Cytological Diagnosis of Pancreatic Solid-Pseudopapillary Neoplasm: A Single-Institution Community Practice Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klöppel, G.; Basturk, O.; Klimstra, D.S.; Lam, A.K.; Notohara, K. Solid pseudopapillary neoplasm of the pancreas. In WHO Classification of Tumors: Digestive System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board; IARC Press: Lyon, France, 2019; Volume 1, pp. 340–342. [Google Scholar]
- Hruban, R.H.; Pitman, M.B.; Klimstra, D.S. Solid-pseudopapillary neoplasms. In Atlas of Tumor Pathology, Series 4: Tumors of the Pancreas; Hruban, R.H., Pitman, M.B., Klimstra, D.S., Eds.; Armed Forces Institute of Pathology: Washington, DC, USA, 2007; pp. 231–250. [Google Scholar]
- Klimstra, D.S.; Wenig, B.M.; Heffess, C.S. Solid-pseudopapillary tumor of the pancreas: A typically cystic carcinoma of low malignant potential. Semin. Diagn. Pathol. 2000, 17, 66–80. [Google Scholar] [PubMed]
- Dinarvand, P.; Lai, J. Solid pseudopapillary neoplasm of the pancreas: A rare entity with unique features. Arch. Pathol. Lab. Med. 2017, 141, 990–995. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, S.; Bongiovanni, M. Pancreatic solid pseudopapillary neoplasm: Key pathologic and genetic features. Arch. Pathol. Lab. Med. 2020, 144, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Estrella, J.S.; Li, L.; Rashid, A.; Wang, H.; Katz, M.H.; Fleming, J.B.; Abbruzzese, J.L.; Wang, H. Solid pseudopapillary neoplasm of the pancreas: Clinicopathologic and survival analyses of 64 cases from a single institution. Am. J. Surg. Pathol. 2014, 38, 147–157. [Google Scholar] [CrossRef]
- Pelosi, G.; Iannucci, A.; Zamboni, G.; Bresaola, E.; Iacono, C.; Serio, G. Solid and cystic papillary neoplasm of the pancreas: A clinico-cytopathologic and immunocytochemical study of five new cases diagnosed by fine-needle aspiration cytology and a review of the literature. Diagn. Cytopathol. 1995, 13, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Di Vizio, D.; Manivel, J.C.; Pambuccian, S.E.; Somma, P.; Insabato, L. Solid-pseudopapillary tumor of the pancreas: A neoplasm with distinct and highly characteristic cytological features. Diagn. Cytopathol. 2002, 27, 325–334. [Google Scholar] [CrossRef]
