Clinicopathologic Analysis of Sinonasal Inverted Papilloma, with Focus on Human Papillomavirus Infection Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HPV DNA Testing
2.3. Statistical Analysis
3. Results
3.1. Overall Outcomes
3.2. Association between Recurrence and Clinical Characteristics
3.3. Association between HPV Infection and Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirza, S.; Bradley, P.J.; Acharya, A.; Stacey, M.; Jones, N.S. Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous malignancy. J. Laryngol. Otol. 2007, 121, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaensen, G.F.; Lim, K.H.; Georgalas, C.; Reinartz, S.M.; Fokkens, W.J. Challenges in the Management of Inverted Papilloma: A Review of 72 Revision Cases. Laryngoscope 2016, 126, 322–328. [Google Scholar] [CrossRef]
- Lisan, Q.; Laccourreye, O.; Bonfils, P. Sinonasal inverted papilloma: From diagnosis to treatment. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 337–341. [Google Scholar] [CrossRef]
- Roh, H.J.; Mun, S.J.; Cho, K.S.; Hong, S.L. Smoking, not human papilloma virus infection, is a risk factor for recurrence of sinonasal inverted papilloma. Am. J. Rhinol. Allergy 2016, 30, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Yang, H.S.; Hong, M.K. Detection of human papillomavirus (HPV) in sinonasal inverted papillomas using polymerase chain reaction (PCR). Am. J. Rhinol. 1998, 12, 363–366. [Google Scholar] [CrossRef]
- Beck, J.C.; McClatchey, K.D.; Lesperance, M.M.; Esclamado, R.M.; Carey, T.E.; Bradford, C.R. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol. Head Neck Surg. 1995, 113, 558–563. [Google Scholar] [CrossRef]
- Beck, J.C.; McClatchey, K.D.; Lesperance, M.M.; Esclamado, R.M.; Carey, T.E.; Bradford, C.R. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol. Head Neck Surg. 1995, 113, 49–55. [Google Scholar] [CrossRef]
- Kraft, M.; Simmen, D.; Casas, R.; Pfaltz, M. Significance of human papillomavirus in sinonasal papillomas. J. Laryngol. Otol. 2001, 115, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Jenko, K.; Kocjan, B.; Zidar, N.; Poljak, M.; Strojan, P.; Zargi, M.; Blatnik, O.; Gale, N. In inverted papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Arch. 2011, 459, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Krouse, J.H. Development of a staging system for inverted papilloma. Laryngoscope 2000, 110, 965–968. [Google Scholar] [CrossRef]
