Abstract
Natural killer (NK)/T-cell lymphoma (NKTCL) is an aggressive malignancy with unique epidemiological, histological, molecular, and clinical characteristics. It occurs in two pathological forms, namely, extranodal NKTCL (ENKTCL) and aggressive NK leukemia, according to the latest World Health Organization (WHO) classification. Epstein–Barr virus (EBV) infection has long been proposed as the major etiology of lymphomagenesis. The adoption of high-throughput sequencing has allowed us to gain more insight into the molecular mechanisms of ENKTCL, which largely involve chromosome deletion and aberrations in Janus kinase (JAK)-signal transducer and activator of transcription (STAT), programmed cell death protein-1 (PD-1)/PD-ligand 1 (PD-L1) pathways, as well as mutations in tumor suppressor genes. The molecular findings could potentially influence the traditional chemoradiotherapy approach, which is known to be associated with significant toxicity. This article will review the latest molecular findings in NKTCL and recent advances in the field of molecular diagnosis in NKTCL. Issues of quality control and technical difficulties will also be discussed, along with future prospects in the molecular diagnosis and treatment of NKTCL.
1. Introduction
Extranodal natural killer (NK)/T-cell lymphoma (NKTCL) is a highly aggressive non-Hodgkin lymphoma that is prevalent in Asia and South America and is characterized by a high relapse rate as well as a poor prognosis [1]. ENKTCL is notorious for its resistance to anthracycline-based chemotherapy, including cyclophosphamide, adriamycin, and vincristine. High expression of P-glycoprotein in ENKTCL has been suggested to be the mechanism for its intrinsic drug resistance [2]. Conventional chemotherapy using CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens is associated with an overall response rate (ORR) of less than 60% and a 5-year overall survival (OS) rate of less than 50% [3,4]. Although combination chemotherapy regimens that do not include anthracyclines, such as DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin), SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide), and AspaMetDex (L-asparaginase, methotrexate, and dexamethasone) and radiotherapy have been shown to improve complete remission (CR) rate and OS, the 5-year OS in advanced stage disease remains around 15–25% [5]. Prognostic models have been developed to categorize ENKTCL patients into risk groups to assist with prognosis and patient counselling. One of the commonly used models was the prognostic index for natural killer cell lymphoma (PINK), which consists of the following four independent factors impacting OS: age > 60 years old, stage III/IV disease, distant lymph node involvement, and non-nasal type disease [6]. Subsequently, Epstein–Barr virus (EBV) viral titer was shown to be a biomarker for tumor load and was incorporated into the PINK model, namely, PINK-E [7]. However, these prognostic models do not include tumor biological characteristics nor provide information relevant to targeted treatment decisions.
EBV infection is almost always detected in ENKTCL tumor cells. The detection of EBV-encoded RNA (EBER) aids in the diagnosis of ENKTCL in extranodal sites such as bone marrow and liver, especially when EBV-infected lymphoma cells have a low-grade appearance [8]. However, previous studies on the cytogenetic profile of NK cell lines in non-malignant and malignant NK lymphoproliferative diseases suggested that additional genetic abnormalities may be involved in the multistep transformation of EBV-infected cells into malignant lymphoma [9]. Other studies suggested that NK cells might have acquired CD21 from EBV-infected B cells through “trogocytosis”, an active transfer phenomenon where lymphocytes can exchange their surface molecules [10,11]. Activated EBV-infected T-cells subsequently produce interleukin (IL)-2, IL-9, and IL-10 in an autocrine manner and proliferate [10,11]. The EBV-infected NK cells may first manifest as chronic active EBV infection, but when more genomic alterations such as deletion of tumor suppressor genes and constitutive activation of specific growth or transcription factors occur, the infected NK cells will progress to ENKTCL. The development of molecular techniques, including genomics, transcriptomics, epigenomics, and metabolomics, has shed light on the molecular pathogenesis of ENKTCL, paving the way for the development of new biomarkers and targeted therapy.
2. Molecular-Based Research on ENKTCL
Cutting-edge molecular diagnostic tools have drastically improved our understanding of the molecular pathogenesis of ENKTCL. However, it is clear that these techniques cannot replace the traditional diagnostic tools of light microscopy and immunohistochemistry (IHC) (Figure 1). Nevertheless, the integration of cellular and molecular data is key to developing more comprehensive prognostic models and aid in the development of more personalized treatment approaches with the aim of improving OS and progression-free survival (PFS).
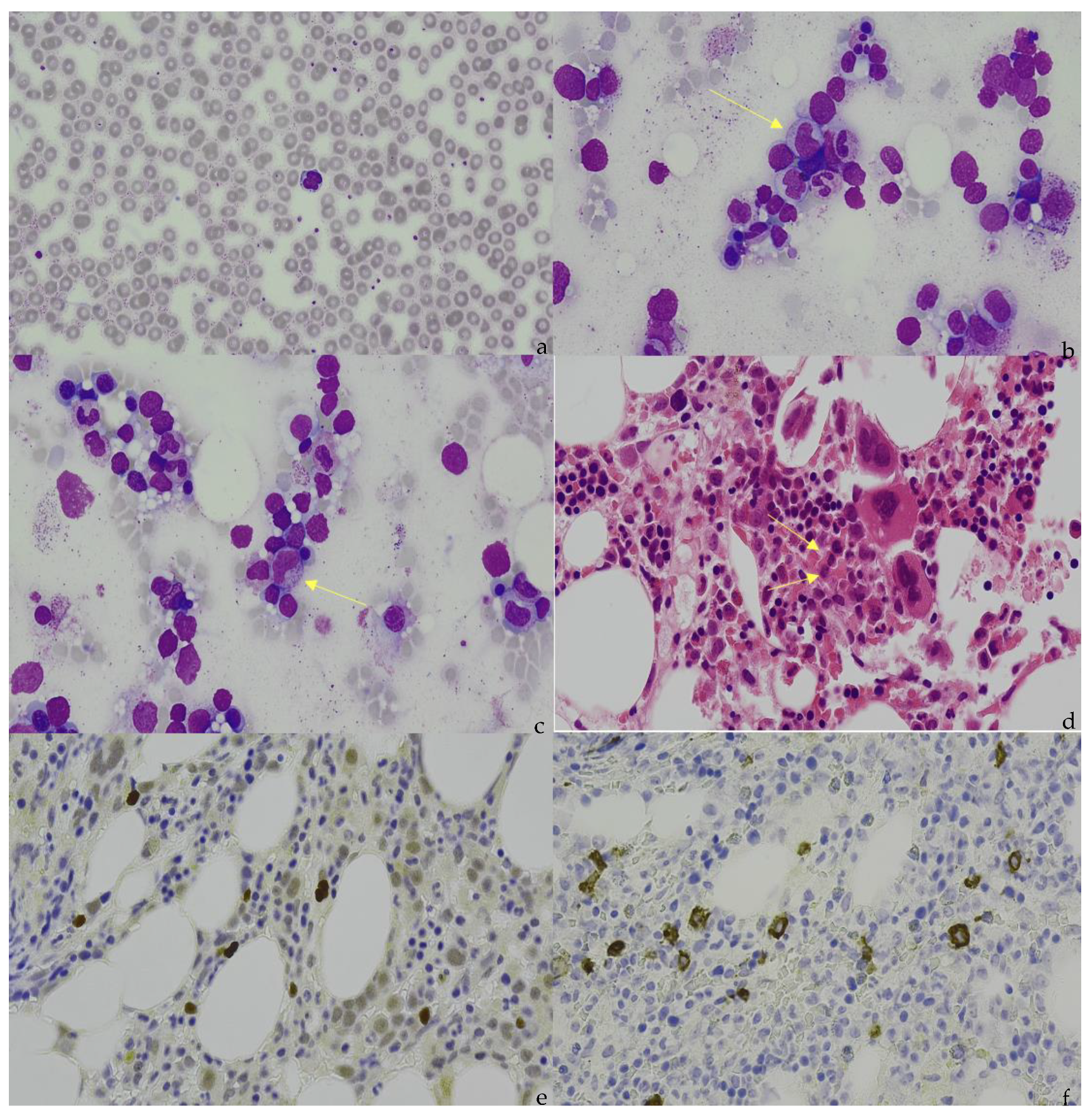
Figure 1.
(a) Peripheral blood appearance of extranodal NK/T-cell lymphoma (ENKTCL). The ENKTCL cells are of medium size with irregular nuclei, slightly dispersed chromatin, inconspicuous nucleolus, and moderate amounts of granulated cytoplasm. (b) (Marrow aspirate), (c) (Marrow aspirate), and (d) (Trephine). Arrows indicate the infiltration of ENKTCL into the bone marrow. The ENKTCL cells have similar appearance as described in the peripheral blood smear. Trilineage hematopoiesis is represented in this patient. (e). Immunohistochemical staining shows that the ENKTCL is positive for CD56. (f). In situ hybridization (ISH) staining shows that ENKTCL is positive for EBV-encoded RNAs.
2.1. Array-Based Comparative Genomic Hybridization (aCGH)
Microarray is a high-throughput, powerful biotechnology that provides a detailed view of the entire genome, transcriptome, and proteome. The principles of microarray are based on the complementary binding of immobilized oligonucleotides, or antigens, on a chip with fluorescently labelled nucleotides or proteins, respectively, in the samples. A signal is produced post-hybridization and is compared with an unaffected control. This method can produce either quantitative (gene expression) or qualitative (diagnostic) data [12].
Microarrays differ according to the nature of the probe, the solid-surface support used, and the specific method used for probe addressing and/or target detection [12]. Five microarrays have emerged for use in both research and clinical settings, namely, printed microarrays, in vitro-synthesized microarrays, high-density bead microarrays, electronic microarrays, and suspension bead microarrays [12].