- Bardales, R.H.; Centeno, B.; Mallery, J.S.; Lai, R.; Pochapin, M.; Guiter, G.; Stanley, M.W. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: A rare neoplasm of elusive origin but characteristic cytomorphologic features. Am. J. Clin. Pathol. 2004, 121, 654–662. [Google Scholar] [CrossRef]
- Jhala, N.; Siegal, G.P.; Jhala, D. Large, clear cytoplasmic vacuolation: An under-recognized cytologic clue to distinguish solid pseudopapillary neoplasms of the pancreas from pancreatic endocrine neoplasms on fine-needle aspiration. Cancer 2008, 114, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Shah, A.A.; Stelow, E.B.; Alsharif, M.; Cameron, S.E.H.; Pambuccian, S.E. Cercariform cells: Another cytologic feature distinguishing solid pseudopapillary neoplasms from pancreatic endocrine neoplasms and acinar cell carcinomas in endoscopic ultrasound-guided fine-needle aspirates. Cancer Cytopathol. 2012, 121, 298–310. [Google Scholar] [CrossRef]
- Burford, H.; Baloch, Z.; Liu, X.; Jhala, D.; Siegal, G.P.; Jhala, N. E-cadherin/β-catenin and CD10: A limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am. J. Clin. Pathol. 2009, 132, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Bal, M.M.; Deodhar, K.; Shrikhande, S.; Shukla, P.; Arya, S.; Ramadwar, M. Solid pseudopapillary tumor of the pancreas: “Experiences” and “Lessons” at a tertiary-care oncology center. Diagn. Cytopathol. 2012, 41, 599–606. [Google Scholar] [CrossRef]
- Misra, S.; Saran, R.K.; Dm, S.S.; Barman, S.; Dahale, A. Utility of cytomorphology in distinguishing solid pseudopapillary neoplasm of pancreas from pancreatic neuroendocrine tumor with emphasis on nuclear folds and nuclear grooves. Diagn. Cytopathol. 2019, 47, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, P.; Rohilla, M.; Gupta, P.; Gupta, N.; Dey, P.; Srinivasan, R.; Rajwanshi, A.; Nada, R. Fine needle aspiration cytology with the aid of immunocytochemistry on cell-block confirms the diagnosis of solid pseudopapillary neoplasm of the pancreas. Cytopathology 2020, 32, 57–64. [Google Scholar] [CrossRef]
- Wu, J.; Mao, Y.; Jiang, Y.; Song, Y.; Yu, P.; Sun, S.; Li, S. Sex differences in solid pseudopapillary neoplasm of the pancreas: A population-based study. Cancer Med. 2020, 9, 6030–6041. [Google Scholar] [CrossRef] [PubMed]