- Makihara, S.; Kariya, S.; Naito, T.; Uraguchi, K.; Matsumoto, J.; Noda, Y.; Okano, M.; Nishizaki, K. Attachment-oriented endoscopic surgical management for inverted papillomas in the nasal cavity and paranasal sinuses. Auris Nasus Larynx 2019, 46, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Katori, H.; Nozawa, A.; Tsukuda, M. Histopathological parameters of recurrence and malignant transformation in sinonasal inverted papilloma. Acta Otolaryngol. 2006, 126, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Orita, Y.; Gion, Y.; Miki, K.; Ikegami, K.; Marunaka, H.; Makino, T.; Akagi, Y.; Akisada, N.; Tsumura, M.; et al. Young adult patients with squamous cell carcinoma of the tongue strongly express p16 without human papillomavirus infection. Acta Otolaryngol. 2019, 139, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Orita, Y.; Gion, Y.; Tachibana, T.; Ikegami, K.; Marunaka, H.; Makihara, S.; Yamashita, Y.; Miki, K.; Makino, T.; Akisada, N.; et al. Laryngeal squamous cell papilloma is highly associated with human papillomavirus. Jap. J. Clin. Oncol. 2018, 48, 350–355. [Google Scholar] [CrossRef]
- Saiki, Y.; Gion, Y.; Nishikori, A.; Norimatsu, Y.; Sato, Y. Comparison of the Hybrid Capture II Method with a PCR-Based Screening Method Using a Carboxyfluorescein-Labeled Primer for Detecting Human Papillomavirus in Cervicovaginal Liquid-Based Cytology. J. Mol. Pathol. 2020, 1, 9–18. [Google Scholar] [CrossRef]
- Sengüven, B.; Baris, E.; Oygur, T.; Berktas, M. Comparison of methods for the extraction of DNA from formalin-fixed, paraffin-embedded archival tissues. Int. J. Med. Sci. 2014, 11, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Panchal, N.K.; Bhale, A.; Chowdary, R.; Verma, V.K.; Beevi, S.S. PCR Amplifiable DNA from Breast Disease FFPE Section for Mutational Analysis. J. Biomol. Tech. 2020, 31, 1–6. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transp. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Pähler Vor der Holte, A.; Fangk, I.; Glombitza, S.; Wilkens, L.; Welkoborsky, H.J. Prognostic factors and risk factors for development and recurrence of sinonasal papillomas: Potential role of different HPV subtypes. Eur. Arch. Otorhinolaryngol. 2020, 277, 767–775. [Google Scholar] [CrossRef]
- Moon, I.J.; Lee, D.Y.; Suh, M.W.; Han, D.H.; Kim, S.T.; Min, Y.G.; Lee, C.H.; Rhee, C.S. Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am. J. Rhinol. Allergy 2010, 24, 325–329. [Google Scholar] [CrossRef]
- Lisan, Q.; Laccourreye, O.; Bonfils, P. Sinonasal Inverted Papilloma: Risk Factors for Local Recurrence After Surgical Resection. Ann. Otol. Rhinol. Laryngol. 2017, 126, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Heard, I.; Palefsky, J.M.; Kazatchkine, M.D. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antivir. Ther. 2004, 9, 13–22. [Google Scholar] [PubMed]