The advantage of microarray is that it is easy to use and allows parallel detection of thousands of genes or RNA products in multiple samples. In addition, a wide collection of sub-genome arrays are available commercially, including a collection of promoters, coding regions, transcript 3′ ends, alternative spliced exons, single nucleotide polymorphisms (SNPs), and disease-gene arrays [13]. However, compared with next-generation sequencing (NGS), microarrays are limited by requiring prior knowledge of the genome or genomic features of a particular cancer, and carry a high signal-to-noise ratio due to cross hybridization between similar sequences as well as difficulty in detecting low-frequency mutations [14]. Nevertheless, DNA microarrays have been widely utilized in ENKTCL research and have the potential to be useful in clinical practice. Huang et al. used an in vitro synthesis microarray (Affymetrix GeneChip array) to extensively delineate the molecular pathways and factors involved in ENKTCL and confirmed the role of del6q21 and underexpression of tumor suppressor genes in the 6q21 region, namely, ATG5 and AIM1 in these tumors [15]. HACE1, a target of epigenetic inactivation in Wilms’ tumor, was discovered to be markedly reduced. Lastly, expression of the PDGF-α receptor was markedly increased, significantly implicating the associated AKT and JAK-STAT pathways were in the pathogenesis of NKTCL. Interestingly, TNFAIP3, encoding an inhibitor of the NF-κB pathway, was underexpressed in the study, highlighting the potential involvement of the NF-κB pathway in the pathogenesis of NKTCL [15].
2.2. Next-Generation Sequencing (NGS)
With the ability to sequence multiple fragments in parallel and generate a large amount of data rapidly and inexpensively, NGS offers an unprecedented opportunity to study the human genome, including the whole genome, exome, and transcriptome. NGS enables us to study the molecular pathogenesis of ENKTCL using a multi-omics approach by investigating genomics, transcriptomics, metabolomics, proteomics, and interactomics.
Many NGS platforms currently exist for massive data analysis, including Illumina (Solexa), Roche 454, Ion torrent: Proton/PGM, and SOLiD sequencing. A comprehensive literature search by the authors found that illumine and Ion Torrent panels have been extensively used in the field of NK/T-cell lymphoma.
Illumina acquired the Solexa company, which was the first inventor of a genome analyzer, in 2007. This sequencer uses the principle of sequencing by synthesis. Essentially, the technology involved sequential steps of DNA library construction by fragmentation of template DNA by either sonication, nebulization, or shearing. This is followed by DNA repair, end polishing, and ligation of platform-specific adaptors. Then the DNA library is denatured into single strands and hybridized onto the flow cell, followed by bridge amplification and finally the incorporation of four different nucleotides (ddATP, ddGTP, ddCTP, and ddTTP) onto the complementary strands. With each cycle of incorporation of a nucleotide, a cleavable fluorescent dye attached to the nucleotide is released. The fluorescent signal is subsequently captured by a charge-coupled device. With continual improvements in polymerase, buffer, flow cell, and software, the output drastically increased from a 1 G/run in Solexa GA to 2000 GB/run in NovaSeq 6000 [16]. Moreover, the minimum DNA input amount is as low as 1 ng, and the use of index labels during DNA library construction allows multiple samples to be sequenced at one time. However, the shortcomings of the Illumina sequencer are related to its intrinsic short read length, which poses difficulties in de novo sequencing. In addition, Kircher et al. reported that substitution type miscalls are the dominant source of errors as a result of similar emission spectra of the fluorophores between A and C as well as G and T [17]. Nakamura et al. also identified two major sequence patterns that trigger the sequence specific error (SSE), namely, inverted repeats and GGC sequences. SSE could be a potential cause of false single nucleotide polymorphism (SNP) calls and hinders accurate de novo assembly [18]. Nevertheless, with the features of high throughput and low cost, Illumina sequencers remain the most popular molecular tools for genome analysis in the era of precision medicine.
ENKTCL research using the Solexa/Illumina NGS platforms has produced interesting results. In the field of genomics, Jiang et al. reported that DDX3X (RNA helicase) is the most frequently mutated gene, occurring in 20% of study subjects. Moreover, mutations of DDX3X seldom overlapped with those of TP53, implying that DDX3X might be a target of TP53 or might cooperate with TP53 as tumor suppressor genes. Importantly, multivariate analysis showed that DDX3X mutations and TP53 mutations, along with International Prognostic Index (IPI) scores, were independent prognostic variables affecting OS and PFS [19]. Dobashi et al. utilized Illumina MiSeq with targeted capture sequencing to study the mutation spectrum in 25 cases of ENKTCL samples in Japan. They found that the most frequently mutated gene was BCOR, a chromatin modifying gene, accounting for 32% of the mutations found, followed by TP53 (16%), DDX3X (12%), FAT4 (8%), and NRAS (8%) [20]. Of note, DDX3X mutations were less frequent in this study than in the study by Jiang lu et al., which might be due to different ethnic groups and different bioinformatic pipelines. No associations between BCOR mutations and OS or PFS were demonstrated [20]. Lee et al. performed whole exome sequencing (WES) and RNA-sequencing on ENKTCL tissues and cell lines using the HiSeq2000 sequencer. Again, genomic analysis revealed enrichment of mutations in the JAK/STAT pathway, tumor suppressor genes, and chromatin-modifying genes. STAT3 was the most frequently mutated gene (26.5%), followed by BCOR (20.6%), MLL2 (17.6%), and TP53 (11.8%). Cluster analysis of both cancer tissues and cancer cell lines also highlighted upregulations in genes related to the JAK/STAT signaling pathway, the mechanistic target of rapamycin (mTOR) signaling pathway (downstream of JAK/STAT), and genes related to the PRC2 polycomb complex, which are involved in epigenetic modification (H3K27 trimethylation). Interestingly, genes related to angiogenesis were upregulated only in ENKTCL tissues but not in ENKTCL cell lines. This could possibly be explained by angiodestruction in ENKTCL tissues with compensated angiogenesis, which was not observed in cell lines [21].
In the field of epigenomics, Zhang et al. identified abnormal miRNA expression profiles in ENKTCL cell lines compared with normal NK cells using Solexa high-throughput sequencing. In particular, the level of miRNA-155 expression was abnormally elevated in ENKTCL cell lines and was shown to correlate with the expression of inflammatory mediators such as IL-6, IL-13, and tumor necrosis factor (TNF), suggesting a potential role in promoting the transformation of inflammatory lesions into malignant cells as well as changing the tumor’s microenvironment. Combination treatment with anti-miRNA-155 and doxorubicin increased the sensitivity of ENKTCL cell lines to doxorubicin, which suggests that anti-miRNA-155 may have a role in overcoming drug resistance [22]. Kucuk et al. used the Illumina Genome Analyzer IIx sequencer to study global methylation patterns by methylation-sensitive cut counting (MSCC) [23]. This technique uses the restriction enzyme HpaII, which cleaves CCGG sites only if cytosines are not methylated. Digestion fragments were size-selected and subjected to NGS [24]. The cut sites were then counted and used to infer the number of methylation sites in particular regions of interest. The higher number of cut sites implied fewer methylation sites. Relative to normal NK cells, malignant samples had promoter hypermethylation, gene body hypomethylation, or usually both. In addition, the promoters of tumor suppressor genes (BIM, DAPK), negative regulators of the JAK/STAT pathway (SOCS6, ZNFX3), epigenetics regulator (TET2), cytokine receptor (IL12RB2), and signaling molecule SHP1 (PTPN6) were aberrantly hypermethylated, and mRNA expression of the aforementioned genes was also downregulated as proven by real-time quantitative polymerase chain reaction (qRT-PCR) [23].
Released by Life Technologies at the end of 2010, the Ion PGM and Ion Proton represent groundbreaking molecular tools that utilize semiconductor technology to sequence nucleic acids and are suited for sequencing amplicons or small genomes. To construct a DNA library in Ion PGM and Ion Proton, sample DNA is fragmented and linked to specific adapter sequences and clonally amplified by emulsion PCR on the surface of 3-micron diameter beads (Ionsphere). Enriched particles are then primed for sequencing by annealing a sequencing primer, and subsequently, the Ionsphere with the DNA products is loaded into proton-sensing wells. Sequencing is started by introducing each type of dideoxynucleotide triphosphate (ddNTP) into the microfluidic conduit on the chips sequentially. When bases of that type are incorporated, a hydrogen ion is released, and a hydrogen ion detector translates the released hydrogen ions from each well into a quantitative readout of the nucleotide base [25]. As a result, Ion PGM and Ion Proton are based on “sequencing by synthesis,” but the detection involves counting hydrogen ions that are released during polymerization of DNA and not fluorescence. Because it does not require scanning or measurement of light, an Ion Torrent sequencer such as Ion PGM has a short run time (2 h) and an acceptable read length (~200 bases) [25]. However, compared with the Illumina platform, the Ion Proton platform has a higher rate of misidentifying nucleotide substitutions in the junctional areas of two homopolymers [26]. Possible reasons are related to miscounting of signal intensity when multiple hydrogen ions are released during nucleotide hybridization in homopolymer areas. Choi et al. reported 25 mutations in 21 genes in 5 patients with ENKTL using the Ion Proton platform and identified three recurrent mutations in actionable genes, namely, KMT2D p.P1194H, ARID1A p.P494T, and TP53 p.R273P [27], which were consistent with the results of other studies [19,20,21]. In addition, several other mutations in genes with unknown significance were identified including chromatin modification genes (SETD2 and KAT6B), transcription regulators (MBD1, BCL11B, ZNF521, and PER1), genes that regulated cytokines and structural integrity (CDH11, MYH9, and SYNE1), and the DNA damage checkpoint gene (ATR) [27]. Montes-Mojarro et al. utilized Ion PGM to investigate the mutational profile of 71 patients with ENKTCL in Latin America and compared the results with other studies. STAT3 mutation was the most frequent mutation in Latin American cases (23% of cases) [28], compared with BCOR mutation in a Japanese study (32% of cases) [20] and DDX3X mutation (20% of cases) in a Chinese study [19]. Meanwhile, mutations in tumor suppressor genes (BCOR, DDX3X, TP53) were again confirmed in the Latin American study. In addition, mutations in genes regulating cytoskeleton structure (MSN) and transcription (MGA) were again identified. Interestingly, mutations in STAT3, BCOR, and DDX3X were mutually exclusive, indicating their role in the pathogenesis of ENKTCL. Lastly, none of the mutations in this study were of prognostic value [28].