- Lanke, G.; Ali, F.S.; Lee, J.H. Clinical update on the management of pseudopapillary tumor of pancreas. World J. Gastrointest. Endosc. 2018, 10, 145–155. [Google Scholar] [CrossRef]
- Ardengh, J.C.; Lopes, C.V.; Venco, F.E.; Machado, M.A. Diagnosis of pancreatic solid pseudopapillary neoplasms using cell-blocks and immunohistochemical evaluation of endoscopic ultrasound-guided fine needle aspiration biopsy specimens. Cytopathology 2020, 32, 50–56. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Simpson, K.W.; Bilello, S.J. The clear cell variant of solid pseudopapillary tumor of the pancreas: A previously unrecognized pancreatic neoplasm. Am. J. Surg. Pathol. 2006, 30, 1237–1242. [Google Scholar] [CrossRef]
- Zhao, P.; Debrito, P.; Ozdemirli, M.; Sidawy, M.K. Solid-pseudopapillary neoplasm of the pancreas: Awareness of unusual clinical presentations and morphology of the clear cell variant can prevent diagnostic errors. Diagn. Cytopathol. 2013, 41, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kato, K.; Notohara, K.; Hojo, H.; Ijiri, R.; Miyake, T.; Nagahara, N.; Sasaki, F.; Kitagawa, N.; Nakatani, Y.; et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001, 61, 8401–8404. [Google Scholar]
- Kim, M.-J.; Jang, S.-J.; Yu, E. Loss of E-cadherin and cytoplasmic-nuclear expression of β-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum. Pathol. 2008, 39, 251–258. [Google Scholar] [CrossRef]
- Li, L.; Othman, M.; Rashid, A.; Wang, H.; Li, Z.; Katz, M.H.; Lee, J.E.; Pisters, P.W.; Abbruzzese, J.L.; Fleming, J.B.; et al. Solid pseudopapillary neoplasm of the pancreas with prominent atypical multinucleated giant tumour cells. Histopathology 2012, 62, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kim, M.-S.; Kim, S.C.; Choi, J.; Yu, E.; Hong, S.-M. Pleomorphic solid pseudopapillary neoplasm of the pancreas: Degenerative change rather than high-grade malignant potential. Hum. Pathol. 2014, 45, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Policarpio-Nicolas, M.L.C.; McHugh, K.E.; Sae-Ow, W.; Brainard, J.A. Pleomorphic and atypical multinucleated giant cells in solid pseudopapillary neoplasm of pancreas: A diagnostic pitfall in cytology and a review of the literature. Diagn. Cytopathol. 2018, 47, 488–493. [Google Scholar] [CrossRef] [PubMed]