- Yong, M.; Parkinson, K.; Goenka, N.; O’Mahony, C. Diabetes and genital warts: An unhappy coalition. Int. J. STD AIDS 2010, 21, 457–459. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef]
- Reinholdt, K.; Thomsen, L.T.; Munk, C.; Dehlendorff, C.; Aalborg, G.L.; Carstensen, B.; Jørgensen, M.E.; Kjaer, S.K. Incidence of human papillomavirus-related anogenital precancer and cancer in women with diabetes: A nationwide registry-based cohort study. Int. J. Cancer 2021, 148, 2090–2101. [Google Scholar] [CrossRef]
- Mor, A.; Dekkers, O.M.; Nielsen, J.S.; Beck-Nielsen, H.; Sørensen, H.T.; Thomsen, R.W. Impact of Glycemic Control on Risk of Infections in Patients with Type 2 Diabetes: A Population-Based Cohort Study. Am. J. Epidemiol. 2017, 186, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ashour, W.; Twells, L.; Valcour, J.; Randell, A.; Donnan, J.; Howse, P.; Gamble, J.M. The association between diabetes mellitus and incident infections: A systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res. Care 2017, 5, e000336. [Google Scholar] [CrossRef]
- Han, J.K.; Smith, T.L.; Loehrl, T.; Toohill, R.J.; Smith, M.M. An evolution in the management of sinonasal inverting papilloma. Laryngoscope 2001, 111, 1395–1400. [Google Scholar] [CrossRef]
- Cannady, S.B.; Batra, P.S.; Sautter, N.B.; Roh, H.J.; Citardi, M.J. New staging system for sinonasal inverted papilloma in the endoscopic era. Laryngoscope 2007, 117, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Gras-Cabrerizo, J.R.; Montserrat-Gili, J.R.; Massegur-Solench, H.; León-Vintró, X.; De Juan, J.; Fabra-Llopis, J.M. Management of sinonasal inverted papillomas and comparison of classification staging systems. Am. J. Rhinol. Allergy 2010, 24, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, D.; Xiong, X.S. Roles of human papillomavirus infection and stathmin in the pathogenesis of sinonasal inverted papilloma. Head Neck 2016, 38, 220–224. [Google Scholar] [CrossRef]
- Alvarez-Aldana, A.; Martínez, J.W.; Sepúlveda-Arias, J.C. Comparison of five protocols to extract DNA from paraffin-embedded tissues for the detection of human papillomavirus. Pathol. Res. Pract. 2015, 211, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, B.J.; Hošnjak, L.; Poljak, M. Detection of alpha human papillomaviruses in archival formalin-fixed, paraffin-embedded (FFPE) tissue specimens. J. Clin. Virol. 2016, 76, S88–S97. [Google Scholar] [CrossRef]
