeh, A.; Sabour, S. Quantitative assessment of vascular density in diabetic retinopathy subtypes with optical coherence tomography angiography. BMC Ophthalmol. 2021, 21, 82. [Google Scholar] [CrossRef]
- Abreu-Gonzalez, R.; Diaz-Rodriguez, R.; Rubio-Rodriguez, G.; Gil-Hernandez, M.A.; Abreu-Reyes, P. Macular vascular flow area and vascular density in healthy population using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6713. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Ma, J.; Li, Y.; Zhang, Z. Association between retinal neuronal degeneration and visual function impairment in type 2 diabetic patients without diabetic retinopathy. Sci. China Life Sci. 2015, 58, 550–555. [Google Scholar] [CrossRef]
- Hirai, F.E.; Tielsch, J.M.; Klein, B.E.K.; Klein, R. Ten-year change in vision-related quality of life in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology 2011, 118, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Orduna-Hospital, E.; Sanchez-Cano, A.; Perdices, L.; Acha, J.; López Alaminos, E.M.; Pinilla, I. Changes in retinal layers in type 1 diabetes mellitus without retinopathy measured by spectral domain and swept source OCTs. Sci. Rep. 2021, 11, 10427. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, H.W.; Kok, P.H.; Garvin, M.; Sonka, M.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; Verbraak, F.D.; Abramoff, M.D. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Sokol, S.; Moskowitz, A.; Skarf, B.; Evans, R.; Molitch, M.; Senior, B. Contrast Sensitivity in Diabetics With and Without Background Retinopathy. Arch. Ophthalmol. 1985, 103, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ismail, G.M.; Whitaker, D. Early detection of changes in visual function in diabetes mellitus. Ophthalmic Physiol. Opt. 1998, 18, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Haulani, H.; Jürgens, I. quantitative and qualitative analysis of the three capillary plexuses and choriocapillaris in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy: A prospective pilot study. Retina 2020, 40, 333–344. [Google Scholar] [CrossRef]
- Alam, M.; Zhang, Y.; Lim, J.I.; Chan, R.V.P.; Yang, M.; Yao, X. Quantitative optical coherence tomography angiography features for objective classification and staging of diabetic retinopathy. Retina 2020, 40, 322–332. [Google Scholar] [CrossRef]
- Nesper, P.L.; Roberts, P.K.; Onishi, A.C.; Chai, H.; Liu, L.; Jampol, L.M.; Fawzi, A.A. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO307–BIO315. [Google Scholar] [CrossRef]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 190–196. [Google Scholar] [CrossRef]
- Ong, J.X.; Kwan, C.C.; Cicinelli, M.V.; Fawzi, A.A. Superficial capillary perfusion on optical coherence tomography angiography differentiates moderate and severe nonproliferative diabetic retinopathy. PLoS ONE 2020, 15, e0240064. [Google Scholar] [CrossRef]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef]
- Fryczkowski, A.W.; Sato, S.E.; Hodes, B.L. Changes in the diabetic choroidal vasculature: Scanning electron microscopy findings. Ann. Ophthalmol. 1988, 20, 299–305. [Google Scholar] [PubMed]
- Fryczkowski, A.W.; Hodes, B.L.; Walker, J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int. Ophthalmol. 1989, 13, 269–279. [Google Scholar] [CrossRef]