2.3. Proteomics
Proteomics refers to the analysis of the structure and function of proteins to acquire a global and integrated view of the biology of a cell. Protein expression is regulated at the transcription, translation, and post-translation levels. Compared with the relatively stable state of the genome, proteomics represents a more dynamic state and an ongoing process that underlies the initiation and progression of cancer cells [29]. Apart from traditional methods of studying protein expression, such as flow cytometry or Western blot, a few high-throughput methods are now increasingly used in cancer research, including the search for novel biomarkers, minimal residual disease (MRD) analysis, and deciphering signaling pathways of leukemogenesis; these high-throughput methods include mass spectrometry by electrospray ionization or matrix-assisted laser desorption/ionization (MALDI), protein microarrays, and mass cytometry (CyTOF) [30]. To date, only two groups have utilized proteomics to study molecular aspects of ENKTCL. Xu et al. performed a metabolomic assay on serum samples from 24 ENKTCL patients using liquid chromatography (LC)-mass spectrometry (MS), highlighting an association between aberrant metabolic programming and cancer progression [31]. Interestingly, ENKTCL patients with lower levels of alanine, aspartic acid, glutamate, and succinic acid, which are the amino acids for asparagine synthesis, have a poor response to asparaginase-based therapy and inferior survival. A prognostic score, namely, the ASPM score, was developed based on the above metabolite levels and was shown to independently predict PFS and OS in multivariate analysis [31]. Another group used LC-MS to study proteomic markers of changes in the cerebrospinal fluid (CSF) of ENKTCL patients [32]. After hierarchical cluster analysis by computer software (STRING), the following nine core proteins: HRG, TIMP-1, SERPINA3, FGA, FGG, TF, FGB, APP, and AGT were all higher in ENKTCL patients than in healthy individuals [32]. These proteins have a role in cell adhesion, regulating growth factors and cytokines, tumor invasion, metastasis, and angiogenesis. However, the significance of these proteins in ENKTCL remains to be explored. Nevertheless, proteomic studies offer an additional choice to precisely identify the pathogenesis of ENKTCL.
3. Quality Control in NGS
With increasing use of NGS in both clinical and research settings in ENKTCL diagnosis, the first and most important step is to implement a quality control process to ensure data accuracy and precision.
3.1. Pre-Analytical Process
The pre-analytical stages of NGS include collection of patients’ clinical details, choosing appropriate sequencing methods and platforms, specimen acquisition, transport, processing, preservation, and storage, as well as NGS library preparation [33]. These steps involve multidisciplinary collaboration between laboratory technicians, surgeons, clinicians, nurses, pathologists, and patients themselves, and play a key role in the success of subsequent steps in NGS analysis. Collection of the patient’s details and communication with patients is also crucial to ensure an appropriate test or sequencing platform is chosen.
Specimen acquisition, transport, processing, and preservation are important steps for reliable and accurate biomarker analysis [33]. Goswami et al. identified five key factors for successful NGS analysis: type of tumor sample, tumor size, tumor location, presence of necrosis, and treatment effect [34]. Specimens collected by resection or excisional biopsy yield the highest NGS success rate. In addition, in order to obtain a minimum of 10 ng recommended DNA input for Ion AmpliSeq Cancer Hotspot Panel, approximately 2000 whole cells are needed and at least 60–100 mm2 selected tumor-rich tissue is required [35]. Specimens from tumors in deep-seated organs or lungs, and those from endoscopic biopsies, are generally small, often resulting in tiny, circled tumors (<10 mm2) for NGS mutation testing [34]. Of note, head and neck tumors have the highest success rate (100%) whereas bone specimens, which require decalcification with strong acids, have a lower NGS success rate of 62% [34]. Time interval between specimen acquisition and fixation should also be minimized. In general, specimens used for DNA and RNA analysis should be transported to the testing lab within 8 h and 10 min, respectively [36]. Tissue processing and preservation are critical steps as well to ensure sufficient quantity and quality of specimens for optimal morphological diagnosis and NGS analysis. Formalin is a commonly used tissue fixative in most anatomical pathology laboratories due to its superior ability to preserve morphological details by fixing both cytoskeletal and soluble proteins [37]. However, formalin fixation can potentially lead to cross-linking between nucleic acids, DNA denaturation, DNA fragmentation, and creating artificial mutation [38]. Methanol-based fixation is associated with higher yield of DNA fragments and shows better correlation than formalin-based fixation in calling copy number variations in snap-frozen biopsies. Thus, methanol is suggested as a better preservation method for subsequent NGS analysis than formalin-based fixation. However, more studies are required to evaluate the effect of long-term methanol fixation on DNA quality and quantity as the only available study utilized samples collected no more than 6 months previously [39]. Extracted DNA should be stored at −20 °C and RNA at −80 °C to maintain sample integrity. Sequencing libraries and PCR products may be stored at −20 °C [40].
3.2. Analytical Process
Whilst NGS is a promising molecular technique in the diagnosis, prognostication, and treatment of ENKTCL, it generates a massive amount of bioinformatic data compared with conventional Sanger sequencing. Because of the huge amount of data, a tightly regulated analytical sequence and process have to be implemented and maintained to ensure the data and results obtained are accurate and precise. The data analysis pipeline (also called bioinformatics pipeline) typically involves the following four major steps: base calling, read alignment, variant identification, and variant annotation. The complex analytical process requires different bioinformatics software and tools that are available commercially as well as on the internet. Essentially, the very first step of NGS involves translating the fluorescence generated during nucleotide incorporation into different base pairs, i.e., base calling. After filtering out bad reads, eliminating the sequencer’s bubble effects, and trimming reads at front and tail by bioinformatics software such as AfterQC, various parameters can be determined in order to conduct the subsequent analysis. “Per base sequence quality score” looks at the probability that a base has been identified incorrectly. The Illumina platform introduced the Q score (Q = −log10(e)) where “e” is the estimated probability that a base is called incorrectly. A good quality read, especially for mutation detection, should ideally have a score of Q30 or above. Of note, sequences at the start and end of each read may have a lower Q score due to ineffective synthesis by DNA polymerase at the beginning and end of the reactions. Santani et al. recommended >80% of bases should be Q ≥ 30 [41]. A sufficient quantity of reads and depth of coverage are also important. Coverage refers to the number of times each nucleotide is sequenced [42]. Regions with low depth of coverage have a higher false negativity rate and should be eliminated from analysis. In general, whole-genome sequencing (WGS) and targeted gene sequencing should at least achieve 30x and 500x depth of coverage to ensure read quality. However, the total number of required reads and depth of coverage vary between laboratories as different laboratories have different analytical sensitivity and different pre-analytical requirements for minimum tumor content. The appropriate number of reads and depth of coverage should be determined in the NGS validation step, which is outside the scope of the current review. “Per sequence GC content” distribution curve, which plots the percentage of GC content vs. number of reads, is also an essential parameter. Theoretically, in a random DNA library, the distribution of GC content should be close to normal distribution. Abnormal peaks or a shift of the actual curve relative to the theoretical curve may indicate another source of contamination such as DNA from bacteria and rRNA contamination. These sources of contamination must be eliminated before further downstream analysis. “Uniformity of coverage” which measures variability in sequence coverage across target regions is an important parameter in NGS. Certain regions in the gene sequence such as at the edges of targeted regions, regions with high GC content as well as regions with homopolymers, may have lower coverage depth which affects the quality of the data. Strategies have been devised to tackle this issue in hybridization-based enrichment techniques, such as a repetitive bait design process, in which a less-dense bait tiling is tested first and underperforming regions are then optimized [41]. Moreover, one can consider extending the baited region beyond the actual region of interest to increase the coverage at the edges of targeted regions [41]. One should also be aware of the “mapping quality score” which is basically the probability that a read is aligned with the wrong position in the human genome. The probability is calculated as P = 10(−q/10) where q is the mapping quality. Multiple mappings are typically seen in repetitive regions especially if using sequencers that produce short reads such as HiSeq2000 (2 × 100 base pairs in length). PCR duplicates should be removed prior to analysis to ensure that duplicated sequences, which may sometimes contain artificial mutations, will not be overrepresented [42]. During library preparation for NGS, we first fragment the DNA, e.g., by sonicator or enzymes, and then ligate the adapters to both ends of the fragments and amplify the fragments with the adapters. During PCR amplification, multiple copies of the same DNA fragments will be generated and thus a certain number of PCR duplicates are unavoidable. However, abnormally high proportions of PCR duplicates may suggest possible bias generated during DNA library preparation especially in sequences with low GC content and short read lengths. Several bioinformatics tools are now available to remove DNA duplicates, including Ion AmpliSeq Cancer Hotspot. To increase the confidence in calling variants, confirmatory tests by Sanger sequencing or a combination of two sequence platforms such as WES and WGS should be performed [43].
3.3. Post-Analytical Process
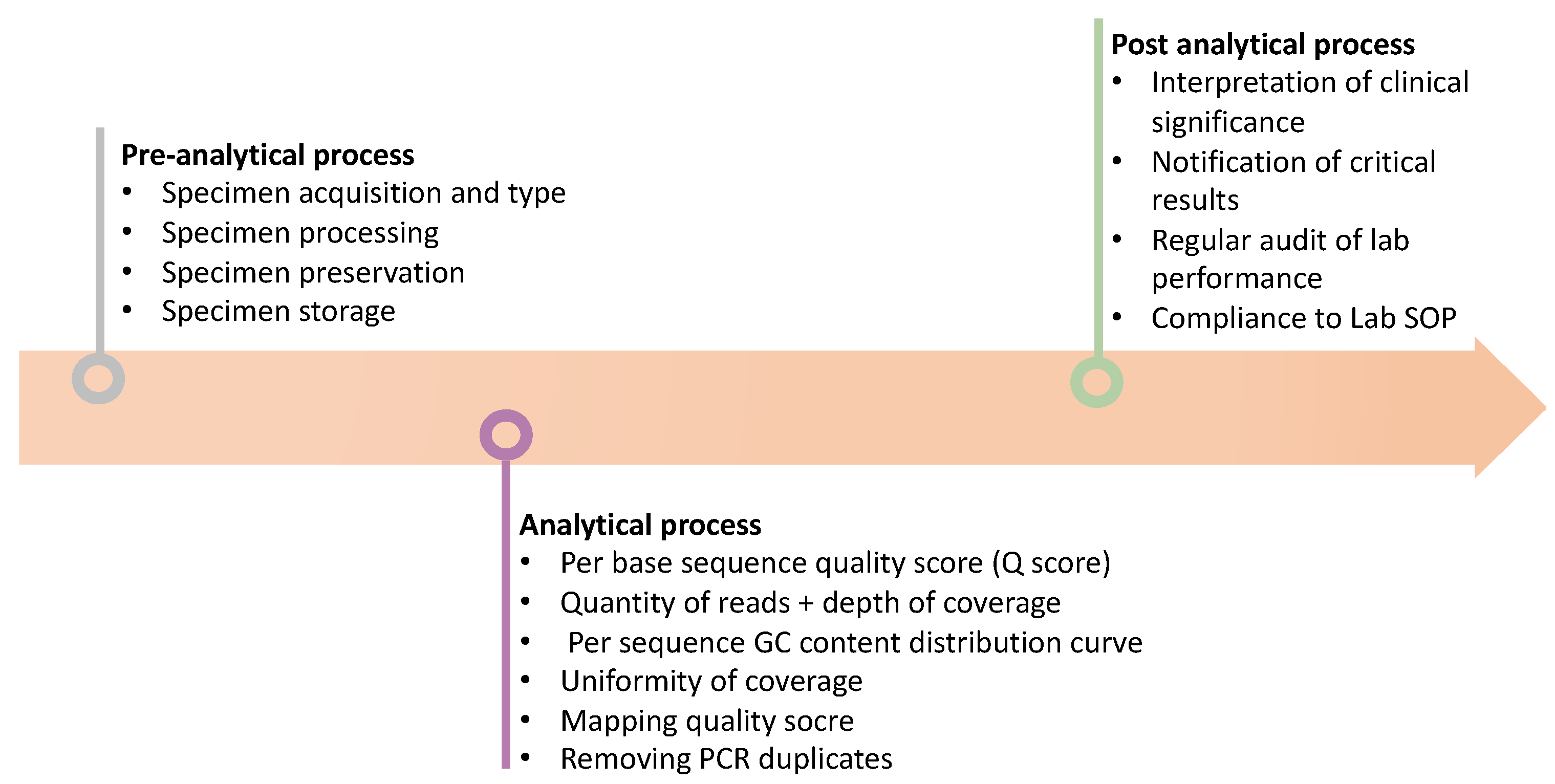
Post-analytical processing involves converting the results into a written report, immediate notification of critical results to the responsible clinician, assessment of the significance of the results based on established reference values, transmission of reports without delay to the requested location, and finally a regular audit of laboratory performance [44]. A molecular laboratory should have a standard operating procedure (SOP) document to tightly regulate the aforementioned processes and ensure traceability of records. In general, the turnaround time from sample collection to result reporting should be around 1–2 months and an annual review as well as update of the previous report based on the most recent molecular findings are needed [41]. A summary of these processes is shown in Figure 2.