- Božić, L.; Jovanović, T.; Šmitran, A.; Janković, M.; Knežević, A. Comparison of HPV detection rate in formalin-fixed paraffin-embedded tissues of head and neck carcinoma using two DNA extraction kits and three amplification methods. Eur. J. Oral. Sci. 2020, 128, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Noel, J.E. Etiology of sinonasal inverted papilloma: A narrative review. World J. Otorhinolaryngol. Head Neck Surg. 2017, 3, 54–58. [Google Scholar] [CrossRef]
- Scheel, A.; Lin, G.C.; McHugh, J.B.; Komarck, C.M.; Walline, H.M.; Prince, M.E.; Zacharek, M.A.; Carey, T.E. Human papillomavirus infection and biomarkers in sinonasal inverted papillomas: Clinical significance and molecular mechanisms. Int. Forum Allergy Rhinol. 2015, 5, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Stepp, W.H.; Farzal, Z.; Kimple, A.J.; Ebert, C.S., Jr.; Senior, B.A.; Zanation, A.M.; Thorp, B.D. HPV in the malignant transformation of sinonasal inverted papillomas: A meta-analysis. Int. Forum Allergy Rhinal. 2021, 11, 1461–1471. [Google Scholar] [CrossRef]
- McCormick, J.P.; Suh, J.D.; Lee, J.T.; Wells, C.; Wang, M.B. Role of High-Risk HPV Detected by PCR in Malignant Sinonasal Inverted Papilloma: A Meta-Analysis. Laryngoscope 2021. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yoon, J.K.; Citardi, M.J.; Batra, P.S.; Roh, H.J. The prevalence of human papilloma virus infection in sinonasal inverted papilloma specimens classified by histological grade. Am. J. Rhinol. 2007, 21, 664–669. [Google Scholar] [CrossRef]
- Nishino, K.; Sekine, M.; Kodama, S.; Sudo, N.; Aoki, Y.; Seki, N.; Tanaka, K. Cigarette smoking and glutathione S-transferase M1 polymorphism associated with risk for uterine cervical cancer. J. Obstet. Gynaecol. Res. 2008, 34, 994–1001. [Google Scholar] [PubMed]
- Goudakos, J.K.; Blioskas, S.; Nikolaou, A.; Vlachtsis, K.; Karkos, P.; Markou, K.D. Endoscopic Resection of Sinonasal Inverted Papilloma: Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2018, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, D.G., Jr.; Keeney, M.G.; Gao, G.; Smith, D.I.; García, J.J.; O’Brien, E.K. Transcriptional activity of HPV in inverted papilloma demonstrated by in situ hybridization for E6/E7 mRNA. Otolaryngol. Head Neck Surg. 2015, 152, 752–758. [Google Scholar] [CrossRef] [PubMed]
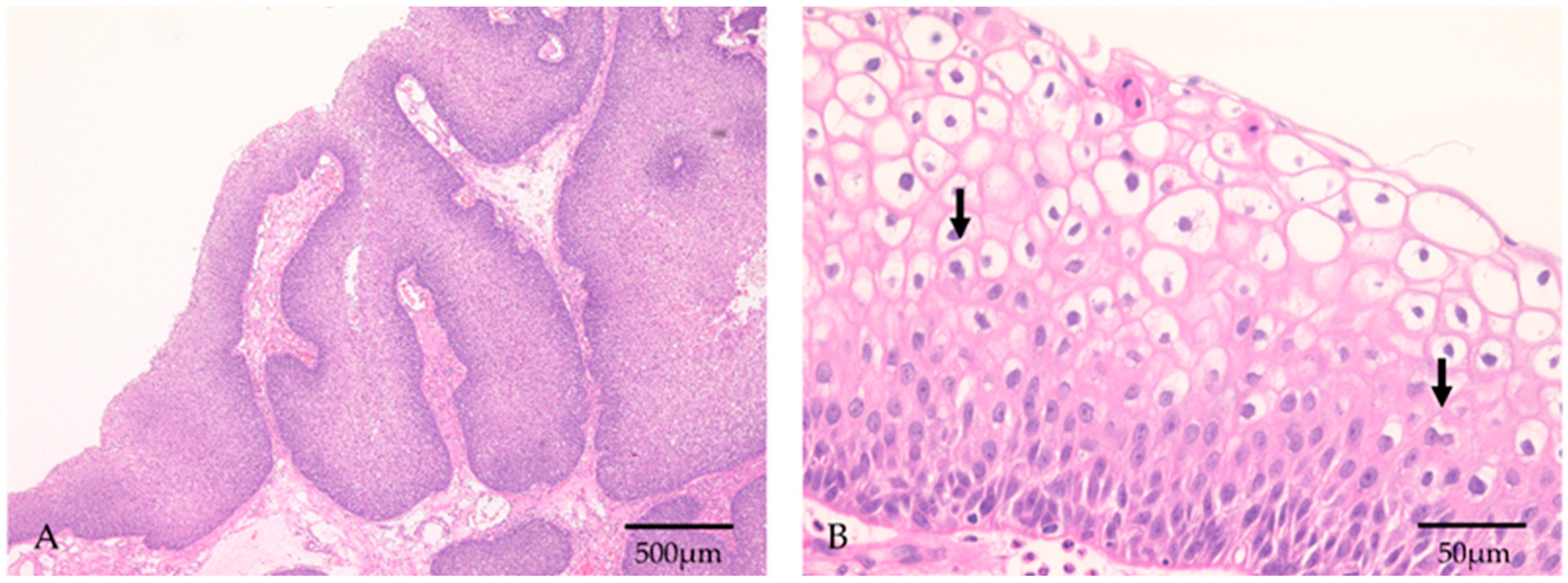
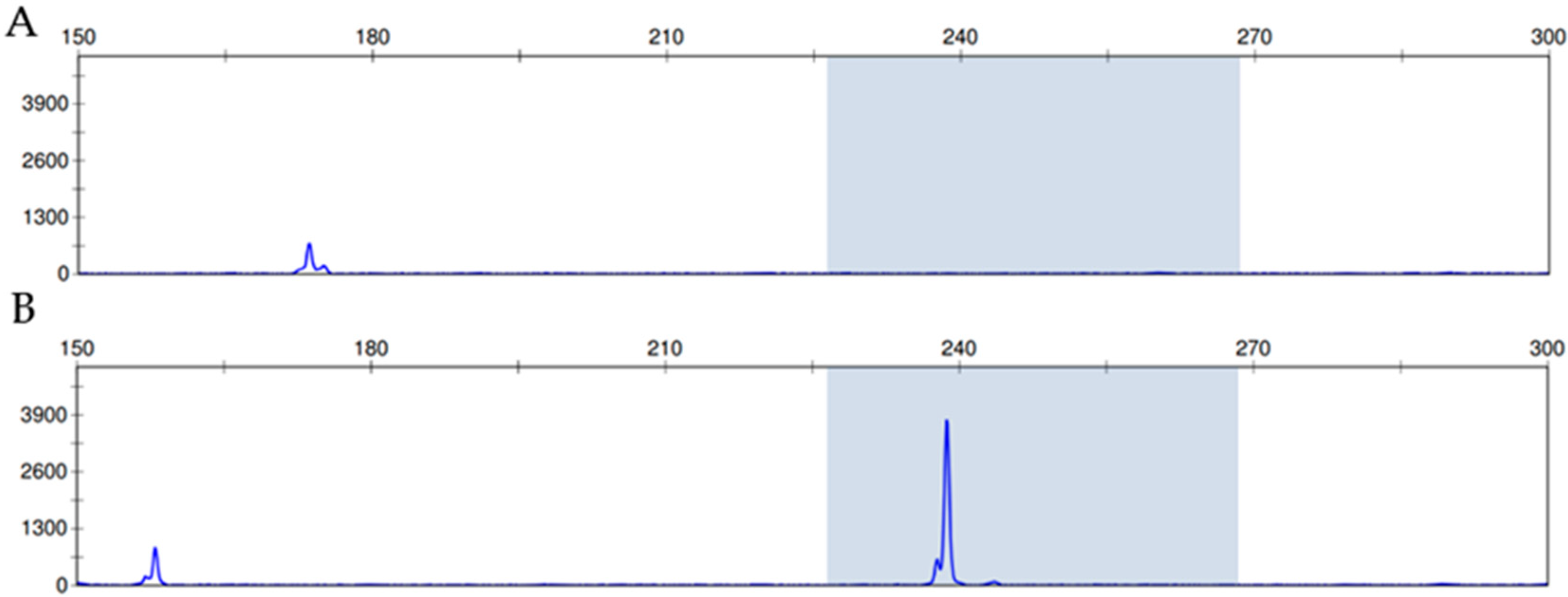
| Recurrence | ||||
|---|---|---|---|---|
| (+) | (−) | p Value | ||
| Sex | Male | 6 | 18 | |
| Female | 1 | 7 | 0.46 | |
| Age | ≥40 | 4 | 23 | |
| <40 | 3 | 2 | 0.02 | |
| Smoker | Yes | 5 | 14 | |
| No | 2 | 11 | 0.46 | |
| Drinker | Yes | 3 | 6 | |
| No | 4 | 19 | 0.33 | |
| History of allergic rhinitis | Yes | 3 | 7 | |
| No | 4 | 18 | 0.45 | |
| History of DM | Yes | 4 | 1 | |
| No | 3 | 24 | <0.01 | |
