PRAME Immunocytochemistry for the Diagnosis of Melanoma Metastases in Cytological Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
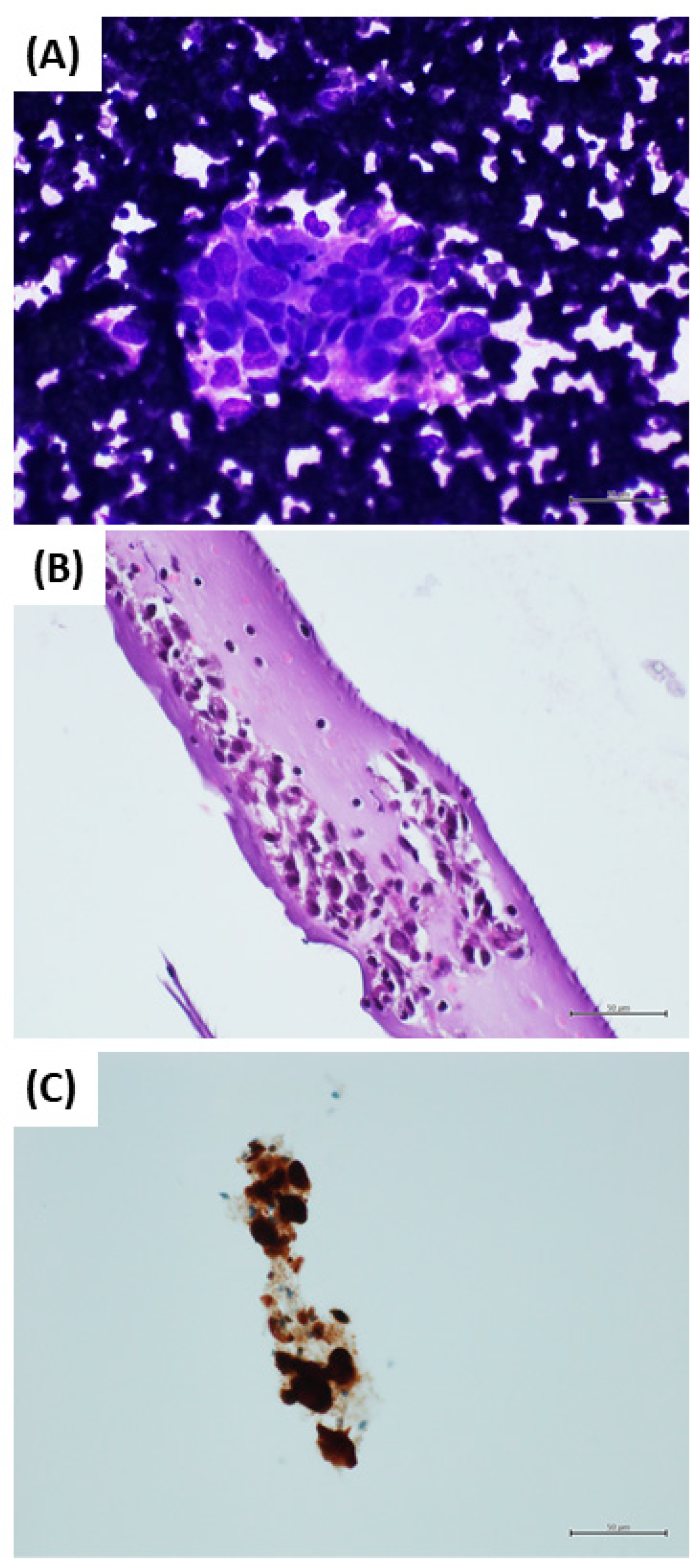
2.2. Sample Management
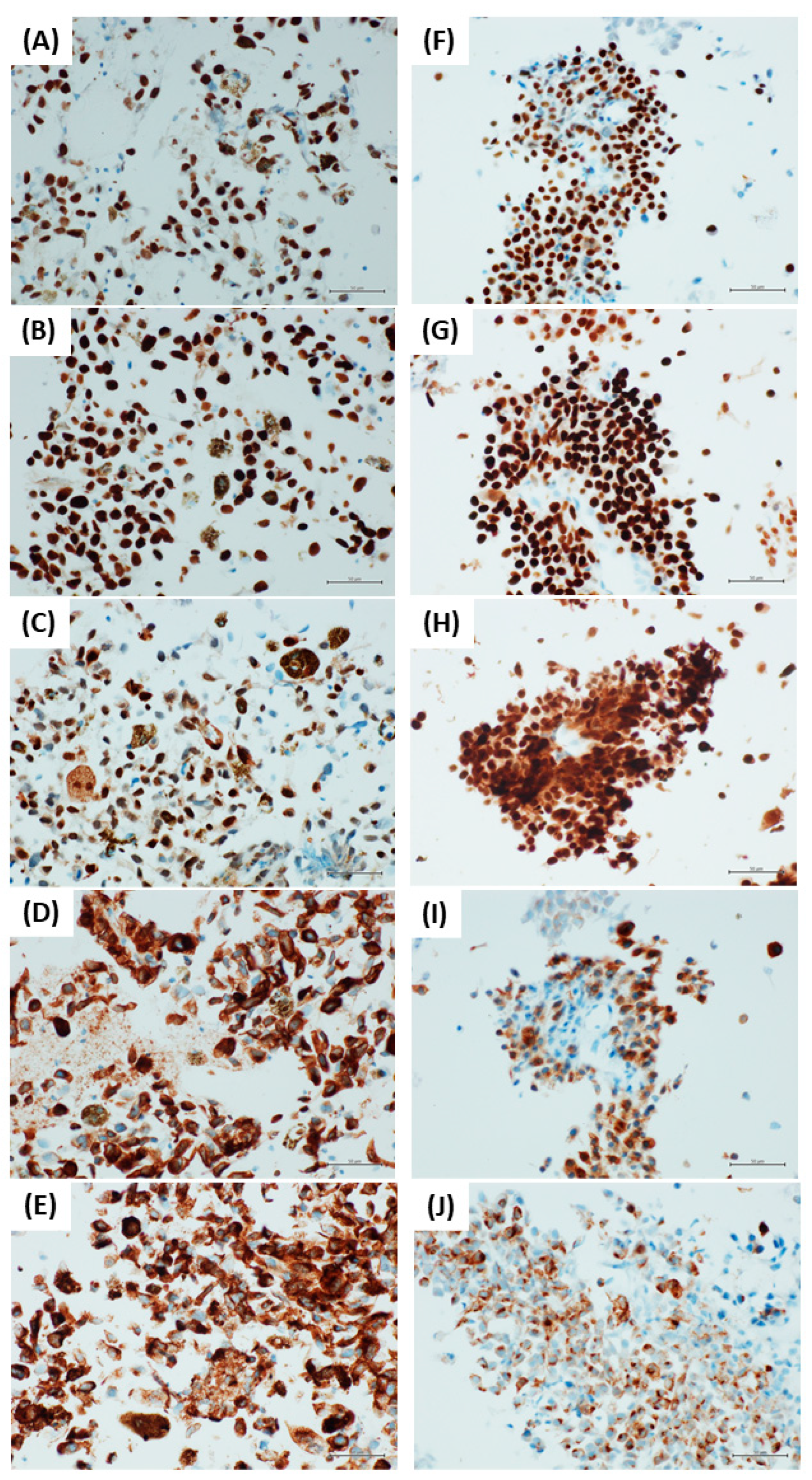
2.3. Immunocytochemistry
2.4. Statistical Analysis
2.5. Ethical Consideration
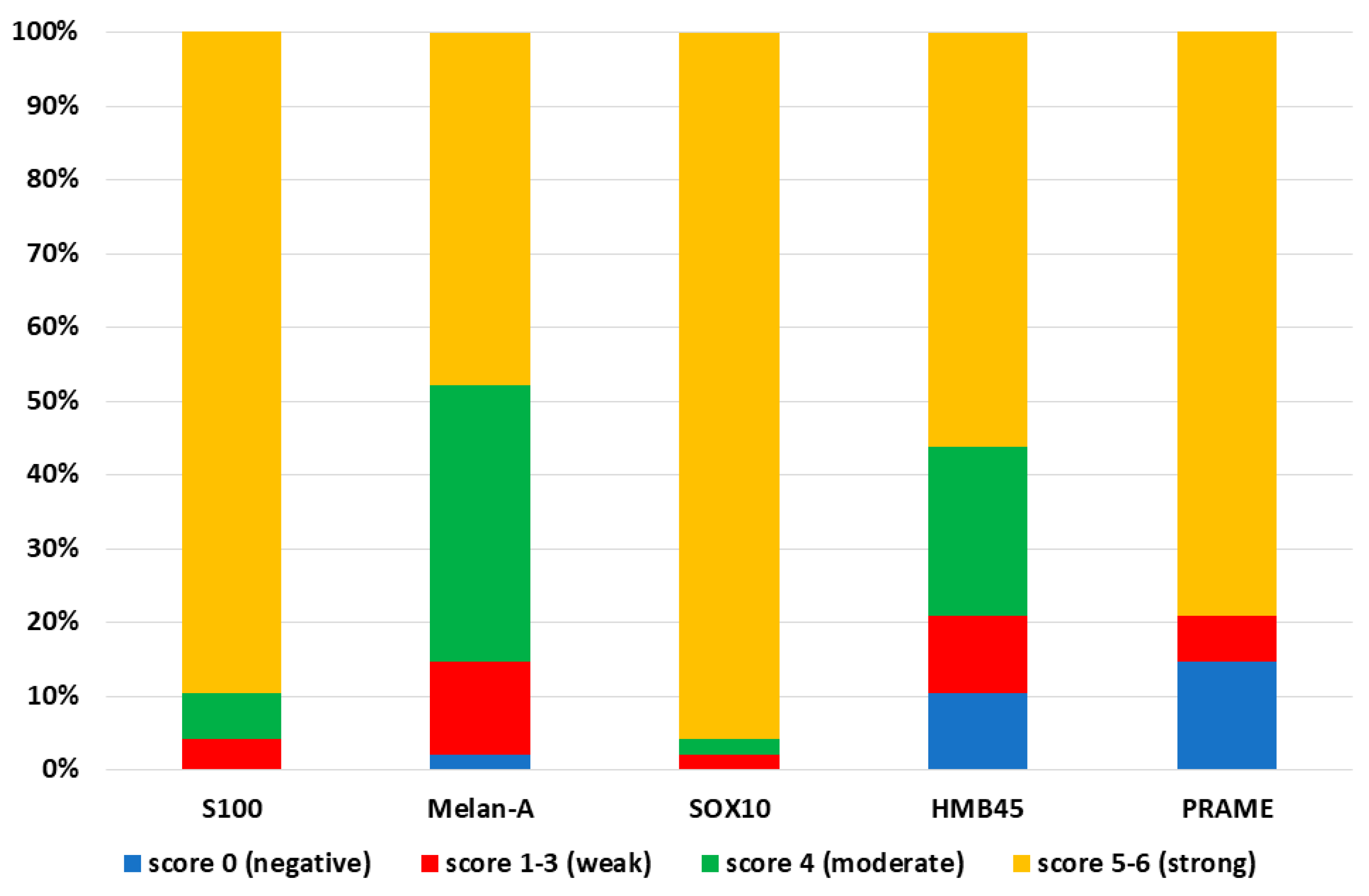
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, Z.; Yousaf, N.; Larkin, J. Melanoma epidemiology, biology and prognosis. EJC Suppl. 2013, 11, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network (NCCN). NCCN Website. Available online: http://www.nccn.org/index.asp (accessed on 5 November 2021).
- Ronchi, A.; Montella, M.; Zito Marino, F.; Caraglia, M.; Grimaldi, A.; Argenziano, G.; Moscarella, E.; Brancaccio, G.; Troiani, T.; Napolitano, S.; et al. Predictive evaluation on cytological sample of metastatic melanoma: The role of BRAF immunocytochemistry in the molecular era. Diagnostics 2021, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Montella, M.; Zito Marino, F.; Argenziano, G.; Moscarella, E.; Brancaccio, G.; Ferraro, G.; Nicoletti, G.F.; Troiani, T.; Franco, R.; et al. Cytologic diagnosis of metastatic melanoma by FNA: A practical review. Cancer Cytopathol. 2021, 130, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.; Ronchi, A.; Cozzolino, I.; Montella, M.; Zito Marino, F.; Franco, R. Mesenchymal neoplasms: Is it time for cytology? New perspectives for the pre-operative diagnosis of soft tissue tumors in the molecular era. Pathol. Res. Pract. 