Figure 2.
Schematic diagram briefly illustrates the procedure to qualify the NGS results. Firstly, perform the pre-analytical analysis before processing NGS, such as observing the specimen type and preservation etc. Secondly, observe the quality of the NGS results, such as Q score, read depth, and coverage rate etc. Finally, interpretate the NGS results based on the guideline and the clinical indication.
4. Potential Sources of Error of Using NGS in ENKTCL Diagnosis
ENKTCL is most commonly present in the upper aerodigestive tract, particularly the nasal cavity. One potential source of error in NGS testing lies in the sample preparation steps in formalin-fixed and paraffin-embedded (FFPE) samples from the nasal cavity. Formalin, a liquid containing 37–40% formaldehyde and 10% methanol, can cause artificial DNA cross-linking, DNA fragmentation and degradation, deamination of cytosine (resulting in either a C→T or a G→A on the sense strand), and hydrolysis of N-glycosyl bonds [45,46,47,48]. These artificial sequence artifacts could interfere with the detection of low-frequency mutations. Lin et al. reported the highest level of baseline noise level could be up to 1.3% in the EGFR gene in cancer-free FFPE samples, attributable to artificial cytosine deamination [49]. Williams et al. demonstrated that 97% of artificial mutations in FFPE samples occurred at guanosine or cytosine positions and resulted in C→T or G→A transitions [45]. Several methods have been devised to tackle this artifact, including duplicate experiments, pre-treatment of FFPE samples with uracil-DNA-glycosylase, and heating the FFPE samples at a high temperature and alkaline pH to increase the yield of DNA from FFPE samples [50].
DNA fragmentation is another potential source of errors during the NGS study for ENKTCL. During library preparation, ultra-sonication as a non-biased manner has been widely used to prepare DNA fragments of an appropriate size. However, during ultra-sonication, the physical scattering of DNA solution leads to a loss of DNA sample. In view of this, enzymatic fragmentation with DNA endonucleases has been developed to minimize the loss of DNA, especially for nano-quantity samples. The downside of enzymatic fragmentation occurs when the DNA fragments produced by this method are too long to be sequenced by NGS.
Barcode contamination is another issue to be paid attention to. During NGS data analysis, some non-target fragments that came from the last round of NGS samples but with the same barcode utilized are detected. These non-target fragments will be misread and contaminate the target data. Thus, different barcodes should be used after each round of sequencing.
5. Designing NGS Panel Content
NGS yields a tremendous amount of genetic information. However, the complexity of genetic information can sometimes hamper the diagnostic process, especially when some gene mutations could just represent a constitutional change. Thus, designing the NGS panel must take several factors into consideration. In a research setting, NGS could be useful in discovering novel mutations. A more comprehensive panel that includes coding regions of several hundred genes or a pan-cancer gene panel will be more useful. However, in clinical settings, genes with known mutation hotspots and those that carry diagnostic, therapeutic, and prognostic information will be more desirable. Meanwhile, we have to consider the quality of the sequencing data as well as the financial cost [51]. As more genes are sequenced, the quality of sequencing will be compromised in terms of read depth and depth of coverage. In addition, it will involve more complex analytical issues and a longer turnaround time. Moreover, the technical limitations of NGS have to be considered when designing a gene panel. Due to the short read lengths of NGS technology and the complexity of the genome, challenges arise from the detection of copy number variations (CNV) of the disease-related genes. Different approaches have been recently devised to detect CNVs, namely, paired-end mapping, the split read-based method, the read depth-based method, and the assembly-based method [52]. However, a validated approach/guideline to detect CNVs is still lacking. Thus, one has to take into consideration whether your laboratory has sufficient bioinformatic support when designing a gene panel involving CNVs.
An exhaustive literature review by the authors identified the five most recently published large-scale NGS studies on ENKTCL as shown in Table 1 below. Mutated genes that were identified in ≥2 patient samples/cell lines are listed in the table. This table of genes is not validated in the author’s laboratories, but each genetic mutation could possibly be a future topic in later research. At the time of writing this review, only DDX3X and TP53 mutations have been shown to have a prognostic impact [19].

Table 1.
Summary of mutated genes from five NGS studies in patients with ENKTCL [19,20,21,27,28]. Only mutated genes that were identified in ≥2 patient samples/cell lines in the reference studies are listed in the table. Tiers of gene mutations are classified according to guideline from Association of Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists [53]. Only DDX3X and TP53 mutations have been shown to have prognostic impact.
6. Future Directions in the Molecular Diagnosis of ENKTCL
The detection and analysis of mutations is a very important biomarker in tumor diagnosis and prognostic prediction. Currently available prognostic indexes for ENKTCL, e.g., IPI, PINK, and PINK E, do not incorporate molecular mutations as one of the prognostic markers. Recently, three molecular subtypes, namely, MB, TSIM, and HEA subtypes are described to account for different genomic alterations profile and different EBV pattern in ENKTCL based on genomic and transcriptomic data [54]. MB subtypes are enriched for MYC-associated aberrations, mainly as MGA (a tumor suppressor) mutations and loss of heterozygosity (LOH) at the BRDT locus. TSIM subtypes are characterized by aberrations in tumor suppressors (TP53 mutation and del6q21) and immune modulators (Jak-STAT mutation/amplification and amp9p24.1/PD-L1/2 locus). Lastly, the HEA subtype is enriched for T cell gene expression and mutations in epigenetic regulators (EP300, HDAC1, and ARID1A) [54]. Interestingly, the MB subtype was associated with much poorer prognosis than the TSIM and HEA subtypes with 3-year survival rates as 38.5%, 79.1%, and 91.7%, respectively [54]. Since MB subtypes presented with MYC-associated aberrations, homoharringtonine (HHT), which is a protein translation inhibitor that exhibits an antileukemia effect through regulating MYC transcriptional expression [55], was shown to exhibit an inhibitory effect on both the MGA-knockdown cell model and the xenograft zebrafish model [54]. With the advancement of sequencing technology, featured by longer read length and faster processing time, a plethora of different molecular mutations and thus different molecular subtypes will be illustrated for prognostication and eventually therapeutic targets for ENKTCL.
From a technical point of view, when the biopsy tissue is too necrotic, particularly in ENKTCL, and NGS testing of the biopsy specimen is unfeasible, cell-free circulating tumor DNA (ctDNA) could possibly be an important complementary source for NGS testing in patients with NKTCL. CtDNA results from tumor cell apoptosis and necrosis with a characteristic peak at 166 bp [56]. Li et al. successfully used ctDNA to identify mutations verified in NKTCL tumors and achieved satisfactory sensitivity (72.4%) [57]. Furthermore, additional somatic mutations can be detected in ctDNA due to tumor spatial heterogeneity. Because of its ease of repeatability and simplicity, ctDNA is likely to become a convenient tool for NKTCL diagnosis and assessment in the clinical and research setting in the future. However, attention must be paid to several important aspects in handling ctDNA. For instance, single centrifugation alone cannot effectively remove all the nucleated cells in plasma, and this can potentially lead to the contamination of ctDNA. Chiu et al. reported that double centrifugation (1600× g for 10 min, then 16,000× g for 10 min) is the best and most cost-effective method to confidently obtain cell-free plasma [58]. Another method is centrifugation followed by filtration, but this method is more expensive [58]. Plasma should be stored at −20 °C or −80 °C if ctDNA is not extracted immediately. Chan et al. also demonstrated that ctDNA will become more fragmented if the plasma undergoes freezing-thawing more than twice [59]. The choice of anticoagulant used in blood collection is also an essential element. Beutler et al. demonstrated that heparin could inhibit PCR, which makes it an unfavorable choice for samples that are to undergo NGS [60]. Lam et al. showed that contaminated DNA content was lower in plasma samples collected in ethylenediaminetetraacetic acid (EDTA) tubes than in heparin or citrate tubes, likely because EDTA causes less leucocyte necrosis or apoptosis when samples are stored for 24 h. Thus, EDTA is suggested to be a better anticoagulant for blood samples that may be processed for plasma DNA analysis up to 6 h after venesection [61].
7. Molecular Pathogenesis of ENKTCL and Its Implication in Personalized Treatment of ENKTCL
In the past, ENKTCL was treated using conventional chemotherapy and radiotherapy. There are more treatment choices for ENKTCL nowadays with the increased understanding of the pathogenesis of the disease, and novel agents are now available targeting different molecular pathways discovered in ENKTCL. Such advances are important since conventional chemotherapy may not achieve long-term remission in advanced-stage disease.