- Cao, J.; McLeod, D.S.; Merges, C.A.; Lutty, G.A. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch. Ophthalmol. (Chic. Ill. 1960) 1998, 116, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Hua, R.; Liu, L.; Wang, X.; Chen, L. Imaging evidence of diabetic choroidopathy in vivo: Angiographic pathoanatomy and choroidal-enhanced depth imaging. PLoS ONE 2013, 8, e83494. [Google Scholar] [CrossRef] [PubMed]
- Adhi, M.; Brewer, E.; Waheed, N.K.; Duker, J.S. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013, 131, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melancia, D.; Vicente, A.; Cunha, J.P.; Abegão Pinto, L.; Ferreira, J. Diabetic choroidopathy: A review of the current literature. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Ashour, D.M.; El-Shazly, A.A.E.-F.; Abdelgawad, R.H.A.; Saleh, M.I. Choroidal thickness in relation to urinary albumin excretion rate in type 2 diabetes mellitus without retinopathy. Int. J. Retin. Vitr. 2021, 7, 61. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, H.; Zhang, Q.; Chu, Z.; Olmos de Koo, L.C.; Chao, J.R.; Rezaei, K.A.; Saraf, S.S.; Wang, R.K. Quantitative assessment of choriocapillaris flow deficits in diabetic retinopathy: A swept-source optical coherence tomography angiography study. PLoS ONE 2020, 15, e0243830. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Chin, A.T.; Bonini Filho, M.A.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 2015, 35, 2364–2370. [Google Scholar] [CrossRef]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H.; Yoshida, M.; Ogura, Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef]
- Di, G.; Weihong, Y.; Xiao, Z.; Zhikun, Y.; Xuan, Z.; Yi, Q.; Fangtian, D. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Couturier, A.; Mané, V.; Bonnin, S.; Erginay, A.; Massin, P.; Gaudric, A.; Tadayoni, R. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina 2015, 35, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Zhang, M.; Bhavsar, K.; Zhang, X.; Campbell, J.P.; Lin, P.; Bailey, S.; Flaxel, C.; Laurer, A.; Wilson, D.; et al. Visualization of 3 Distinct Retinal Plexusses by Projection-Resolved Optical Coherence Tomogaphy Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2018, 134, 1411–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, N.; Tan, Y.; Liu, S.; Xie, Y.; Shuai, S.; Wang, W.; Huang, W. Macular vessel density in diabetes and diabetic retinopathy with swept-source optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. Albr. Graefes Arch. Klin. Exp. Ophthalmol. 2020, 258, 2671–2679. [Google Scholar] [CrossRef]
- Lavia, C.; Feldman-Billard, S.; Erginay, A.; Guérin, O.; Virgili, G.; Gaudric, A.; Tadayoni, R.; Dupas, B. Rapid macular capillary loss in patients with uncontrolled type 1 diabetes. Retina 2020, 40, 1053–1061. [Google Scholar] [CrossRef]
- Ciloglu, E.; Unal, F.; Sukgen, E.A.; Koçluk, Y. Evaluation of Foveal Avascular Zone and Capillary Plexuses in Diabetic Patients by Optical Coherence Tomography Angiography. Korean J. Ophthalmol. 2019, 33, 359. [Google Scholar] [CrossRef]