2020, 216, 152923. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Zito Marino, F.; Toni, G.; Pagliuca, F.; Russo, D.; Signoriello, G.; Moscarella, E.; Brancaccio, G.; Argenziano, G.; Franco, R.; et al. Diagnostic performance of melanocytic markers for immunocytochemical evaluation of lymph-node melanoma metastases on cytological samples. J. Clin. Pathol. 2021, 75, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Umano, G.R.; Errico, M.E.; D’Onofrio, V.; Delehaye, G.; Trotta, L.; Spinelli, C.; Strambi, S.; Franco, R.; D’Abbronzo, G.; Ronchi, A.; et al. The challenge of melanocytic lesions in pediatric patients: Clinical-pathological findings and the diagnostic value of PRAME. Front. Oncol. 2021, 11, 688410. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.; Chung, H.J. Diagnostic utility of preferentially expressed antigen in melanoma immunohistochemistry in the evaluation of melanomas with a co-existent nevoid melanocytic population: A single-center retrospective cohort study. J. Am. Acad. Dermatol. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Fattori, A.; De La Fouchardière, A.; Cribier, B.; Mitcov, M. PRAME immunohistochemistry as an adjunct for evaluating ambiguous melanocytic proliferation. Hum. Pathol. 2022; in press. [Google Scholar] [CrossRef]