7.1. Brentuximab Vedotin (Anti-CD30 Antibody-Drug Conjugate)
CD30 (TNFRSF8) is a glycoprotein of the tumor necrosis factor receptor (TNFR) expressed on activated B, T, and NK cells. CD30 can activate the NF-κB signaling pathway and the mitogen-activated protein kinases (MAPKs) to modulate cell growth, proliferation, and apoptosis.
Between 31.2% and 47.3% of patients with ENKTCL are positive for CD30 [62], but the relationship between CD30 expression and the prognosis or clinical characteristics of ENKTCL remains unclear. The expression of CD30 was shown to be significantly associated with longer OS in a study of 72 patients with NKTCL who were treated with non-anthracycline-based chemotherapy [63]. Another study suggested that CD30 was an independent prognostic factor for OS and PFS [64].
A multicenter, phase II study of brentuximab vedotin was conducted in 33 patients with relapsed/refractory CD30-positive non-Hodgkin lymphoma, seven of whom had ENKTCL. Brentuximab vedotin was administered once every 3 weeks. The overall response rate for patients with ENKTC was 29%, with one patient obtaining complete remission and another achieving partial remission; the response lasted for more than one year in both patients [65].
Further studies are needed for the assessment of the efficacy of brentuximab vedotin, particularly in combination with chemotherapy, in the treatment of NK/T-cell lymphoma.
7.2. Immune Checkpoint Inhibitors (PD1 and PDL1 Inhibitors)
PD-L1 expression is higher in patients with EBV-positive compared with EBV-negative non-Hodgkin lymphoma [66], and the expression of PD-L1 in NKTCL ranges from 39% to 100% [67,68,69,70,71].
Programmed cell death protein-1 (PD-1)/PD-L1 pathways act as a “brake” to control and maintain immune tolerance within the tumor microenvironment by decreasing T cell activation, proliferation, cytokine secretion, and survival [72]. An association and interactions between EBV and the PD-1/PD-L1 pathway has been noted in different malignancies [73,74,75]. Moreover, EBV-driven LMP1 upregulated PD-L1 expression by activating the MAPK/NF-κB pathway [76]. There was overexpression of PD-L1 in both serum and tumor specimens from patients with ENKTCL, and this carried adverse prognostic impacts [69,76].
Studies using PD-1/PD-L1 inhibitors have shown promising results. Kwong et al. showed that PD-1 blockade with pembrolizumab was useful for the treatment of relapsed or refractory ENKTCL. Seven patients with relapsed or refractory ENKTCL (1 with stage I and 6 with stage IV disease) who had previously received L-asparaginase-based regimens were treated with pembrolizumab. The pembrolizumab dose was 2 mg/kg every 3 weeks in all but one patient, who received pembrolizumab every 2 weeks. The ORR was 100%; five patients (71.4%) achieved CR, and two even achieved molecular remission. The five patients who achieved CR were still in remission after a median follow-up of 6 months [77].
Li et al. also reported the efficacy of pembrolizumab in a series of seven patients with refractory/relapsed NKTCL [78]. Pembrolizumab was administered at a dose of 100 mg every 3 weeks for a median of four cycles (range 2–18). The ORR was 57.1%, with two patients obtaining CR (28.6%) and two achieving a partial response (28.6%). The response duration was 4.1 months, PFS 4.8 months, and OS 5.0 months. The combination of immune checkpoint inhibitors with brentuximab vedotin could also potentially increase antitumor activity [79].
It is clinically important to identify biomarkers that identify patients with a higher chance of response to immune checkpoint inhibitors. PD-L1 structural rearrangement (PD-1MUT) level is a potential biomarker for clinicians to choose anti-PD-1 therapy for patients with NKTCL [80]. Clinicians may consider selective use of immune checkpoint inhibitors based on PD-1MUT level so as to minimize potential side effects.
7.3. Daratumumab (CD38 Monoclonal Antibody)
Daratumumab, a CD38 monoclonal antibody, was approved as therapy for patients newly diagnosed or relapsed with multiple myeloma [81,82,83,84]. CD38 may also be a novel target for the treatment of patients with NKTCL since CD38 expression was found in 95% of tumor samples in a study of 94 patients with NKTCL. Clinical data suggests that CD38 may also be a novel prognostic biomarker in patients with NKTCL [85].
Daratumumab was used as monotherapy in a phase 2 study in 32 patients with ENKTCL. Daratumumab was given in 28-day cycles at a dosage of 16 mg/kg intravenously once weekly for cycles one and two, every other week for cycles three through six, and every 4 weeks thereafter until progression or intolerable toxicity. The results showed an ORR of 25.0%, although CR was not achieved in the study. The median OS and PFS were 141 days and 53 days, respectively, with a median follow-up of 10.2 months [86]. Daratumumab monotherapy may not be adequate to treat patients with aggressive features as it has modest activity as a single agent against NKTCL. Combination with other chemotherapy will be the future direction of clinical trials.
7.4. CC Chemokine Receptor 4 (CCR4)
Chemokines can promote progression, metastasis, and neovascularization of EBV-related hematologic malignancies [87,88]. Levels of chemokine (CC motif) ligand (CCL) 17 and CCL22 were significantly elevated in the sera of patients with ENKTCL. CCR4 is the receptor for CCL17 and CCL22 and was detected in the cell lines and tumor tissues of patients with ENKTCL [89].
Mogamulizumab is a humanized monoclonal antibody shown to be effective in killing CCR4-positive cells through the antibody-dependent cellular cytotoxicity, and to inhibit the growth of EBV-positive NK-cell lymphomas in murine models [88]. This suggests that blocking the interaction of CCR4 with its ligands CCL17 and CCL22 may have potential as a therapeutic option for ENKTCL.
7.5. Signal Transduction Pathway Targets
7.5.1. Platelet-Derived Growth Factor (PDGF) and PDGFR Antagonists
The platelet-derived growth factor receptor α (PDGFRα) is a receptor tyrosine kinase that mediates the interaction between external cell signals and the intracellular PI3K-AKT and JAK-STAT3 pathway proteins [90]. Abnormal expression of PDGFR and aberrations in the PDGF pathway are common in the oncogenesis of different types of tumors [91,92,93].
High PDGFRα expression is a marker of a poor prognosis (lower OS and PFS) in patients with ENKTCL [94], and PDGFR is correlated with reduced PFS [15]. PDGFR antagonists have been shown to induce G1 cell cycle arrest and inhibit cell proliferation in ENKTCL cell lines in vitro [15,95]. The results could indicate a potential role for small molecular tyrosine kinase inhibitors targeting the PDGF pathway in ENKTCL.
7.5.2. Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) and JAK-STAT Inhibitors (WP1066, CP-690550, AG490, Tofacitinib, and Chidamide)
Both in vitro studies and gene expression profiling data revealed recurrent mutations in Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway genes in ENKTL [21,96], leading to aberrant pro-proliferative signaling. It was found that 75.0% of ENKTCL biopsy specimens showed expression of pSTAT3, and pSTAT3 expression was correlated with Ki67 overexpression. The selective inhibitor, WP1066, induced apoptosis of ENKTCL cells via STAT3 suppression [96]. A functional JAK3 mutation associated with JAK-STAT activation was present in 23 of 65 (35.4%) patients with ENKTCL. Interestingly, JAK3 was shown to mediate a functional switch of EZH2 from histone methyltransferase to a transcriptional coactivator in ENKTCL and upregulates a set of genes involved in DNA replication, cell cycle regulation, biosynthesis, cell stemness, and invasiveness. [97]
The use of pan-JAK inhibitor CP-690550 and JAK2 inhibitor AG490 resulted in dose-dependent reduction of pSTAT5 and pSTAT3 together with increased apoptosis in ENKTCL cell lines [98,99]. Moreover, the JAK1/3 inhibitor, tofacitinib, induces G1/S arrest and inhibits cell growth in STAT3-mutant ENKTCL cells [100].
An ongoing phase 2 clinical trial (NCT03598959) is investigating the efficacy and safety of a combination of tofacitinib and chidamide in patients with relapsed or refractory ENKTCL.
7.5.3. NF-κB Signaling and Proteosome Inhibitors
One of the hallmarks of ENKTCL is the persistent activation of the NF-κB signaling pathway. It is thought that activation of NF-κB signaling may account for the transformation of EBV-infected cells into ENKTCL cells [101,102].
Proteasome inhibitors have latency-reversing properties that can reactivate EBV into the lytic cycle [103]. They will then help the immune system or anti-EBV drugs to kill the EBV-infected tumor cells (i.e., the “shock and kill theory”) [104].
Bortezomib is a first-generation proteasome inhibitor. The combination of bortezomib and GIFOX (gemcitabine, ifosfamide, oxaliplatin) chemotherapy resulted in an ORR of 43% and a median PFS of 4.3 months in seven patients with newly diagnosed ENKTCL, four of whom had advanced-stage disease [105].
Through activation of NF-κB pathway, LMP-1 was shown to upregulate CD25 expression in ENKTCL, which would then induce resistance to asparaginase in ENKTCL cell lines [101]. Thus, the combination of proteasome inhibitors and asparaginase-based chemotherapy may further improve the treatment responses of patients with relapsed/refractory ENKTCL.
7.6. Histone Acetylation and Histone Deacetylase Inhibitor (Chidamide)
Histone deacetylase (HDAC) inhibitors can induce different cytotoxic effects in cancer cells by histone acetylation of candidate tumor suppressors to initiate gene transcription [106]. Chidamide is a novel oral agent belonging to the benzamide class and is a subtype-selective inhibitor of HDAC 1, 2, 3, and 10. It also interferes with PI3K/Akt/mTOR and MAPK signaling and inhibits cell proliferation [107].
In a prospective phase 2 trial, chidamide monotherapy was studied in 15 patients with relapsed/refractory ENKTCL. Four of the 12 evaluable patients (33%) obtained CR with a median follow-up of 3.7 months; all CR patients remained disease-free at 6.9 months [107].
7.7. Chimeric Antigen Receptor T-Cell (CART) Therapy
Chimeric antigen receptor T-cell (CART) therapy is a new form of immunotherapy and has shown success in the treatment of relapses or refractory acute lymphocytic leukemia, diffuse large B-cell lymphoma, and multiple myeloma [108,109,110,111,112,113,114,115]. There are currently limited data on the use of CART therapy for ENKTCL, but the results of a phase 1 clinical trial of CD30 CART therapy for patients with advanced CD30-expressing lymphomas are pending (NCT03049449). It may be a promising treatment option.