- Tang, F.Y.; Chan, E.O.; Sun, Z.; Wong, R.; Lok, J.; Szeto, S.; Chan, J.C.; Lam, A.; Tham, C.C.; Ng, D.S.; et al. Clinically relevant factors associated with quantitative optical coherence tomography angiography metrics in deep capillary plexus in patients with diabetes. Eye Vis. 2020, 7, 7. [Google Scholar] [CrossRef]
- Boned-Murillo, A.; Albertos-Arranz, H.; Diaz-Barreda, M.D.; Orduna-Hospital, E.; Sánchez-Cano, A.; Ferreras, A.; Cuenca, N.; Pinilla, I. Optical Coherence Tomography Angiography in Diabetic Patients: A Systematic Review. Biomedicines 2022, 10, 88. [Google Scholar] [CrossRef]
- Xiong, H.; You, Q.S.; Guo, Y.; Wang, J.; Wang, B.; Gao, L.; Flaxel, C.J.; Bailey, S.T.; Hwang, T.S.; Jia, Y. Deep learning-based signal-independent assessment of macular avascular area on 6 × 6 mm optical coherence tomography angiogram in diabetic retinopathy: A comparison to instrument-embedded software. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Nazir, T.; Irtaza, A.; Shabbir, Z.; Javed, A.; Akram, U.; Mahmood, M.T. Diabetic retinopathy detection through novel tetragonal local octa patterns and extreme learning machines. Artif. Intell. Med. 2019, 99, 101695. [Google Scholar] [CrossRef]



| DM2 Group (n = 54) | Mean | SD |
|---|---|---|
| Disease duration (years) | 2.50 | 2.88 |
| HbA1c (%) | 7.58 | 1.30 |
| Total cholesterol total (mg/dL) | 148.04 | 33.18 |
| HDL cholesterol HDL (mg/dL) | 47.83 | 15.21 |
| LDL cholesterol LDL (mg/dL) | 71.47 | 23.10 |
| TG (mg/dL) | 122.24 | 51.71 |
| GF (CKD-EPI) (mL/min/1.73) | 73.57 | 20.52 |
| Creatinine (mg/dL) | 1.05 | 0.50 |
| Control Group (n = 73) | DM2 Group (n = 54) | p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| BCVA (LogMAR) | 0.04 | 0.05 | 0.10 | 0.12 | 0.001 |
| SE (D) | 0.04 | 1.58 | 0.37 | 1.70 | 0.110 |
| AL (mm) | 24.00 | 2.80 | 23.23 | 0.84 | 0.075 |
| IOP (mmHg) | 15.30 | 2.90 | 14.76 | 2.49 | 0.676 |
| FAZ Area (μm2) | |||||
|---|---|---|---|---|---|
| Control Group | DM2 Group | p | |||
| Mean | SD | Mean | SD | ||
| SCP | 242.37 | 85.36 | 333.59 | 161.03 | <0.0001 |
| DCP | 278.85 | 103.25 | 307.18 | 141.16 | 0.301 |
| Anatomical Alterations in NPDR DM2 Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral Disruption SCP | Dilatation SCP | MA SCP | IRMAs SCP | Flow Changes SCP | Peripheral Disruption DCP | Dilatation DCP | MA DCP | IRMAs DCP | Flow Changes DCP | Lack of CC Perfusion | |
| Age | −0.082 (0.558) | −0.027 (0.847) | 0.081 (0.565) | −0.162 (0.246) | 0.080 (0.571) | −0.043 (0.762) | 0.286 * (0.038) | −0.122 (0.386) | −0.182 (0.193) | 0.096 (0.493) | 0.251 (0.070) |
| Duration DM2 | 0.094 (0.500) | 0.113 (0.417) | 0.010 (0.945) | 0.130 (0.349) | 0.202 (0.143) | −0.092 (0.506) | 0.254 (0.064) | −0.021 (0.881) | −0.056 (0.686) | −0.025 (0.858) | −0.075 (0.590) |
| HbA1C% | −0.008 (0.954) | 0.111 (0.425) | −0.064 (0.648) | 0.138 (0.318) | −0.040 (0.772) | 0.042 (0.764) | −0.186 (0.178) | 0.066 (0.633) | −0.052 (0.710) | −0.155 (0.264) | −0.052 (0.708) |
| Cholesterol | 0.059 (0.672) | −0.092 (0.510) | −0.084 (0.545) | −0.236 (0.086) | −0.189 (0.171) | 0.022 (0.874) | 0.020 (0.886) | 0.133 (0.338) | 0.084 (0.545) | −0.240 (0.080) | −0.121 (0.384) |
| HDL | 0.067 (0.630) | −0.241 (0.080) | −0.155 (0.263) | −0.308 * (0.024) | −0.415 ** (0.002) | −0.076 (0.583) | −0.176 (0.203) | −0.096 (0.490) | −0.015 (0.916) | −0.078 (0.575) | −0.225 (0.102) |