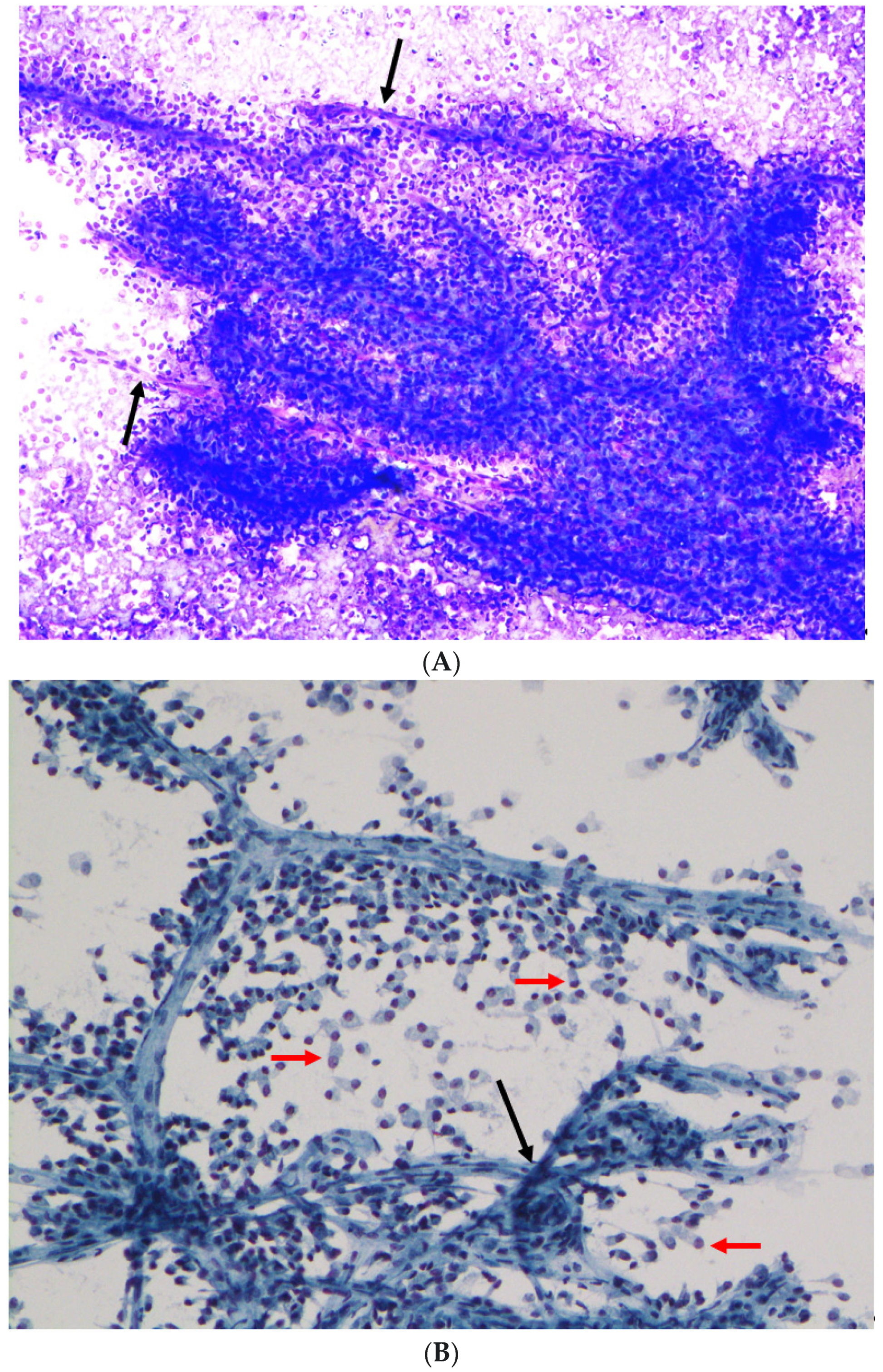






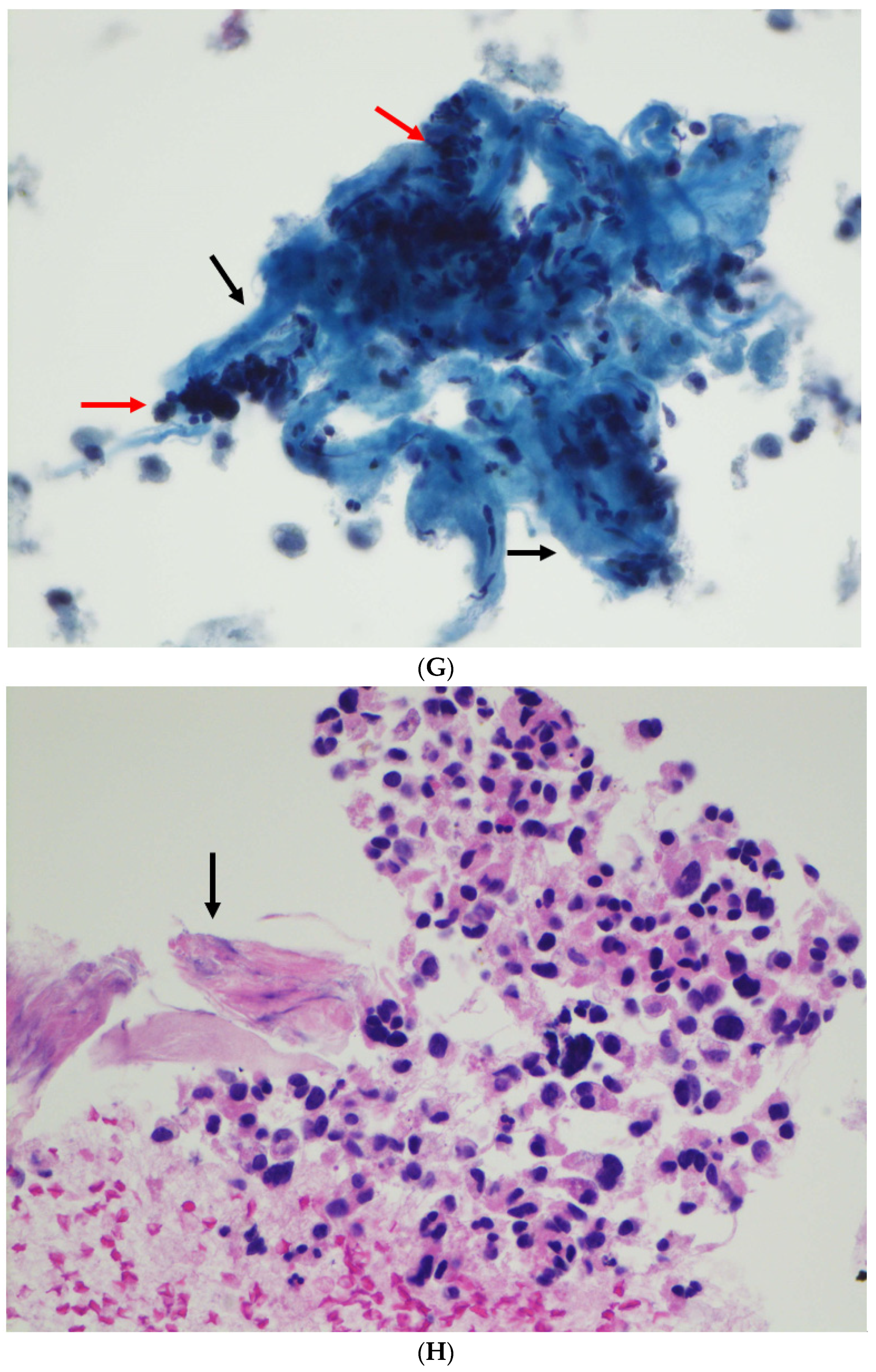



| Case | Age (Years) | Sex | Site | Radiology/Gross Features | Size (cm) | ROSE Diagnosis | FNA/Biopsy Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 65 | F | Body | Solid and cystic | 4.0 | Atypical cells with mucinous features | SPN |
| 2 | 39 | F | Tail | Cystic | 3.0 | Serous papillary lesion | SPN |
| 3 | 28 | F | Body | Solid and cystic | 4.0 | NA | SPN |
| 4 | 26 | F | Tail | Solid and cystic | 4.0 | NA | SPN |
| 5 | 33 | F | Body | Solid and cystic | 6.5 | Lesional tissue | SPN |
| 6 | 26 | F | Tail | Solid and cystic | 1.5 | Suspicious for SPN vs. PanNET | SPN |
| 7 | 24 | F | Tail | Solid and cystic | 4.0 | SPN | SPN |
| 8 | 26 | M | Tail | Solid and cystic | 3.5 | NA | SPN |
| 9 | 25 | M | Body | Solid | 2.2 | Favor PanNET | SPN |
| 10 | 29 | F | Body | Solid | 2.9 | Lesional tissue | SPN |
| 11 | 23 | M | Body | Solid and cystic | 4.8 | NA | SPN |
| 12 | 41 | F | Tail | Solid and cystic | 2.2 | NA | SPN |
| 13 | 20 | F | Head | Solid | 6.0 | NA | SPN |
| 14 | 73 | F | Body | Solid and cystic | 1.2 | NA | SPN |
| 15 | 21 | F | Tail | Solid and cystic | 15 | Round cells | SPN (biopsy) |
| 16 | 21 | F | Tail | Solid and cystic | 12 | Lesional cells | SPN (biopsy) |
| 17 | 47 | F | Body | Solid and cystic | 4.7 | Tumor | SPN (biopsy) |
| 18 | 46 | F | Tail | Solid and cystic | 4.5 | NA | NA |
| 19 | 14 | F | Tail | Solid and cystic | 3.7 | NA | NA |
| 20 | 71 | F | Body | Solid and cystic | 15 | NA | NA |
| 21 | 12 | F | Tail | Solid and cystic | 3.5 | NA | NA |
| 22 | 16 | F | Tail | Solid and cystic | 3.5 | NA | NA |
| Case | Papillation | Monomorphic Cells | Central Capillaries | Nuclear Groove | Myxoid Fibrovascular Stroma | Cytoplasmic Hyaline Globules | Atypical Multinucleated Cells |
|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | - |
| 2 | + | + | + | + | + | + | - |
| 3 | + | + | + | + | + | + | - |
| 4 | + | + | + | + | + | + | - |
| 5 | + | + | + | + | + | + | - |
| 6 | + | + | + | + | + | + | - |
| 7 | + | + | + | + | + | + | - |
| 8 | + | + | + | + | - | + | - |
| 9 | + | + | + | + | + | + | - |
| 10 | + | + | + | + | + | + | - |