7.8. Apoptotic Pathways and Other Tumor Suppressor Genes
Deregulation of apoptotic pathways is another mechanism by which ENTKL arises and progresses. Overexpression of surviving and mutations of TP53 conferred a survival advantage on the ENKTL cells [116]. Mutated TP53 was detected in 40% of cases in one study in China [117]. Mutated TP53 correlates with advanced stage presentation and poor prognosis in ENKTCL [118].
In addition, deletion at chromosome region 6q21–23, the most common cytogenetic aberrations have been implicated in the loss of several tumor suppressor genes including PRDM1, FOXO3, HACE1, and PTPPK [116]
At the time of writing this manuscript, no target therapies acting against the aforementioned pathways and genes have been developed in the treatment of ENKTCL.
A summary of novel agents in development or being investigated for ENKTCL is shown in Table 2 [15,65,77,86,88,95,100,105,107].

Table 2.
Novel agents and their targets in the treatment of ENKTCL.
8. Conclusions
ENKTCL is an aggressive malignancy with unique epidemiological, histological, molecular, and clinical characteristics. The use of high-throughput sequencing has allowed us to gain more insight into the molecular mechanisms of ENKTCL, which largely involves chromosome deletion and aberrations in the JAK/STAT and PD-1/PD-L1 pathways, as well as mutations in tumor suppressor genes. This information has provided new perspectives on immunotherapy and targeted therapy within the spectrum of ENKTCL treatment. These molecular findings could potentially revolutionize the traditional chemoradiotherapy approach, which is known to be associated with significant toxicity. The use of immunotherapy in combination with other mechanism-based targeted therapies is a promising strategy to treat ENKTCL.
Author Contributions
Conceptualization, acquisition, analysis, and interpretation of data/references; drafting and approving the manuscript, K.-H.S.; Y.-T.W.; K.-M.C. and M.-F.L. Interpretation of data/references; revising critically and approving the manuscript, C.Y.; Y.-T.C.; W.-Y.L.; C.C.; W.-S.F.; Y.-K.C. and H.-C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tse, E.; Kwong, Y.-L. How I treat NK/T-cell lymphomas. Blood 2013, 121, 4997–5005. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Oki, Y. Novel immunotherapy options for extranodal NK/T-cell lymphoma. Front. Oncol. 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhu, Y.; Cao, J.Z.; Zhang, Y.J.; Xu, L.M.; Yuan, Z.Y.; Wu, J.X.; Wang, W.; Wu, T.; Lu, B.; et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: Analysis from a multicenter study. Blood 2015, 126, 1424–1432. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; Chapter 8; IARC: Lyon, France, 2017. [Google Scholar]
- Liu, W.; Yang, Y.; Qi, S.; Wang, Y.; He, X.; Zhang, L.; Qu, B.; Qian, L.; Hou, X.; Qiao, X.; et al. Treatment, Survival, and Prognosis of Advanced-Stage Natural Killer/T-Cell Lymphoma: An Analysis From the China Lymphoma Collaborative Group. Front. Oncol. 2021, 10, 583050. [Google Scholar] [CrossRef]
- Lee, J.; Suh, C.; Park, Y.H.; Ko, Y.H.; Bang, S.M.; Lee, J.H.; Lee, D.H.; Huh, J.; Oh, S.Y.; Kwon, H.C.; et al. Extranodal natural killer T-cell lymphoma, nasal-type: A prognostic model from a retrospective multicenter study. J. Clin. Oncol. 2006, 24, 612–618. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, J.Y.; Hyun, S.H.; Ki, C.S.; Oh, D.; Ahn, Y.C.; Ko, Y.H.; Choi, S.; Jung, S.H.; Khong, P.L.; et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: A multicentre, retrospective analysis. Lancet Haematol. 2015, 2, e66–e74. [Google Scholar] [CrossRef]
- Greer, J.P.; Mosse, C.A. Natural killer-cell neoplasms. Curr. Hematol. Malign. Rep. 2009, 4, 245–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Nagata, H.; Ikeuchi, T.; Mukai, H.; Oyoshi, M.K.; Demachi, A.; Morio, T.; Wakiguchi, H.; Kimura, N.; Shimizu, N.; et al. Common cytological and cytogenetic features of Epstein-Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br. J. Haematol. 2003, 121, 805–814. [Google Scholar] [CrossRef]
- Smith, N.A.; Coleman, C.B.; Gewurz, B.E.; Rochford, R. CD21 (Complement Receptor 2) is the receptor for Epstein-Barr virus entry into T cells. J. Virol. 2020, 94, e00428-20. [Google Scholar] [CrossRef]
- Tabiasco, J.; Vercellone, A.; Meggetto, F.; Hudrisier, D.; Brousset, P.; Fournié, J.J. Acquisition of viral receptor by NK cells through immunological synapse. J. Immunol. 2003, 170, 5993–5998. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.B.; Tang, Y.W. Basic concepts of microarrays and potential applications in clinical microbiology. Clin. Microbiol. Rev. 2009, 22, 611–633. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.; Zegar, C.; Giordano, A. Advantages and limitations of microarray technology in human cancer. Oncogene 2003, 22, 6497–6507. [Google Scholar] [CrossRef] [Green Version]
- Hurd, P.J.; Nelson, C.J. Advantages of next-generation sequencing versus the microarray in epigenetic research. Brief. Funct. Genom. Proteom. 2009, 8, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; de Reyniès, A.; de Leval, L.; Ghazi, B.; Martin-Garcia, N.; Travert, M.; Bosq, J.; Brière, J.; Petit, B.; Thomas, E.; et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 2010, 115, 1226–1237. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, S.; Hu, N.; He, Y.; Pong, R.; Lin, D.; Lu, L.; Law, M. Comparison of next-generation sequencing systems. J. Biomed. Biotech. 2012, 2012, 251364. [Google Scholar] [CrossRef]
- Kircher, M.; Stenzel, U.; Kelso, J. Improved base calling for the Illumina Genome Analyzer using machine learning strategies. Genome Biol. 2009, 10, R83. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Oshima, T.; Morimoto, T.; Ikeda, S.; Yoshikawa, H.; Shiwa, Y.; Ishikawa, S.; Linak, M.C.; Hirai, A.; Takahashi, H.; et al. Sequence-specific error profile of Illumina sequencers. Nucleic Acids Res. 2011, 39, e90. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Gu, Z.H.; Yan, Z.X.; Zhao, X.; Xie, Y.Y.; Zhang, Z.G.; Pan, C.M.; Hu, Y.; Cai, C.P.; Dong, Y.; et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 2015, 47, 1061–1066. [Google Scholar] [CrossRef]
- Dobashi, A.; Tsuyama, N.; Asaka, R.; Togashi, Y.; Ueda, K.; Sakata, S.; Baba, S.; Sakamoto, K.; Hatake, K.; Takeuchi, K. Frequent BCOR aberrations in extranodal NK/T-cell lymphoma, nasal type. Genes Chromosomes Cancer 2016, 55, 460–471. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.Y.; Kang, S.Y.; Kim, S.J.; Hwang, J.; Lee, S.; Kwak, S.H.; Park, K.S.; Yoo, H.Y.; Kim, W.S.; et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget 2015, 6, 17764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ji, W.; Huang, R.; Li, L.; Wang, X.; Li, L.; Fu, X.; Sun, Z.; Li, Z.; Chen, Q.; et al. MicroRNA-155 is a potential molecular marker of natural killer/T-cell lymphoma. Oncotarget 2016, 7, 53808. [Google Scholar] [CrossRef] [Green Version]
- Küçük, C.; Hu, X.; Jiang, B.; Klinkebiel, D.; Geng, H.; Gong, Q.; Bouska, A.; Iqbal, J.; Gaulard, P.; McKeithan, T.W.; et al. Global promoter methylation analysis reveals novel candidate tumor suppressor genes in natural killer cell lymphoma. Clin. Cancer Res. 2015, 21, 1699–1711. [Google Scholar] [CrossRef] [Green Version]
- Pereira, W.J.; Pappas, M.C.R.; Grattapaglia, D.; Pappas, G.J., Jr. A cost-effective approach to DNA methylation detection by Methyl Sensitive DArT sequencing. PLoS ONE 2020, 15, e0233800. [Google Scholar] [CrossRef]
- Quail, M.A.; Smith, M.; Coupland, P.; Otto, T.D.; Harris, S.R.; Connor, T.R.; Bertoni, A.; Swerdlow, H.P.; Gu, Y. A tale of three next generation sequencing platforms: Comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genom. 2012, 13, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Zhao, S.; Xue, D.; Song, F.; Li, Z.; Chen, D.; He, B.; Hao, Y.; Wang, Y.; Liu, Y. Improving alignment accuracy on homopolymer regions for semiconductor-based sequencing technologies. BMC Genom. 2016, 17, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Go, J.H.; Kim, E.K.; Lee, H.; Lee, W.M.; Cho, C.S.; Han, K. Mutational analysis of extranodal NK/T-cell lymphoma using targeted sequencing with a comprehensive cancer panel. Genom. Inform. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes-Mojarro, I.A.; Chen, B.J.; Ramirez-Ibarguen, A.F.; Quezada-Fiallos, C.M.; Pérez-Báez, W.B.; Dueñas, D.; Casavilca-Zambrano, S.; Ortiz-Mayor, M.; Rojas-Bilbao, E.; García-Rivello, H.; et al. Mutational profile and EBV strains of extranodal NK/T-cell lymphoma, nasal type in Latin America. Mod. Pathol. 2020, 33, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Citalan-Madrid, A.F.; Cabral-Pacheco, G.A.; Martinez-de-Villarreal, L.E.; Villarreal-Martinez, L.; Ibarra-Ramirez, M.; Garza-Veloz, I.; Cardenas-Vargas, E.; Marino-Martinez, I.; Martinez-Fierro, M.L. Proteomic tools and new insights for the study of B-cell precursor acute lymphoblastic leukemia. Hematology 2019, 24, 637–650. [Google Scholar] [CrossRef] [Green Version]
- López Villar, E.; Wu, D.; Cho, W.C.; Madero, L.; Wang, X. Proteomics-based discovery of biomarkers for paediatric acute lymphoblastic leukaemia: Challenges and opportunities. J. Cell. Mol. Med. 2014, 18, 1239–1246. [Google Scholar] [CrossRef]