| LDL | −0.033 (0.810) | −0.020 (0.885) | −0.072 (0.603) | −0.248 (0.071) | −0.262 (0.055) | 0.009 (0.951) | −0.044 (0.750) | 0.198 (0.152) | 0.046 (0.743) | −0.219 (0.112) | −0.106 (0.447) |
| TG | −0.217 (0.115) | 0.104 (0.456) | 0.254 (0.064) | 0.181 (0.190) | 0.077 (0.580) | 0.108 (0.436) | 0.159 (0.252) | 0.080 (0.567) | 0.108 (0.438) | −0.047 (0.733) | 0.008 (0.956) |
| GF | 0.198 (0.152) | 0.080 (0.563) | 0.117 (0.401) | 0.200 (0.148) | 0.018 (0.895) | −0.037 (0.790) | −0.061 (0.661) | 0.238 (0.083) | 0.258 (0.060) | −0.275 * (0.044) | −0.328 * (0.015) |
| Creatine | −0.097 (0.484) | −0.007 (0.959) | −0.123 (0.377) | −0.107 (0.440) | 0.098 (0.479) | 0.103 (0.457) | 0.034 (0.805) | −0.227 (0.098) | −0.205 (0.137) | 0.271 * (0.048) | 0.305 * (0.025) |
| VA (LogMAR) | −0.051 (0.716) | −0.111 (0.426) | −0.220 (0.110) | −0.076 (0.587) | −0.065 (0.639) | −0.017 (0.903) | 0.009 (0.948) | −0.027 (0.849) | −0.039 (0.779) | −0.094 (0.500) | 0.165 (0.233) |
| SE (D) | −0.053 (0.704) | 0.140 (0.314) | −0.033 (0.815) | −0.051 (0.712) | −0.134 (0.333) | −0.200 (0.147) | −0.142 (0.305) | −0.135 (0.331) | 0.030 (0.832) | 0.214 (0.121) | −0.003 (0.982) |
| AL (mm) | 0.115 (0.408) | −0.180 (0.194) | 0.089 (0.524) | −0.054 (0.700) | 0.421 ** (0.002) | 0.073 (0.602) | −0.014 (0.918) | 0.136 (0.328) | 0.199 (0.149) | 0.144 (0.300) | 0.024 (0.861) |
| IOP (mmHg) | −0.003 (0.982) | 0.112 (0.421) | 0.039 (0.781) | −0.218 (0.113) | −0.149 (0.282) | −0.090 (0.520) | 0.092 (0.506) | −0.172 (0.215) | 0.015 (0.915) | −0.186 (0.179) | −0.315 * (0.020) |
| Anatomical Alterations in NPDR DM2 Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral Disruption SCP | Dilatation SCP | MA SCP | IRMAs SCP | Flow Changes SCP | Peripheral Disruption DCP | Dilatation DCP | MA DCP | IRMAs DCP | Flow Changes DCP | Lack of CC Perfusion | |
| SCP | |||||||||||
| C | 0.018 (0.900) | 0.033 (0.811) | 0.199 (0.149) | −0.113 (0.415) | −0.048 (0.731) | 0.212 (0.125) | −0.054 (0.697) | 0.081 (0.560) | 0.136 (0.328) | 0.052 (0.709) | 0.101 (0.468) |
| S | 0.172 (0.213) | −0.260 (0.057) | −0.155 (0.263) | −0.318 * (0.019) | −0.130 (0.350) | −0.148 (0.287) | 0.101 (0.465) | −0.223 (0.105) | −0.103 (0.457) | 0.000 (1.000) | −0.217 (0.115) |
| T | −0.091 (0.513) | 0.002 (0.986) | 0.117 (0.401) | −0.135 (0.332) | −0.272 * (0.046) | −0.076 (0.584) | 0.049 (0.727) | −0.155 (0.263) | −0.043 (0.759) | 0.064 (0.644) | 0.021 (0.878) |
| N | 0.014 (0.918) | −0.112 (0.421) | 0.024 ± 0.865 | −0.166 (0.231) | −0.212 (0.124) | −0.074 (0.596) | −0.170 (0.219) | −0.060 (0.664) | −0.056 (0.687) | −0.014 (0.921) | 0.185 (0.180) |
| I | 0.081 (0.559) | −0.026 (0.851) | 0.007 ± 0.958 | −0.139 (0.315) | 0.018 (0.899) | −0.042 (0.764) | 0.123 (0.376) | −0.040 (0.775) | −0.235 (0.088) | 0.113 (0.415) | 0.084 (0.545) |
| FAZ | 0.002 (0.991) | 0.221 (0.108) | −0.040 (0.775) | 0.142 (0.306) | 0.058 (0.677) | −0.093 (0.501) | 0.003 (0.984) | −0.013 (0.924) | −0.009 (0.949) | 0.107 (0.441) | 0.037 (0.792) |
| DCP | |||||||||||
| C | −0.010 (0.944) | −0.035 (0.806) | 0.131 (0.351) | −0.285 * (0.039) | −0.172 (0.218) | 0.008 (0.957) | −0.068 (0.630) | 0.079 (0.574) | −0.002 (0.991) | −0.195 (0.161) | 0.142 (0.311) |