| 11 | + | + | + | + | + | + | - |
| 12 | + | + | + | + | + | + | + |
| 13 | + | + | + | + | + | + | - |
| 14 | + | - | - | + | + | + | + |
| 15 | + | + | + | + | + | + | - |
| 16 | + | + | + | + | + | + | - |
| 17 | + | + | + | + | + | + | - |
| Case | β-Cat | CyD1 | PR | CD56 | SYN | CHR | CD10 | CK | VIM | AAT | Ki-67 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | NA | NA | + | Focal | NA | + | - | + | + | NA |
| 2 | + | NA | NA | + | NA | NA | + | NA | + | + | NA |
| 3 | NA | NA | NA | + | - | - | + | NA | NA | NA | NA |
| 4 | + | NA | NA | + | Focal | - | + | - | + | + | NA |
| 5 | + | NA | NA | + | Focal | - | + | - | + | + | NA |
| 6 | + | NA | NA | NA | - | - | NA | NA | NA | + | NA |
| 7 | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | NA |
| 8 | + | NA | NA | + | NA | NA | + | NA | NA | + | NA |
| 9 | + | NA | NA | NA | Focal | - | + | + | NA | + | <5% |
| 10 | + | NA | + | + | - | - | + | - | NA | NA | <1% |
| 11 | + | + | + | NA | - | - | + | - | NA | NA | <5% |
| 12 | + | NA | NA | NA | - | - | NA | - | NA | NA | <5% |
| 13 | + | + | + | NA | Focal | - | NA | - | NA | NA | 5% |
| 14 | + | + | + | NA | Focal | - | NA | - | NA | NA | 1% |
| 15 | + | + | + | + | - | - | + | - | NA | + | NA |
| 16 | + | + | + | NA | - | - | + | NA | + | NA | NA |
| 17 | + | + | NA | NA | NA | NA | + | - | NA | NA | NA |
| Total | 15/15 100% | 6/6 100% | 6/6 100% | 8/8 100% | 6/13 46% | 0/12 0% | 12/12 100% | 1/11 9% | 5/5 100% | 9/9 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.G.; Mani, H.; Wang, Z.Q.; Li, W. Cytological Diagnosis of Pancreatic Solid-Pseudopapillary Neoplasm: A Single-Institution Community Practice Experience. Diagnostics 2022, 12, 449. https://doi.org/10.3390/diagnostics12020449
Wang BG, Mani H, Wang ZQ, Li W. Cytological Diagnosis of Pancreatic Solid-Pseudopapillary Neoplasm: A Single-Institution Community Practice Experience. Diagnostics. 2022; 12(2):449. https://doi.org/10.3390/diagnostics12020449
Chicago/Turabian StyleWang, Brant G., Haresh Mani, Zoe Q. Wang, and Wenping Li. 2022. "Cytological Diagnosis of Pancreatic Solid-Pseudopapillary Neoplasm: A Single-Institution Community Practice Experience" Diagnostics 12, no. 2: 449. https://doi.org/10.3390/diagnostics12020449