- Xu, P.P.; Xiong, J.; Cheng, S.; Zhao, X.; Wang, C.F.; Cai, G.; Zhong, H.J.; Huang, H.Y.; Chen, J.Y.; Zhao, W.L. A phase II study of methotrexate, etoposide, dexamethasone and pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage IE to IIE extranodal natural-killer/T-cell lymphoma, nasal-type. EBioMedicine 2017, 25, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zeng, H.; Zhao, Y.; Gong, Y.; Ma, X. Proteomic Analysis of Cerebrospinal Fluid From Patients With Extranodal NK-/T-Cell Lymphoma of Nasal-Type With Ethmoidal Sinus Metastasis. Front. Oncol. 2020, 9, 1489. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Bifulco, C.; Palmieri, G.; Peters, S.; Sidiropoulos, N. Preanalytic variables and tissue stewardship for reliable next-generation sequencing (NGS) clinical analysis. J. Mol. Diagnost. 2019, 21, 756–767. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.S.; Luthra, R.; Singh, R.R.; Patel, K.P.; Routbort, M.J.; Aldape, K.D.; Yao, H.; Dang, H.D.; Barkoh, B.A.; Manekia, J.; et al. Identification of factors affecting the success of next-generation sequencing testing in solid tumors. Am. J. Clin. Pathol. 2016, 145, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luthra, R.; Goswami, R.S.; Singh, R.R.; Roy-Chowdhuri, S. Analysis of pre-analytic factors affecting the success of clinical next-generation sequencing of solid organ malignancies. Cancers 2015, 7, 1699–1715. [Google Scholar] [CrossRef]
- Rocha, L.A.; Aleixo, A.; Allen, G.; Almeda, F.; Baldwin, C.C.; Barclay, M.V.; Bates, J.M.; Bauer, A.M.; Benzoni, F.; Berns, C.M.; et al. Specimen collection: An essential tool. Science 2014, 344, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A. Formalin fixation in the ‘-omics’ era: A primer for the surgeon-scientist. ANZ J. Surg. 2012, 82, 395–402. [Google Scholar] [CrossRef]
- De Giorgi, C.; Finetti Sialer, M.; Lamberti, F. Formalin-induced infidelity in PCR-amplified DNA fragments. Mol. Cell. Probes 1994, 8, 459–462. [Google Scholar] [CrossRef]
- Piskorz, A.M.; Ennis, D.; Macintyre, G.; Goranova, T.E.; Eldridge, M.; Segui-Gracia, N.; Valganon, M.; Hoyle, A.; Orange, C.; Moore, L.; et al. Methanol-based fixation is superior to buffered formalin for next-generation sequencing of DNA from clinical cancer samples. Ann. Oncol. 2016, 27, 532–539. [Google Scholar] [CrossRef]
- Kim, J.; Park, W.Y.; Kim, N.K.D.; Jang, S.J.; Chun, S.M.; Sung, C.O.; Choi, J.; Ko, Y.H.; Choi, Y.L.; Shim, H.S.; et al. Good laboratory standards for clinical next-generation sequencing cancer panel tests. J. Pathol. Transl. Med. 2017, 51, 191. [Google Scholar] [CrossRef]
- Santani, A.; Murrell, J.; Funke, B.; Yu, Z.; Hegde, M.; Mao, R.; Ferreira-Gonzalez, A.; Voelkerding, K.V.; Weck, K.E. Development and validation of targeted next-generation sequencing panels for detection of germline variants in inherited diseases. Arch. Pathol. Lab. Med. 2017, 141, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Ebbert, M.T.; Wadsworth, M.E.; Staley, L.A.; Hoyt, K.L.; Pickett, B.; Miller, J.; Duce, J.; Kauwe, J.S.; Ridge, P.G. Evaluating the necessity of PCR duplicate removal from next-generation sequencing data and a comparison of approaches. BMC Bioinform. 2016, 17, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Gargis, A.S.; Kalman, L.; Berry, M.W.; Bick, D.P.; Dimmock, D.P.; Hambuch, T.; Lu, F.; Lyon, E.; Voelkerding, K.V.; Zehnbauer, B.A.; et al. Assuring the quality of next-generation sequencing in clinical laboratory practice. Nat. Biotechnol. 2012, 30, 1033–1036. [Google Scholar] [CrossRef]
- Bain, B.J.; Bates, I.; Laffan, M.A. Dacie and Lewis Practical Haematology e-Book, 12th ed.; Elsevier Health Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 519–523. [Google Scholar]
- Williams, C.; Pontén, F.; Moberg, C.; Söderkvist, P.; Uhlén, M.; Pontén, J.; Sitbon, G.; Lundeberg, J. A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am. J. Pathol. 1999, 155, 1467–1471. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.Q.; Li, J.; Tan, A.Y.; Vedururu, R.; Pang, J.M.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Quach, N.; Goodman, M.F.; Shibata, D. In vitro mutation artifacts after formalin fixation and error prone translesion synthesis during PCR. BMC Clin. Pathol. 2004, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Campos, P.; Gilbert, T.P. DNA Extraction from Formalin-Fixed Material. In Methods in Molecular Biology; Shapiro, B., Hofreiter, M., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 840, pp. 81–85. [Google Scholar] [CrossRef]
- Lin, M.T.; Mosier, S.L.; Thiess, M.; Beierl, K.F.; Debeljak, M.; Tseng, L.H.; Chen, G.; Yegnasubramanian, S.; Ho, H.; Cope, L.; et al. Clinical Validation of KRAS, BRAF, and EGFR Mutation Detection Using Next Generation Sequencing. Am. J. Clin. Pathol. 2014, 141, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Do, H.; Wong, S.Q.; Li, J.; Dobrovic, A. Reducing sequence artifacts in amplicon-based massively parallel sequencing of formalin-fixed paraffin-embedded DNA by enzymatic depletion of uracil-containing templates. Clin. Chem. 2013, 59, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sujobert, P.; Le Bris, Y.; de Leval, L.; Gros, A.; Merlio, J.P.; Pastoret, C.; Huet, S.; Sarkozy, C.; Davi, F.; Callanan, M.; et al. The need for a consensus next-generation sequencing panel for mature lymphoid malignancies. HemaSphere 2019, 3, e169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Q.; Wang, Q.; Jia, P.; Zhao, Z. Computational tools for copy number variation (CNV) detection using next-generation sequencing data: Features and perspectives. BMC Bioinform. 2013, 14, S1. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologist. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Cui, B.W.; Wang, N.; Dai, Y.T.; Zhang, H.; Wang, C.F.; Zhong, H.J.; Cheng, S.; Ou-Yang, B.S.; Hu, Y.; et al. Genomic and Transcriptomic Characterization of Natural Killer T Cell Lymphoma. Cancer Cell 2020, 37, 403–419. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, W.N.; Chen, B.; Xi, W.D.; Lu, Y.; Huang, J.Y.; Wang, Y.Y.; Long, J.; Wu, S.F.; Zhang, Y.X.; et al. Homoharringtonine deregulates MYC transcriptional expression by directly binding NF-κB repressing factor. Proc. Natl. Acad. Sci. USA 2019, 116, 2220–2225. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Chan, C.W.; Chan, K.C.; Cheng, S.H.; Wong, J.; Wong, V.W.; Wong, G.L.; Chan, S.L.; Mok, T.S.; Chan, H.L.; et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, E1317–E1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, W.; Li, J.; Xiong, J.; Liu, J.; Chen, T.; Wen, Q.; Zeng, Y.; Gao, L.; Gao, L.; et al. Plasma circulating tumor DNA assessment reveals KMT2D as a potential poor prognostic factor in extranodal NK/T-cell lymphoma. Biomark. Res. 2020, 8, 27. [Google Scholar] [CrossRef]
- Chiu, R.W.; Poon, L.L.; Lau, T.K.; Leung, T.N.; Wong, E.M.; Lo, Y.M. Effects of blood-processing protocols on fetal and total DNA quantification in maternal plasma. Clin. Chem. 2001, 47, 1607–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.C.; Yeung, S.W.; Lui, W.B.; Rainer, T.H.; Lo, Y.M. Effects of preanalytical factors on the molecular size of cell-free DNA in blood. Clin. Chem. 2005, 51, 781–784. [Google Scholar] [CrossRef]
- Beutler, E.; Gelbart, T.; Kuhl, W. Interference of heparin with the polymerase chain reaction. Biotechniques 1990, 9, 166. [Google Scholar] [PubMed]
- Lam, N.Y.; Rainer, T.H.; Chiu, R.W.; Lo, Y.M. EDTA is a better anticoagulant than heparin or citrate for delayed blood processing for plasma DNA analysis. Clin. Chem. 2004, 50, 256–257. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.R.; Zhang, L.; Wang, J.W. The landscape of new drugs in extranodal NK/T-cell lymphoma. Cancer Treat. Rev. 2020, 89, 102065. [Google Scholar] [CrossRef]
- Kim, W.Y.; Nam, S.J.; Kim, S.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk Lymphoma 2015, 56, 1778–1786. [Google Scholar] [CrossRef]
- Li, P.; Jiang, L.; Zhang, X.; Liu, J.; Wang, H. CD30 expression is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. BMC Cancer 2014, 14, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Yoon, D.H.; Kim, J.S.; Kang, H.J.; Lee, H.W.; Eom, H.S.; Hong, J.Y.; Cho, J.; Ko, Y.H.; Huh, J.; et al. Efficacy of Brentuximab Vedotin in Relapsed or Refractory High-CD30-Expressing Non-Hodgkin Lymphomas: Results of a Multicenter, Open-Labeled Phase II Trial. Cancer Res. Treat. 2020, 52, 374–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Hyeon, J.; Cho, I.; Ko, Y.H.; Kim, W.S. Comparison of efficacy of pembrolizumab between Epstein-Barr viruspositive and negative relapsed or refractory non-hodgkin lymphomas. Cancer Res. Treat. 2019, 51, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Jung, H.Y.; Nam, S.J.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Archiv. 2016, 469, 581–590. [Google Scholar] [CrossRef]
- Jo, J.C.; Kim, M.; Choi, Y.; Kim, H.J.; Kim, J.E.; Chae, S.W.; Kim, H.; Cha, H.J. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2017, 96, 25–31. [Google Scholar] [CrossRef]
- Panjwani, P.K.; Charu, V.; DeLisser, M.; Molina-Kirsch, H.; Natkunam, Y.; Zhao, S. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum. Pathol. 2018, 71, 91–99. [Google Scholar] [CrossRef]