| S | 0.143 (0.301) | −0.284 * (0.037) | −0.168 (0.224) | −0.193 (0.162) | −0.200 (0.146) | −0.204 (0.139) | −0.033 (0.813) | −0.108 (0.438) | 0.062 (0.656) | −0.167 (0.228) | −0.026 (0.852) |
| T | −0.180 (0.192) | 0.243 (0.077) | −0.058 (0.679) | 0.035 (0.804) | −0.290 * (0.034) | −0.155 (0.263) | 0.020 (0.886) | −0.031 (0.824) | −0.019 (0.891) | −0.199 (0.150) | 0.141 (0.310) |
| N | −0.126 (0.364) | −0.065 (0.638) | 0.037 (0.791) | −0.127 (0.358) | −0.348 ** (0.010) | −0.207 (0.134) | −0.016 (0.910) | 0.041 (0.767) | −0.090 (0.518) | −0.382 ** (0.004) | 0.073 (0.598) |
| I | 0.069 (0.622) | 0.078 (0.573) | −0.162 (0.241) | −0.074 (0.596) | −0.103 (0.457) | −0.044 (0.750) | 0.049 (0.727) | 0.000 (1.000) | −0.208 (0.131) | 0.156 (0.260) | 0.127 (0.360) |
| FAZ | −0.069 (0.622) | −0.045 (0.746) | −0.205 (0.137) | 0.030 (0.831) | 0.149 (0.283) | −0.042 (0.764) | −0.069 (0.622) | −0.205 (0.137) | 0.046 (0.743) | 0.229 (0.095) | 0.049 (0.725) |
| CC | |||||||||||
| C | 0.065 (0.639) | 0.102 (0.462) | −0.074 (0.596) | 0.018 (0.898) | −0.151 (0.275) | 0.197 (0.154) | −0.157 (0.256) | 0.058 (0.679) | 0.173 (0.212) | 0.021 (0.878) | −0.349 ** (0.010) |
| S | 0.118 (0.396) | 0.114 (0.411) | −0.090 (0.518) | 0.139 (0.315) | 0.165 (0.233) | 0.140 (0.312) | 0.100 (0.472) | −0.010 ± 0.941 | −0.068 ± 0.626 | 0.179 ± 0.195 | −0.116 (0.403) |
| T | −0.086 (0.536) | 0.170 (0.219) | 0.128 (0.355) | −0.046 (0.739) | −0.377 ** (0.005) | 0.278 * (0.042) | −0.252 (0.067) | 0.226 ± 0.101 | 0.077 ± 0.581 | −0.145 ± 0.295 | −0.177 (0.199) |
| N | −0.018 (0.900) | −0.100 (0.472) | −0.193 (0.162) | −0.244 (0.075) | −0.237 (0.085) | 0.108 (0.436) | −0.300 * (0.027) | 0.034 ± 0.808 | −0.072 ± 0.603 | 0.037 ± 0.792 | −0.309 * (0.023) |
| I | 0.171 (0.218) | −0.064 (0.645) | −0.075 (0.589) | −0.087 (0.532) | 0.197 (0.154) | 0.032 (0.818) | −0.089 (0.524) | 0.031 ± 0.824 | −0.264 ± 0.054 | 0.162 ± 0.242 | −0.080 (0.568) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Espinosa, G.; Boned-Murillo, A.; Orduna-Hospital, E.; Díaz-Barreda, M.D.; Sánchez-Cano, A.; Bielsa-Alonso, S.; Acha, J.; Pinilla, I. Retinal Vascularization Abnormalities Studied by Optical Coherence Tomography Angiography (OCTA) in Type 2 Diabetic Patients with Moderate Diabetic Retinopathy. Diagnostics 2022, 12, 379. https://doi.org/10.3390/diagnostics12020379
Fernández-Espinosa G, Boned-Murillo A, Orduna-Hospital E, Díaz-Barreda MD, Sánchez-Cano A, Bielsa-Alonso S, Acha J, Pinilla I. Retinal Vascularization Abnormalities Studied by Optical Coherence Tomography Angiography (OCTA) in Type 2 Diabetic Patients with Moderate Diabetic Retinopathy. Diagnostics. 2022; 12(2):379. https://doi.org/10.3390/diagnostics12020379
Chicago/Turabian StyleFernández-Espinosa, Guisela, Ana Boned-Murillo, Elvira Orduna-Hospital, María Dolores Díaz-Barreda, Ana Sánchez-Cano, Sofía Bielsa-Alonso, Javier Acha, and Isabel Pinilla. 2022. "Retinal Vascularization Abnormalities Studied by Optical Coherence Tomography Angiography (OCTA) in Type 2 Diabetic Patients with Moderate Diabetic Retinopathy" Diagnostics 12, no. 2: 379. https://doi.org/10.3390/diagnostics12020379