- Hu, J.; Cai, X.; Lv, J.J.; Wan, X.C.; Zeng, X.Y.; Feng, M.L.; Dai, B.; Kong, Y.Y. Preferentially expressed antigen in melanoma immunohistochemistry as an adjunct for differential diagnosis in acral lentiginous melanoma and acral nevi. Hum. Pathol. 2021, 120, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Grillini, M.; Ricci, C.; Pino, V.; Pedrini, S.; Fiorentino, M.; Corti, B. HMB45/PRAME, a novel double staining for the diagnosis of melanocytic neoplasms: Technical aspects, results, and comparison with other commercially available staining (PRAME and Melan A/PRAME). Appl. Immunohistochem. Mol. Morphol. 2022, 30, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Jungbluth, A.A.; Busam, K.J. PRAME Immunohistochemistry as an ancillary test for the assessment of melanocytic lesions. Surg. Pathol. Clin. 2021, 14, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Epping, M.T.; Wang, L.; Edel, M.J.; Carlée, L.; Hernandez, M.; Bernards, R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 2005, 122, 835–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermes, N.; Kewitz, S.; Staege, M.S. Preferentially expressed antigen in melanoma (PRAME) and the PRAME Family of Leucine-rich repeat proteins. Curr. Cancer Drug Targets 2016, 16, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Stolt, C.C.; Lommes, P.; Hillgärtner, S.; Wegner, M. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 2008, 36, 5427–5440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PRAME | S100 | SOX10 | Melan-A | HMB45 | |
|---|---|---|---|---|---|
| Positive (N. cases) | 41 | 48 | 48 | 47 | 43 |
| Negative (N. cases) | 7 | 0 | 0 | 1 | 5 |
| Strong positivity (N. cases) | 38 | 43 | 46 | 23 | 27 |