- Nagato, T.; Ohkuri, T.; Ohara, K.; Hirata, Y.; Kishibe, K.; Komabayashi, Y.; Ueda, S.; Takahara, M.; Kumai, T.; Ishibashi, K.; et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: A potential rationale for immunotherapy. Cancer Immunol. Immunother. 2017, 66, 877–890. [Google Scholar] [CrossRef]
- Han, L.; Liu, F.; Li, R.; Li, Z.; Chen, X.; Zhou, Z.; Zhang, X.; Hu, T.; Zhang, Y.; Young, K.; et al. Role of programmed death ligands in effective T-cell interactions in extranodal natural killer/T-cell lymphoma. Oncol. Lett. 2014, 8, 1461–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar]
- Fang, W.; Hong, S.; Chen, N.; He, X.; Zhan, J.; Qin, T.; Zhou, T.; Hu, Z.; Ma, Y.; Zhao, Y.; et al. PD-L1 is remarkably overexpressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget 2015, 6, 33019–33032. [Google Scholar] [CrossRef]
- Laurent, C.; Fabiani, B.; Do, C.; Tchernonog, E.; Cartron, G.; Gravelle, P.; Amara, N.; Malot, S.; Palisoc, M.M.; Copie-Bergman, C.; et al. Immunecheckpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: A clinical and pathological study in 82 patients. Haematologica 2016, 101, 976–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Bi, X.W.; Wang, H.; Zhang, W.W.; Wang, J.H.; Liu, W.J.; Xia, Z.J.; Huang, H.Q.; Jiang, W.Q.; Zhang, Y.J.; Wang, L. PD-L1 is upregulated by EBV driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 2016, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Kwong, Y.L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.M.; Mow, B.; Khong, P.L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, Y.; Zhang, M.; Yan, J.; Li, L.; Fu, X.; Zhang, X.; Chang, Y.; Sun, Z.; Yu, H.; et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diefenbach, C.S.; Hong, F.; Ambinder, R.F.; Cohen, J.B.; Robertson, M.J.; David, K.A.; Advani, R.H.; Fenske, T.S.; Barta, S.K.; Palmisiano, N.D.; et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: Phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020, 7, e660–e670. [Google Scholar] [CrossRef]
- Lim, J.Q.; Huang, D.; Tang, T.; Tan, D.; Laurensia, Y.; Peng, R.J.; Wong, E.K.Y.; Cheah, D.M.Z.; Chia, B.K.H.; Iqbal, J.; et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia 2020, 34, 3413–3419. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. ALCYONE Trial Investigators. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Li, P.F.; Lu, Y.; Xia, Z.J.; Huang, H.Q.; Zhang, Y.J. CD38 expression pre- dicts poor prognosis and might be a potential therapy target in extra-nodal NK/T cell lymphoma, nasal type. Ann. Hematol. 2015, 94, 1381–1388. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Yao, M.; Kim, T.M.; Yoon, D.H.; Cho, S.G.; Eom, H.S.; Lim, S.T.; Yeh, S.P.; Song, Y.; et al. Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: An open-label, single-arm, multicenter, phase 2 study. J. Hematol. Oncol. 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Laurence, A.D.J. Location, movement and survival: The role of chemokines in haematopoiesis and malignancy: Review. Br. J. Haematol. 2006, 132, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Hiramatsu, Y.; Iwata, S.; Siddiquey, M.; Sato, Y.; Suzuki, M.; Ito, Y.; Goshima, F.; Murata, T.; Kimura, H. AntiCCR4 Monoclonal Antibody Mogamulizumab for the Treatment of EBV-Associated T- and NK-Cell Lymphoproliferative Diseases. Clin. Cancer Res. 2014, 20, 5075–5084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumai, T.; Nagato, T.; Kobayashi, H.; Komabayashi, Y.; Ueda, S.; Kishibe, K.; Ohkuri, T.; Takahara, M.; Celis, E.; Harabuchi, Y. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol. Immunother. 2015, 64, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Gotlib, J.; Cools, J.; MMalone, J.; LSchrier, S.; Gary Gilliland, D.; ECoutré, S. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: Implications for diagnosis, classification, and management. Blood 2004, 103, 2879–2891. [Google Scholar] [CrossRef]
- Martinho, O.; Longatto-Filho, A.; Lambros, M.B.K.; Martins, A.; Pinheiro, C.; Silva, A.; Pardal, F.; Amorim, J.; Mackay, A.; Milanezi, F.; et al. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br. J. Cancer 2009, 101, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Du, X.; Lazar, A.J.; Pollock, R.; Hunt, K.; Chen, K.; Hao, X.; Trent, J.; Zhang, W. Genetic aberrations of gastrointestinal stromal tumors. Cancer 2008, 113, 1532–1543. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, P.; Liu, W.; Xia, Z.; Shi, F.; Zhong, M. Expression and significance of Ku80 and PDGFR-α in nasal NK/T-cell lymphoma. Pathol.-Res. Pract. 2016, 212, 204–209. [Google Scholar] [CrossRef]
- Lu, L.; Fu, X.; Li, Z.; Qiu, Y.; Li, W.; Zhou, Z.; Xue, W.; Wang, Y.; Jin, M.; Zhang, M. Platelet-derived growth factor receptor alpha (PDGFRα) is overexpressed in NK/T-cell lymphoma and mediates cell survival. Biochem. Biophys. Res. Commun. 2018, 504, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Gouilleux-Gruart, V.; Huang, Y.; Bouhlal, H.; Bouamar, H.; Bouchet, S.; Perrot, C.; Vieillard, V.; Dartigues, P.; Gaulard, P.; et al. STAT3 transcription factor is constitutively activated and is oncogenic in nasal-type NK/T-cell lymphoma. Leukemia 2009, 23, 1667–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Li, B.; Lin, B.; Lee, P.T.; Chung, T.H.; Tan, J.; Bi, C.; Lee, X.T.; Selvarajan, V.; Ng, S.B.; et al. EZH2 phosphorylation by JAK3 mediates a switch to noncanonical function in natural killer/T-cell lymphoma. Blood 2016, 128, 948–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, L.; Li, X.; Zhou, X.; Fang, X.; Yuan, D.; Wang, X. WP1066 exhibits antitumor efficacy in nasal-type natural killer/T-cell lymphoma cells through downregulation of the STAT3 signaling pathway. Oncol. Rep. 2016, 36, 2868–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, G.C.; Tan, S.Y.; Tang, T.; Poon, S.L.; Allen, G.E.; Tan, L.; Chong, S.C.; Ong, W.S.; Tay, K.; Tao, M.; et al. Janus Kinase 3–Activating Mutations Identified in Natural Killer/T-cell Lymphoma. Cancer Discov. 2012, 2, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liang, L.; Li, D.; Nong, L.; Zheng, Y.; Huang, S.; Zhang, B.; Li, T. JAK3/STAT3 oncogenic pathway and PRDM1 expression stratify clinicopathologic features of extranodal NK/T-cell lymphoma, nasal type. Oncol. Rep. 2019, 12, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, X.W.; Zhu, Y.J.; He, Y.Z.; Lai, Q.Y.; Xia, Z.J.; Cai, Q.Q. IL-2Rα up-regulation is mediated by latent membrane protein 1 and promotes lymphomagenesis and chemotherapy resistance in natural killer/T-cell lymphoma. Cancer Commun. 2018, 38, 62. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.-C.; Lay, J.-D.; Hsieh, W.-C.; Su, I.-J. Pathogenesis and mechanism of disease progression from hemophagocytic lymphohistiocytosis to Epstein-Barr virus-associated T-cell lymphoma: Nuclear factor-kappa B pathway as a potential therapeutic target. Cancer Sci. 2007, 98, 1281–1287. [Google Scholar] [CrossRef]
- Takada, H.; Imadome, K.-I.; Shibayama, H.; Yoshimori, M.; Wang, L.; Saitoh, Y.; Uota, S.; Yamaoka, S.; Koyama, T.; Shimizu, N.; et al. EBV induces persistent NF-κB activation and contributes to survival of EBV-positive neoplastic T- or NK-cells. PLoS ONE 2017, 12, e0174136. [Google Scholar]
- Tang, T.; Tay, K.; Tao, M.; Quek, R.H.H.; Farid, M.; Lim, S.T. A Phase II Study of Bortezomib-GIFOX (Gemcitabine, Ifosfamide, Oxaliplatin) in Patients with Newly Diagnosed Natural-Killer/T-Cell Lymphoma. Blood 2016, 128, 5353. [Google Scholar] [CrossRef]
- Granato, M.; Romeo, M.A.; Tiano, M.S.; Santarelli, R.; Gonnella, R.; Gilardini Montani, M.S.; Faggioni, A.; Cirone, M. Bortezomib promotes KHSV and EBV lytic cycle by activating JNK and autophagy. Sci. Rep. 2017, 7, 13052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, P.; Dai, Y.; Grant, S. Histone deacetylase inhibitor (HDACI) mechanisms of action: Emerging insights. Pharmacol. Ther. 2014, 143, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Huang, H.; Li, P.; Bing, B.; Wang, X.; Rong, Q.; Li, Z.; Cai, Q.; Jiang, W. Chidamide, Oral Subtype-Selective Histone Deacetylase Inhibitor (HDACI) Monotherapy Was Effective on the Patients with Relapsed or Refractory Extranodal Natural Killer (NK)/T-Cell Lymphoma. Blood 2017, 130 (Suppl. 1), 2797. [Google Scholar]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- de Mel, S.; Hue, S.S.; Jeyasekharan, A.D.; Chng, W.J.; Ng, S.B. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J. Haematol. Oncol. 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Hongyo, T.; Jia, X.; He, Y.; Hasui, K.; Dong, Z.; Luo, W.J.; Ham, M.F.; Nomura, T.; Aozasa, K. Analysis of p53, K-ras, c-kit, and β-catenin gene mutations in sinonasal NK/T cell lymphoma in northeast district of China. Cancer Sci. 2003, 94, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintanilla-Martinez, L.; Kremer, M.; Keller, G.; Nathrath, M.; Gamboa-Dominguez, A.; Meneses, A.; Luna-Contreras, L.; Cabras, A.; Hoefler, H.; Mohar, A.; et al. p53 Mutations in nasal natural killer/T-cell lymphoma from Mexico: Association with large cell morphology and advanced disease. Am. J. Pathol. 2001, 159, 2095–2105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).