| History of hypertension | Yes | 3 | 8 | |
| No | 4 | 17 | 0.59 | |
| Staging | T1 or T2 | 0 | 11 | |
| T3 | 7 | 14 | 0.03 | |
| Surgical methods | Endoscopic excision | 6 | 23 | |
| Endoscopic excision combined with external approach | 1 | 2 | 0.61 | |
| EH or presence of SH | (+) | 3 | 12 | |
| (−) | 4 | 13 | 0.81 | |
| HPV high or low risk (PCR) | (+) | 3 | 2 | |
| (−) | 4 | 23 | 0.02 | |
| HPV High or Low Risk (PCR) | ||||
|---|---|---|---|---|
| (+) | (−) | p Value | ||
| Sex | Male | 5 | 19 | |
| Female | 0 | 8 | 0.16 | |
| Age | ≥40 | 2 | 25 | |
| <40 | 3 | 2 | <0.01 | |
| Smoker | Yes | 4 | 15 | |
| No | 1 | 12 | 0.31 | |
| Drinker | Yes | 1 | 8 | |
| No | 4 | 19 | 0.66 | |
| History of allergic rhinitis | Yes | 2 | 8 | |
| No | 3 | 19 | 0.65 | |
| History of DM | Yes | 2 | 3 | |
| No | 3 | 24 | 0.1 | |
| History of hypertension | Yes | 2 | 9 | |
| No | 3 | 18 | 0.77 | |
| Staging | T1 or T2 | 0 | 11 | |
| T3 | 5 | 16 | 0.08 | |
| Surgical methods | Endoscopic excision | 5 | 24 | |
| Endoscopic excision combined with external approach | 0 | 3 | 0.43 | |
| EH or presence of SH | (+) | 3 | 12 | |
| (−) | 2 | 15 | 0.52 | |
| Recurrence | Yes | 3 | 4 | |
| No | 2 | 23 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsumura, M.; Makihara, S.; Nishikori, A.; Gion, Y.; Morito, T.; Miyamoto, S.; Naito, T.; Uraguchi, K.; Oka, A.; Tachibana, T.; et al. Clinicopathologic Analysis of Sinonasal Inverted Papilloma, with Focus on Human Papillomavirus Infection Status. Diagnostics 2022, 12, 454. https://doi.org/10.3390/diagnostics12020454
Tsumura M, Makihara S, Nishikori A, Gion Y, Morito T, Miyamoto S, Naito T, Uraguchi K, Oka A, Tachibana T, et al. Clinicopathologic Analysis of Sinonasal Inverted Papilloma, with Focus on Human Papillomavirus Infection Status. Diagnostics. 2022; 12(2):454. https://doi.org/10.3390/diagnostics12020454
Chicago/Turabian StyleTsumura, Munechika, Seiichiro Makihara, Asami Nishikori, Yuka Gion, Toshiaki Morito, Shotaro Miyamoto, Tomoyuki Naito, Kensuke Uraguchi, Aiko Oka, Tomoyasu Tachibana, and et al. 2022. "Clinicopathologic Analysis of Sinonasal Inverted Papilloma, with Focus on Human Papillomavirus Infection Status" Diagnostics 12, no. 2: 454. https://doi.org/10.3390/diagnostics12020454
APA StyleTsumura, M., Makihara, S., Nishikori, A., Gion, Y., Morito, T., Miyamoto, S., Naito, T., Uraguchi, K., Oka, A., Tachibana, T., Orita, Y., Kariya, S., Okano, M., Ando, M., & Sato, Y. (2022). Clinicopathologic Analysis of Sinonasal Inverted Papilloma, with Focus on Human Papillomavirus Infection Status. Diagnostics, 12(2), 454. https://doi.org/10.3390/diagnostics12020454