| Moderate positivity (N. cases) | 0 | 3 | 1 | 18 | 11 |
| Weak positivity (N. cases) | 3 | 2 | 1 | 6 | 5 |
| Sensitivity | 85.4% | 100% | 100% | 97.9% | 89.6% |
| Specificity | 100% | 85% | 100% | 100% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronchi, A.; Zito Marino, F.; Moscarella, E.; Brancaccio, G.; Argenziano, G.; Troiani, T.; Napolitano, S.; Franco, R.; Cozzolino, I. PRAME Immunocytochemistry for the Diagnosis of Melanoma Metastases in Cytological Samples. Diagnostics 2022, 12, 646. https://doi.org/10.3390/diagnostics12030646
Ronchi A, Zito Marino F, Moscarella E, Brancaccio G, Argenziano G, Troiani T, Napolitano S, Franco R, Cozzolino I. PRAME Immunocytochemistry for the Diagnosis of Melanoma Metastases in Cytological Samples. Diagnostics. 2022; 12(3):646. https://doi.org/10.3390/diagnostics12030646
Chicago/Turabian StyleRonchi, Andrea, Federica Zito Marino, Elvira Moscarella, Gabriella Brancaccio, Giuseppe Argenziano, Teresa Troiani, Stefania Napolitano, Renato Franco, and Immacolata Cozzolino. 2022. "PRAME Immunocytochemistry for the Diagnosis of Melanoma Metastases in Cytological Samples" Diagnostics 12, no. 3: 646. https://doi.org/10.3390/diagnostics12030646
APA StyleRonchi, A., Zito Marino, F., Moscarella, E., Brancaccio, G., Argenziano, G., Troiani, T., Napolitano, S., Franco, R., & Cozzolino, I. (2022). PRAME Immunocytochemistry for the Diagnosis of Melanoma Metastases in Cytological Samples. Diagnostics, 12(3), 646. https://doi.org/10.3390/diagnostics12030646









